
Making Hydrogel with Crosslinked Reactions between Chitosan and
Dialdehyide Cellulose from Coconut Fiber as Wound Healers
Firman Sebayang
1*
, Rumondang Bulan
1
, Emma Zaidar Nasution
1,2
,
M. Zulham Efendi Sinaga
1,2
and Windi Anggara Putri
1
1
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Medan, 20155, Indonesia
2
Pusat Unggulan IPTEK (PUI) Kitosan dan Material Maju Universitas Sumatera Utara, Indonesia
Keywords: α-Cellulose, Hydrogel, Chitosan, Dialdehyde Cellulose, Cross-linking.
Abstract: Making hydrogels from crosslinked reactions between cellulose oxidized with chitosan through Schiff base
formation reaction has been investigated as a wound healing drug in vivo. α-Cellulose obtained from the
isolation of coconut fiber by 14.24 g. α-Cellulose is oxidized by using KIO
4
to be dialdehyde cellulose. The
degree of oxidation of cellulose dialdehyde is 86%. Hydrogels were made by schiff base crosslinking
reaction between chitosan and dialdehyde cellulose with temperature variations of 75, 100, 125 and 150 oC.
The optimum temperature in hydrogel synthesis is 100 oC. the formation of a hydrogel is supported by the
presence of FTIR Spectrophotometer where the spectrum of 1643.35 cm-1 is formed where the group -
C=N- which shows the formation of a Schiff base reaction. The hydrogel that is obtained has a good
swelling ability of more than 1000%. Invivo analysis was carried out for 7 days in mice and as a result, the
injured mice have recovered and have not left a mark.
1 INTRODUCTION
Coconut coir is one of the biomass that is easily
obtained and is a by-product of agriculture. The coir
composition in coconuts is about 35% of the overall
weight of the coconut fruit. Coconut coir consists of
fiber (fiber) and cork (pitch) which connects one
fiber to another fiber. Coconut coir consists of 75%
fiber and 25% cork. Coconut coir fibers contain
lignin (35% - 45%) and cellulose (23% -43%)
(Carrijo, et al., 2002).
Cellulose is a linear condensed polymer
composed of D-anhydroglucopiranose units bound
by β-1,4-glycosidic bonds (Kalia, 2011). Cellulose
has one reducing group containing a non-substituted
hemiacetal, and one non-reducing group containing
an additional hydroxy group in C4. There are 2 main
crystalline cellulose arrangements, namely cellulose
I and cellulose II. Almost all initial cellulose consists
of cellulose I. Cellulose which has been dissolved
and deposited (regenerated) or through treatment
with concentrated alkaline solution and rinsed with
water (mercerized) consists of cellulose II. The
change in cellulose I to cellulose II is irreversible
(Wertz, 2010).
Chitosan is a natural polysaccharide obtained
from chitin deacetylation. If most of the acetyl
groups in chitin are substituted by hydrogen atoms
into amine groups by the addition of a strong high
concentration base solution, the result is called
deacetylated chitosan or chitin (Bastman, 1989).
Chitosan is one of the sources of amino natural
polysaccharides known as pH-sensitive properties
(Zhao, 2003).
Cellulose dialdehyde can be produced by
reacting cellulose with potassium periodate. Calcium
periodate is a selective oxidizer that will only break
the C2-C3 bonds so that 2 aldehyde groups are
formed (Höglund, 2015).
Hydrogels are essentially cross-linked polymers
which have the ability to absorb water thousands of
times from their dry weight, but are not soluble in
water due to the presence of a 3-dimensional
structure on the polymer network. Hydrogel is a very
interesting material because of its unique solubility
and water carrying capacity (Erizal, 2010).
The In vivo process is divided into three phases,
namely:
204
Sebayang, F., Bulan, R., Zaidar Nasution, E., Sinaga, M. and Putri, W.
Making Hydrogel with Crosslinked Reactions between Chitosan and Dialdehyide Cellulose from Coconut Fiber as Wound Healers.
DOI: 10.5220/0008869402040210
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 204-210
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

The inflammatory phase lasts from the time of
the wound until the third day. Broken blood vessels
in the wound cause bleeding and the body will try to
stop it with vasoconstriction. Hemostasis occurs
because platelets that come out of blood vessels
stick together and together with fibrin formed
freezes blood coming out of blood vessels.
