
Effect Croslinking on Characteristics of Silica Chitosan Composite
from Vulcanic Ash of Sinabung Mount and Shrimp Husk by Sol Gel
Method
Lisnawaty Simatupang
1,2
, Manihar Situmorang
2
, Rikson Siburian
3
, Basuki Wirjosentono
3*
1
Chemistry Postgraduate Study Programs, Universitas Sumatera Utara, Medan 20155, Indonesia
2
Department of Chemistry, Universitas Negeri Medan, Jl. Willem Iskandar Psr. V Medan Estate 20371, Indonesia
3
Department of Chemistry, Universitas Sumatera Utara, Medan 20155, Indonesia
Keywords: composit material, cross-link, glutaraldehyde, precursor, sol gel
Abstract: This research was carried out to determine the effect of glutaraldehyd croslinking on the synthesis of silica
chitosan composites from the Na
2
SiO
3
precursor of volcanic ash of Sinabung and chitosan from shrimp husk
by sol gel method. The synthesis of the silica-chitosan composite by mixing the 20 mL Na
2
SiO
3
precursors
with (2% : 3%) (w/v) chitosan in the aid of glutaraldehyde as crosslinking agent and without glutaraldehyde.
Modifications with sol gel is done because it is more simple and rapid progress as well as the binding
reaction of the ligands mobilized. Prepared Silica Chitosan Composit using glutaraldehyd labeled (Si-g-
ChC) and Silica Chitosan Composit without glutaraldehyd labeled (SiChC) . The characteristic of the
composit material (Si-g-ChC) and (SiChC) used by FTIR and XRD.
1 INTRODUCTION
The volcanic ash of Sinabung mount has the main
content of Silica (SiO
2
) which is very abundant
based on previous research (Barasa et al., 2013;
Kusmartini et al., 2017; Simatupang et al., 2016).
This potential SiO
2
content can be used as a basic
ingredient in making silica-based adsorbents (Barasa
et al., 2013). Silica can be used as an absorben
because of its high porosity, high mechanical
strength, high thermal stability, high pore surface
area, stable surface in acidic medium, non-fluffy,
resistant to microbes and low prices. Previous
research shows that the silica gel be successful
synthesized by using of volcanic ash base on
Sinabung mount. The silica gel consists of –OH
from Si-OH and Si-O from Si-O-Si. The silica gel is
well generated on this research is amorphous,
average pore radius of 1.5469x10
-1
Å, the surface
area of silica gel is 374,994 m²/g which is it possible
to be applied as an adsorbent (Simatupang et al.,
2018). The susceptibility of using silica gel is the
low ability of its surface to interact with heavy metal
ions so that silica gel is unable to function as an
effective adsorbent for heavy metal ions (Astuti et
al., 2012).
Abundant shrimp husk waste can be used as a
basis for making chitosan. Chitosan can be used in
the adsorption process because it is rich in amino
and hydroxyl groups as chelating, biocompability,
biodegradation and high adhesion power
(Kolodynska D,. 2011; Budnyak ТM, et al., 2013; Li
et al., 2009; Hu et al., 2018; Li et al., 2018; Badwan
et al., 2015). Chitosan without modification has low
mechanical strength and low solubility in acidic
medium. The amine groups in chitosan are unstable
in acidic conditions and cause protonation.
Therefore chitosan in original form generally does
not have specific selectivity for certain types of
heavy metals such as complex pollutants in water or
wastewater, even though the chitosan has high
content of amine and hydroxyl groups. The
modification of silica surface with chitosan by sol
gel method will produce silica chitosan composite
adsorbent in the development of technical
Simatupang, L., Situmorang, M., Siburian, R. and Wirjosentono, B.
Effect Croslinking on Characteristics of Silica Chitosan Composite from Vulcanic Ash of Sinabung Mount and Shrimp Husk by Sol Gel Method.
DOI: 10.5220/0008875902110214
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 211-214
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
211

nanomaterials, due to performance benefits such as
ease of synthesis, reduction in size, weight, and
multifunction. The sol-gel process provides many
advantages such as reaction conditions at room
temperature, easy and simple, high purity and
homogen, uniform and small size due to the binding
process going on simultaneously ( Zhao et al., 2017;
Budnyak et al., 2015; Budnyak et al., 2016). The
improvement effectivity of silica-organic polymer-
based materials because it combines the hardness
properties of silica and the functional properties of
chitosan polymers (Shchipunov et al., 2004). The
purpose of this research was study the effect of the
use of glutaraldehyd croslinking on the formation of
composite materials and their characteristics.