The proliferation phase is also called fibroplation
because what stands out is the process of fibroblast
proliferation. In this phase the fiber is formed and
destroyed again to adjust to the stress on the wound
that tends to constrict. This property, together with
the contractile properties of miofibroblasts, causes
attraction to the edges of the wound. At the end of
this phase the wound strain strength reaches 25% of
normal tissue. Later, in the process of increasing the
strength of collagen fibers increases due to
intramolecular bonds and between molecules. In this
fibroplasias phase, the wound is filled with
fibroblasts and collagen which forms a reddish
colored tissue with a fine-grained surface called
granulation tissue. The wound edge epithelium
consisting of basal cells is released from the base
and moves to fill the wound surface. The place is
then filled with new cells formed from the mitotic
process. The migration process can only occur in a
lower or flat direction, because the epithelium
cannot migrate in a higher direction. This process
only stops after the epithelium touches and closes
the entire wound surface. With the closed surface of
the wound, the process of fibroplasia with the
formation of granulation tissue will also stop and the
maturation process begins in the phase of
completion.
In this phase there is a maturation process that
consists of excessive tissue reabsorption, shrinkage
and finally new tissue is formed. The body tries to
normalize everything that is abnormal because of the
healing process. During this process, the scar tissue
is pale, thin and weak and easily moved from the
bottom. Maximum shrinkage is seen in wounds. At
the end of this phase, skin wound healing can
withstand stretches of approximately 80% of normal
skin ability (Moenadjat, 2003).
2 MATERIALS AND METHODS
2.1 Tools
The tools used in this study include: glassware,
beaker glass, sieve, petri dish, reaction tube,
analytical balance, oven, hot plate stirrer,
thermometer, desiccator, incubator, Fisher
Scientific, vacuum device, universal indicator,
Aluminum foil, Thermometer , pH meter, Alcohol
Meter, FTIR, SEM.
2.2 Materials
The materials that used in this study include:
Coconut coir, Chitosan, Water, Aquadest, HNO
3
,
NaNO
2
, NaOH, Na
2
SO
3
, NaOCl, H
2
O
2
, KIO
4
,
Acetate buffer, KMnO
4.
Chitosan powder (1, 2, 3 and 4 g) was dissolved
in a 100 ml acetic acid 0.2 M solution at 60 ºC and
600 rpm to produce chitosan solution. Agar powder
(5 g) was dissolved in a 100 ml deionized water at
95 ºC and 600 rpm for 60 minutes. An amount of
20% of glycerol was added to the agar and chitosan
solution as plasticizer. The agar-chitosan film was
fabricated by mixing chitosan solution (1, 2, 3 and 4
g/100 mL) and agar solution (5 g/100 ml) with a
ratio of 1:1. The mixture was stirred at 60 ºC and
600 rpm for 60 minutes. An amount of film solution
was distributed into the template for drying and
casting for 24 hours at 40 ºC. The films were stored
in a desiccator.
2.3 Research Procedure
2.3.1 Preparation of Coconut Fiber Powder
Coconut coir is separated from the outer skin. Then
the fiber is washed with running water. Then the
clean coconut fiber is dried in the sun for 1 day. Dry
coconut coir is cut into smaller pieces and then in a
blender. Then the coconut husk that has been finely
filtered uses an 80 mesh sieve.
2.3.2 Isolation of α-Cellulose from Coconut
Coir
Weighed as much as 75 grams of coconut fiber
powder is put into a glass beaker, then added 2000
ml of 3,5% HNO
3
and 10 mg NaNO
2
then heated
at 90 ℃ for 2 hours while stirring on a hot plate.
Filtered and washed residue until neutral filtrate.
Then added 375 ml of 2% NaOH and 375 ml of 2%
Na2SO3, heated at 50 ℃ for 1 hour while stirring on
the hot plate then filtered and washed away until
neutral filtrate. Then it was bleached with 500 ml of
1.75% NaOCl solution, heated at 70℃ for 30
minutes while stirring on a hot plate. Filtered and
washed residue until neutral filtrate. Then added
with 500 ml of 17.5% NaOH and then heated at
80℃ for 30 minutes while stirring on a hot plate.
Filtered and washed residue until neutral filtrate.
Making Hydrogel with Crosslinked Reactions between Chitosan and Dialdehyide Cellulose from Coconut Fiber as Wound Healers
205

Then added with 250 ml of 10% H
2
O
2
, heated at
60℃ for 15 minutes while stirring on a hot plate.
Filtered and washed residue with aquadest until the
filtrate is neutral. Dried the residue in the oven at
60℃ then stored in the desiccator. (Ohwoavworhua,
2009)
2.3.3 α-Cellulose Oxidation with Potassium
Periodate (KIO
4
)
1 gr of isolated cellulose was immersed in a mixture
of KIO
4
solution and 0.1 M acetate buffer, with a
ratio of KIO
4
and acetate buffer 1: 100 (b / v) and
variation of KIO4 concentration of 0.2; 0.4; 0.6; 0.8;
1.0 mg / ml. Then stir it slowly without light and the
reaction conditions pH 4.5 and temperature 40o C
for 60 minutes. After oxidation, it is washed with
aquadest. The oxidized cellulose is characterized by
FT-IR. (Liu, 2004)
2.3.4 Determination of the Degree of
Oxidation (D.O) of Cellulose
A total of 0.1 grams of sample was dissolved with
10 ml of aquadest. Then added with 10 mL of NaOH
0.1 N. The solution is then heated to complete
dissolution. After that, the solution is cooled. Added
to the 10 mL 0.15 N HCl solution to the pH of the
solution <7. Then add aquadest as much as the
volume decreases during the first heating. The
solution is reheated for 1 minute, then the PP
indicator 2 drops is added. Titrated with 0.1 N
NaOH solution, and observed color changes.
Determination of the degree of oxidation can be
determined through the following equation:
%DO=((C
NaOH
× V
NaOH
)-(C
HCl
× V
HCl
))
/(m/162)×100%
2.3.5 Crosslinking between Oxidized
Chitosan and Cellulose
Chitosan solution was made by: as much as 8.0
grams of chitosan were put into 400 ml of acetic
acid solution 2% (v / v) with constant stirring for 1
hour at 60oC then oxidized cellulose was immersed
in chitosan solution with stirring for 15 minutes at
sushu 60
o
C so that a thick brownish yellow solution
is formed. Then poured into a glass beaker and dried
at a temperature variation of 75, 100, 125 and 150
o
C for 2 hours. (Pratama, A 2018)
2.3.6 Characterization of Hydorgels
Chitosan-dialdehyde Cellulose
FT-IR Analysis.
The sample is prepared in the form of pulp. Porridge
is examined in a thin film placed between flat plates
of salt. The test is done by clamping the mixed film
on the sample site. Then the film is placed on the
plate in the direction of infrared light. The results
will be recorded periodic paper in the form of a
wave flow curve 4000-200 cm-1 to the intensity.
Test of Water Absorption Percentage.
Testing the percentage of water absorption was
carried out by determining the percent swelling ratio
by measuring the initial weight (Wd) of the sample
which was then immersed in distilled water for 24
hours. The soaked samples are then filtered using
filter paper and measured the final weight (Ws).
Measuring the percentage of water absorption in the
hydrogel can be determined by the following
formula (Muthoharoh, 2012):
% S=(Ws-Wd)/Wd x100 % (1)
Where :
% S = Percentage of water absorption (%) (g/g)
Ws = Swollen hydrogel weight (g)
Wd = Dry weight of hydrogel (g)
Cross-linking Test.
The percentage value of crosslinking can be done by
determining the crosslink percent percent where the
dry weight of the resulting hydrogel is weighed.
Then the hydrogel is soaked with a solvent
(chloroform) for 24 hours. After immersion, the
hydrogel is heated at a temperature of 60oC to dry
for 3 hours. The dry weight of the hydrogel after
immersion is determined by weighing using an
analytical balance. The degree of crosslinking can be
determined by the following formula (Muthoharoh,
2012):
% DC= Wg/(Wo ) x 100 (2)
Where,
% DC = degree of crosslinking
Wg = Dry weight of hydrogel (g)
Wo = Swollen hydrogel weight (g)
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
206
Morphological Analysis using Scanning Electron
Microscope (SEM).