2 RESEARCH METHODS
2.1 Materials
The Na2SiO3 solution precursor from volcanic ash
of Sinabung mount and shrimp husk were obtained
on previous research. Reagents consist of HNO
3
,
NaOH, HCl, CH
3
COOH, etanol, and glutaraldehyd
25% were Analytical Grade (AR) from E-Merck..
.
2.2 Instrumentations
Equipment includes: analytic balance, mortar, 200
mesh sieve, desiccator, filter paper whatman 42,
magnetic strirer, universal indicator, buchner funnel,
glassware and plastic glass container. The
measurements was made by using a Fourier
Transform Infra Red (FTIR) Bruker spectrometer
equipped with a Digitec detector (Shimadzu),
Rigaku ZSX X- Ray Difraction (XRD) (Shimadzu
XRD 6000).
2.3 Procedure
2.3.1 Synthesis of Precursor Na
2
SiO
3
Solution
The volcanic ash as much as 20 g was soaked into
120 mL HNO
3
for 24 hours. Dried on the oven at T
= 120°C for 6 hours and then weight was recorded.
Subsequently, the volcanic ash was destructed with
156 mL NaOH 4M until the viscous on the furnace
at T = 500 °C during 30 minutes. After that, it was
added 200 mL water for 24 hours. Finally the
solution was filtered as Na-Silicat (Na
2
SiO
3
)
(Simatupang et al., 2018).
2.3.2 Synthesis of Silica-based Chitosan
Composite
The Na
2
SiO
3
solution as much as 20 mL put into a
plastic glass containers. The other plastic glass
containers were put (0.2; 0.3) g chitosan and
dissolved in 10 ml of acetic acid (2%, v / v) and then
stirred for 1 hour to form (2%, 3% ) w/v chitosan
solution. The glutaraldehyde of 5% as much as 1
mL was put into a chitosan solution (2%, 3%) and
stirred vigorously for 5 minutes and in another
plastic glass containers (2%, 3%) chitosan solution
without glutaraldehyde. Then the mixture of
chitosan and glutaraldehyde is poured into a sodium
silicate solution while stirring with a magnetic
stirrer. Chitosan solution without glutaraldehyde is
added to the other Na
2
SiO
3
solution. Gel formation
is done by adding 3M HCl drops to pH 7. The gel is
left overnight, filtered, washed with distilled water.
The obtained gel is dried at 70° with the use of a
vacuum pump. The obtained gel was sieved with a
200 mesh sieve. The end product is named as silica
chitosan composite material. Silica chitosan
composite are labelled based on the composition of
the chitosan, successively the composites material
prepared from 20 mL of Na
2
SiO
3
with 2% chitosan
used glutaraldehyd as crosslinking is labeled as
composite S-g-ChC1, with 3% chitosan is composite
S-g-ChC2. While 20 mL of Na
2
SiO
3
and 2%
chitosan without glutaraldehyd is labeled as SChC1,
and 3% chitosan without glutaraldehyd as SChC2.
Characterizations of Silica Chitosan
Composit
The methods for silica chitosan composit research
are versatile. X-Ray Diffraction (XRD) data at room
temperature using an X-ray diffractometer (Siemens
D 500) with copper anticathode radiation
(λCuKα=1.541838
Å) at 2θ from 7 to 70°. Fourier
Transform Infrared (FTIR) spectra were obtained on
a Vertex 70 spectrometer equipped with a digital
detector, via the conventional KBr pellet method.
The samples were scanned in transmission mode
with 2 and 4 cm
-1
resolution, at the range of 4000 to
400 cm
-1
.
3 RESULTS AND DISSCUSION
The preparation of silica chitosan composite (S-g-
ChC) was done by mixing 20 mL of Na
2
SiO
3
and
chitosan with variation (20 mL: 2%), (20 mL: 3%)
using glutaraldehyde as crosslinker, and silica
chitosan composite without glutaraldehyd (SChC)
with variation (20 mL: 2%), (20 mL, 3%). The
modification of the silica surface was done using sol
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
212
gel method. Chitosan has a high affinity to the
surface due to the interaction between part of
protonated amino groups of polymer and dissociated
hydroxyl groups of silica.
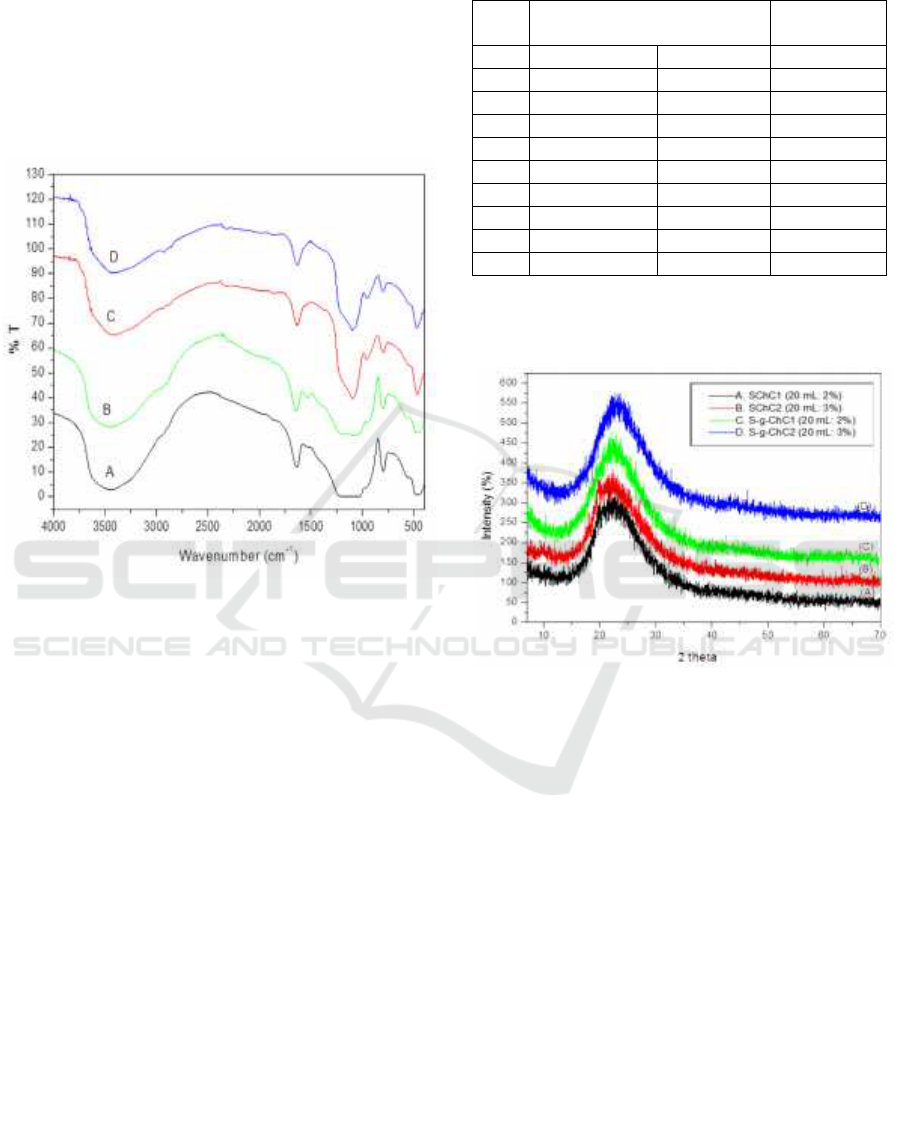
The FTIR spectrum of the synthesized silica
chitosan composit with glutaraldehyd as
crosslinking (S-g-ChC) show fig 1(A) and (B),
without glutaraldehyd (SChC) show fig 1 (C) and
(D) .
Figure 1: The FTIR spectra of: (A) S-g-ChC1 (20
mL:2%), (B) S-g-ChC2 (20 mL:3%), (C) SChC1 (20
mL:2%), and (D) SChC2 (20 mL:3%).