The microscopic observation process using SEM
begins with glueing the sample with a stick made of
older metal specimens. Then the sample is cleaned
with a blower, then the sample is coated with gold
and palladium with a dionspater machine
pressurized 1492x10
-2
atm. The sample is then put
into a special room and then illuminated with 10
Kvolt-powered electrons so that the sample emits
secondary electrons and the electrons are detected by
a scientor detector which is then amplified by an
electric circuit that causes a chatode ray tube (CRT)
image. Shooting is done after selecting a specific
part of the object (sample) and the desired
magnification so that a clear photo is obtained
(Negulescu, 2004).
Antimicrobial Activity.
Antimicrobial activity of hydrogels was performed
on two types of microbes, i.e., S. Aureus and E.
Coli. The microbes culture were diluted in
accordance with the standards McFarland, each
inoculated into petri dishes containing Mueller
Hinton agar. Then the disc was inserted into the
blends of dialdehyde cellulose/chitosan in a petri
dish aseptically. The petri dish then put in an
incubator at 35 °C for 24 h. After 24 h, the
antimicrobial zone (clear zone) was observed and
measured in diameter by using a caliper.
In Vivo.
Before cutting, the hair around the femur was shaved
and anesthetized using lidocaine 0.2 cc in 2 cc
aquadest, then injured. The injury is done at the
femur of the mouse by making an incision with a
length of 20.0 mm and a width of 1.8 mm using a
sterile scalpel then handled. Handling is done twice
a day, before giving the sample to the wound,
always clean it first using distilled water. Giving the
sample is done by applying it to the part of the
wound on the femur of the mice, ie in the morning
and evening, for 7 days after induction of the wound
using a cotton bud. As a comparison, negative
controls and positive controls were used using
Salticin Gentamicin Sulfate.
3 RESULTS AND DISCUSSIONS
In this study α-Cellulose can be isolated from
coconut fiber waste. Where from 75 grams of
coconut coir powder isolated 14.24 grams of pure
cellulose were obtained or about 18.98% of the
initial mass of coconut husk samples used
α-Cellulose is oxidized using potassium periodate
which is a selective oxidizer. Potassium periodate
will break the C2-C3 bond which has a secondary
alcohol group so that it becomes 2 aldehyde groups.
Figure 1: Cellulose Oxidation Reaction with Periodate.
The reaction between watershed and chitosan is
an imine group formation reaction (Schiff base),
where the primary amine group in chitosan will react
with carbonyl aldehyde groups found in the
watershed to form an imine group (R-C = N-).
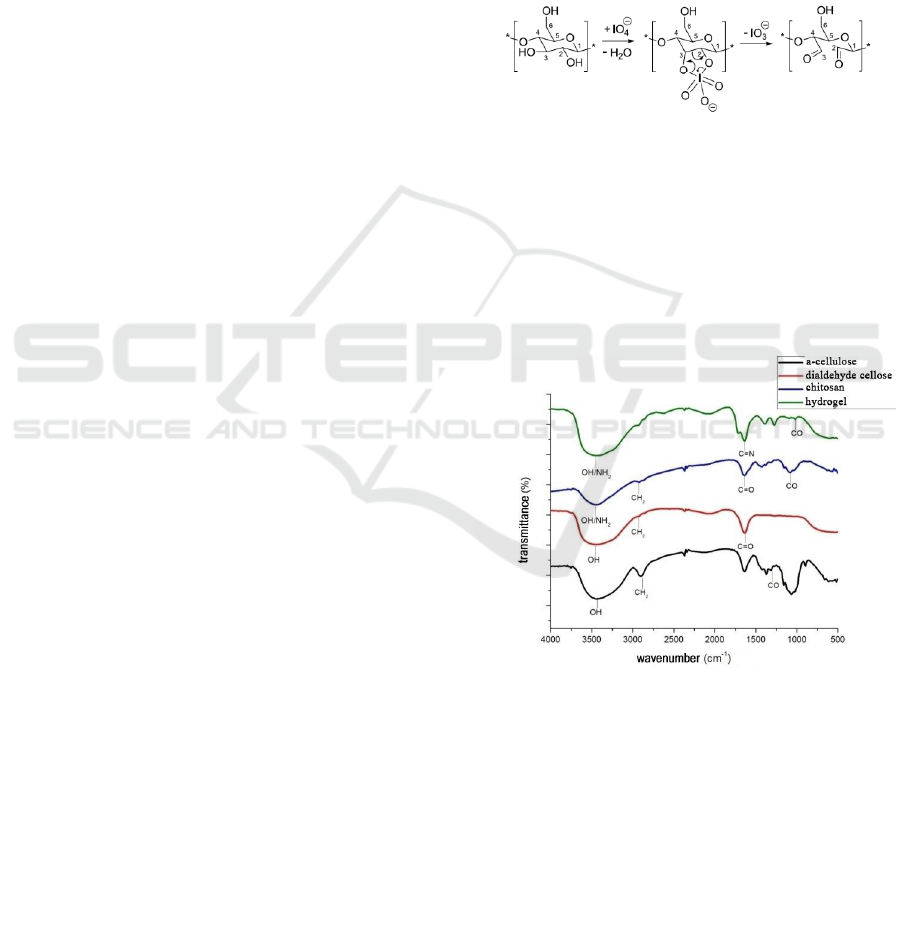
3.1 FT-IR Analysis
Figure 2: Spectrum FT-IR α-Cellulose, DAS, Chitosan,
Hydrogel.