The formation of silica chitosan composites
occurs because silanol groups on the surface of silica
take hold of important role in the modification of
chitosan copolymers. FTIR results show a
significant difference in wave numbers between
silica chitosan composites using crosslinking
glutaraldehydes S-g-ChC and without crosslinking
glutaraldehydes (SChC). At S-g-ChC, the wave
number for O-H, C-H, and C-O group stretch
vibrations appears to shift to a higher wave number
compared to SChC. The wave number shift is due to
the cross link formed between the chitosan polymer
and silica. When silica gel is introduced into
chitosan copolymers, the number of hydroxyl groups
(Si-OH) increases which results in an increase in
hydrogen interactions between the copolymer matrix
and silica gel (Nithya et al., 2016). This cross
linking causes the movement of molecules to be
more limited, so that more energy is needed to
conduct vibrations (Bin et al., 2013)
Table 1: Analysis of S-g-ChC and SChC function groups
based on FTIR spectrum.
No
Wave number
Streaching
vibration
1
S-g-ChC1
SChC1
3447,44
3421,72
-OH
2928,57
2927,94
-CH
1637.71
1631.78
-C=N
1099.42
1095.57
-Si-O
2
S-g-ChC2
SChC2
3436,05
3421.72
-OH
2927,35
2927.94
-CH
1639,48
1631.78
-C=N
1095,10
1091.71
-Si-O
This result was further supported by the XRD.
The XRD of SChC and S-g-ChC show Fig 2.
Figure 2: The XRD pattern of: (A) and (B) komposit silika
chitosan without crosslinking glutaraldehyde (SChC); (C)
and (D) komposit silika chitosan wih crosslinking
glutaraldehyde S-g-ChC
Figure 2 that silica chitosan composites with
various variations of chitosan have a peak pattern
widened which represent a low degree of
crystallization (amorphous). The decrease in
chitosan crystallinity evidence the conjugation
between chitosan and silica polymer chains so that it
suppresses crystallinity to a certain extent. The silica
and chitosan polymer chains are well mixed at the
molecular level wherein that the peak of SChC is
more lowest than S-g-ChC (Gandhi et al., 2012).
There is also a 2θ difference between SChC and S-g-
ChC where SChC at 2θ = 20-22 while S-g-ChC at 2θ
= 22-23. This is due the silica chitosan composite S-
g-ChC has greater conjugation of silica and chitosan
compared to SChC. This can occur because the
glutaraldehyde as cross-linker on the composit S-g-
ChC will cause a higher density form so that the
Effect Croslinking on Characteristics of Silica Chitosan Composite from Vulcanic Ash of Sinabung Mount and Shrimp Husk by Sol Gel
Method
213

chemical bond will be much stronger compared to
SChC which has a lower density.
4 CONCLUSIONS
The silica chitosan composites from precursors of
sodium silicate (Na
2
SiO
3
) from volcanic ash of
Sinabung mount and chitosan from shrimp husk
combined with glutaraldehyde crosslink (S-g-ChC)
and without cronsslink glutaraldehyde (SChC) by
sol gel method have been successfull. The results of
the FTIR and XRD analysis showed differences in
the characteristics of the two composites due to the
glutaraldehyde crosslink effect.
ACKNOWLEDGEMENTS
Acknowledgment our sincere thanks to BOPTN
Universitas Negeri Medan for their support and
funds provided for the completion of this work,
Rector of Universitas Negeri Medan and Head of
Unimed Research Institute and other peoples who
helped a lot in this research.
REFERENCES
Astuti, M. D., Nurmasari, R., Mujiyanti DR., 2012.
Imobilisasi 1,8 Dihidroxyanthraquinon Pada Silika
Gel Melalui Proses Sol-Gel. Sains dan Terapan Kimia.
6(1), 25-34.
Badwan, A.A., Rashid, I., Al Omari, M.M.H. and Darras,
F.H. 2015. Chitin and Chitosan as Direct Compression
Excipients in Pharmaceutical Applications. Mar.
Drugs. 13, 1519-1547.
Li, B., Shan, C.L., Zhou, Q., Fang, Y., Wang, Y.L., Xu,
F., Han, L.R., Ibrahim, M., Guo, L.B., Xie, G.L.,
2013. Synthesis, characterization, and antibacterial
activity of cross-linked chitosan-glutaraldehyde. Mar.