The results of the analysis of the α-cellulose
functional group used for the study showed that the
absorption of wavelengths of 3448.72 cm
-1
showed
the presence of a bound -OH group found in α-
cellulose compounds, the absorption of a wavelength
of 2900.94cm
-1
indicated the presence of a group -
CH sp3 and the absorption of wavelengths of
1064.71cm
-1
indicates the presence of -CO- groups.
Making Hydrogel with Crosslinked Reactions between Chitosan and Dialdehyide Cellulose from Coconut Fiber as Wound Healers
207

The results of the analysis of the cellulose
dialdehyde (DAS) functional group used for the
study showed that the wavelength absorption of
3441.01cm
-1
showed the presence of a bound -OH
group found in cellulose dialdehyde compound
(DAS), with wavelength uptake of 2924.09cm
-1
shows the presence of -CH sp3 group and the
presence of wavelength uptake of 1026.15cm
-1
shows the presence of -CO- group and at wavelength
absorption of 1635.64cm
-1
indicates the presence of
–C = O-.
In the FTIR spectrum of chitosan powder, there
was an absorption peak at wave number 3448.72 cm
-
1
which showed the free peak absorption of O-H and
nitrogen amine (-NH
2
). With the presence of N-H
absorption peaks, it is the main characteristic of
chitosan structure. In addition, the presence of
absorption peaks in wave numbers can also be
referred to as marking N-H stretching primary
aliphatic amines (Rohman A, 2014). According to
(Fessenden and Fesssenden, 1982) if there are two
hydrogens on a nitrogen amine (-NH
2
), absorption of
N-H appears as a twin peak. This is not proven by
the results of the FTIR spectrum of chitosan powder
analyzed showing that there were no twin peaks on
the absorption band around 3000 - 3700 cm
-1
. In
addition there is an absorption peak at wave number
2924.09 cm
-1
indicating the presence of –C-H sp3
bond. Furthermore, the absorption peak at wave
number 1265.30 cm
-1
indicates the presence of C-O
and C-N groups. The absorption bands of C-O and
C-N groups are usually not easy to identify in the
fingerprint area because this spectrum area often
contains many peaks that overlap and are difficult to
identify (Fessenden and Fesssenden, 1982). But on
the results of the spectrum analysis of chitosan
powder used for research, CO and CN fingerprint
areas are easy to identify. One of the factors that
supports this convenience is that chitosan powder
used in the study can be categorized as pure. In
addition, where this CN group is also a characteristic
typical functional groups found in chitosan
molecules. From the description above, it can be
seen that the results of functional group analysis
using FTIR spectrophotometer on chitosan powder
have no changes in the functional groups composed
of chitosan chemical molecules or there are no other
groups that absorb infrared absorption from FTIR
spectrophotometers so that chitosan powder used in
research is pure chitosan compound.
On the results of the hydrogel functional group
analysis used for the study, the wavelength uptake of
3441.01 cm
-1
showed the presence of a bound -OH
group found in the hydrogel compound, the
wavelength absorption of 2924.09 cm
-1
indicated the
presence of the -CH sp3 group . In the crosslinking
reaction between α-cellulose with chitosan, an imine
bridge (Bases Shiff) is formed as a change that links
between chitosan compounds and α-cellulose. The C
= N group causes vibrations on wavelength
absorption of 1643.35 cm
-1
but unlike chitosan
uptake in the previous FTIR results, the absorption
of these wavelengths does not form a twin peak
because there is no hydrogen group in the nitrogen
atomic bond chain, replaced by a bond duplicate
between the carbon chain on cellulose dialdehyde
and nitrogen in chitosan compounds. The occurrence
of crosslinking process is also reinforced by the
formation of uptake at a wavelength of 1273.02 as
an amplifier of carbon atoms in the chitosan group
still bound to nitrogen as from chitosan compounds.