Drugs. 11, 1534–1552.
Barasa RF, Rauf A, Sembiring M., 2013. Dampak Debu
Vulkanik Letusan Gunung Sinabung Terhadap Kadar
Cu, Pb, Dan B Tanah Di Kabupaten Karo. Jurnal
Online Agroekoteknologi. 1(4), 1288-1297.
Budnyak, ТM., Tetykh, VА., Yanovska, ES., 2013.
Chitosan and its Derivatives as Sorbents for Effective
Removal of Metal Ions. Surface. Suppl, 20, 118–34.
Budnyak, TM., Pylypchuket, LV., Tertykh, VA.,
Yanovska, ES., Kolodynska, D., 2015. Synthesis and
adsorption properties of chitosan-silica nanocomposite
prepared by sol-gel method. Nanoscale Research
Letters. 10, 87.
Budnyak, T.M., Yanovska, E.S., Kichkiruk, O.Y., Sternik,
D., and Tertykh, V.A., 2016. Natural Minerals Coated
by Biopolymer Chitosan: Synthesis, Physicochemical,
and Adsorption Properties. Nanoscale Research
Letters. 11, 492.
Gandhi, MR., Meenakshi., 2012. Preparation and
Characterization of Silica Gel/Chitosan Composite For
the Removal of Cu(II) and Pb(II). International
Journal of Biological Macromolecules. 50(3), 650–
657.
Hu, Z., Zhang, D.Y., Lu, S.T., Li, P.W., and Li, S.D.,
2018. Chitosan-Based Composite Materials for
Prospective Hemostatic Applications, Mar. Drugs. 16,
273.
Kolodynska, D., 2011. Chitosan as an Effective Low-Cost
Sorbent of Heavy Metal Complexes with the
Polyaspartic Acid. Chem Eng J., 173:520–9.
Kusmartini, I., Syahfitri, W.Y.N., Kurniawati, S., Lestiani,
D.D., M. Santoso, M., 2017. Journal of Physics:
Conf. Series. 860.
Li C, Champagne P., 2009. Fixed-Bed Column Study for
the Removal of Cadmium (II) and Nickel (II) Ions
from Aqueous Solutions Using Peat and Mollusk
Shells. Journal of Hazardous Materials. 171, 872.
Li, J., Cai, C., Li, J., Li, J., Li, J., Sun, T., Wang, L., Wu,
H., and Yu, G., 2018. Chitosan-Based Nanomaterials
for Drug Delivery, Molecules. 23, 2661.
Nithyaa. R., Gomathi, T., Sudha, P.N., Venkatesan, J.,
Anil. S., Kim, S.K., 2016. Removal of Cr(VI) from
aqueous solution using chitosan-g-
poly(butylacrylate)/silica gel nanocomposite,
International Journal of Biological Macromolecules.
87, 545-554,
Shchipunov, A.Y., Karpenko, Y.T, Bakunina, Y.I,
Burtseva, V.Y, Zvyagintseva, N.T., 2004. A New
Precursor for the Immobilization of Enzymes Inside
Sol–Gel-Derived Hybrid Silica Nanocomposites
Containing Polysaccharides, J. Biochem. Biophys
Methods. 58, 25–38.
Simatupang L., Devi., 2016. The preparation and
characterization of Sinabung volcanic ash as silica
based adsorbent. Jurnal Pendidikan Kimia, 8(3), 9-13.
Simatupang, L., Siburian, R., Sitanggang, P., Doloksaribu,
M., Situmorang, M., Marpaung, M., 2018. Synthesis
and Application of Silica Gel Base on Mount
Sinabung’s Fly Ash For Cd(II) Removal With Fixed
Bed Column. Rasayan J.Chem. 11(2), 819-827.
Zhou, D., Qi, C., Chen, Y.X., Zhu, Y.J., Sun, T.W., Chen,
F., and Zhang, C.Q., 2017. Comparative study of
porous hydroxyapatite/ chitosan and
whitlockite/chitosan scaffolds for bone regeneration in
calvarial defects. International Journal of
Nanomedicine. 12, 2673–268.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
214