Similar to the results of the previous functional
analysis, there was an absorption peak of 3441.01
nm as the absorption peak of the OH group, 2924.09
nm and 2854.65 nm as the absorption peak of the C-
H sp3 and 1064.71 groups as the peak C-O-H
absorption.
3.2 Percentage of Water Absorption
In making the hydrogel, the crosslinking reaction
works well. With the ability to expand well, the air
will be absorbed in crosslinked molecules between
cellulose dialdehyde and chitosan. On heating 75
o
C
produces a percentage of water absorption below
from heating 100
o
C which is 3.91% g / g. This is
evidenced by the destruction of the hydrogel into a
cluster of fine grains proving that the crosslinking
reaction between cellulose and chitosan dialdehyde
has not been fully formed because the temperature
of the crosslinking has not been fully formed. At an
increase of 125
o
C, and 150
o
C, the percentage of air
absorbency was lower than that of making hydrogels
with an increase of 100
o
C while the percentage of
overall air absorption reached 800.73% g / g and
198.26% g / g. This shows a high increase. A perfect
crosslinking occurs at 100
o
C. It has started to repair
or cut off the crossing. Because with cross bonding
between polymers, it will allow bond bonding with
the polymer where the air is absorbed in the
hydrogel crosslinked polymer polymer. With the
ability to absorb air, the results of this research
hydrogel can be used as a media to store drugs,
namely hydrogels as a healing agent for wound
healing and even wound healing agents.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
208

3.3 Percentage Crosslink Degree
On the results of the crosslinked degree test, the
highest percentage of crosslinking was at 150
o
C
heating. This is very inversely proportional to the
percentage of water absorption. Where a good
hydrogel is a hydrogel with the ability to absorb
water high due to the optimum crosslinking and does
not make the shape of the hydrogel hard and stiff so
that good cross-linking capability will increase the
ability of high water absorption.
At 75
o
C heating, the ability of crosslinking is the
lowest because an imperfect crosslinking process
occurs reinforced in the explanation of the ability of
the absorption of water on the heating. With the
modification of the polymer chain extension,
chloroform as an extracting agent for polymeric
materials is not able to extract hydrogel material
with good cross-linking ability. Whereas at 75
o
C
heating, the ability of low water absorption makes
the average percentage of crosslinking at 18.94% g /
g. The ability of chloroform to extract the remaining
ingredients of the formula for making hydrogels,
resulted in the shape of the hydrogel after extracting
it with chloroform not decreasing its shape and
weight after extracting it from its initial weight or
dry weight. This is indicated by the results of
crosslinking in the middle of the average percentage
of cross-linking at 100
o
C heating which is 78.32%
g/g. This shows that every gram of hydrogel formed,
the crosslinking ability is in the range of 78.32%
which is successfully cross-linked in the formula for
making it.
Whereas the heating of 125
o
C and 150
o
C has a
high percentage of crosslinking capacity, namely
91.61% g / g and 92.93% g / g. The high percentage
of crosslinking does not indicate that the hydrogel is
in a good shape. In fact, the hydrogel is rubbed on
each side so it is possible that the hydrogel has been
crystallized so that it is difficult to extract by
chloroform.
3.4 Sem Analysis
Figure 3: Results of morphological analysis of hydrogels
using SEM with magnifications of 1000 and 2000 time.
3.5 Anti Bacterial Test Results with
Hydrogels
Table 1: Antibacterial Test Results for Hydrogels.
Diameter of
hydrogel
(mm)
Diameter of
inhibition zone
(mm)
E. coli
6.5
17.45
S. aureus
6.5
15.20
(a) (b)
Figure 4: observations of clear zones (a) Escherichia coli,
(b) Staphylococcus aureus.
From Figure 4 it can be seen that hydrogels made
from crosslinked reactions between chitosan and
watershed have good antimicrobial activity against
Escherichia coli bacteria as gram negative bacteria
and Staphylococcus aureus bacteria as gram-positive
bacteria.
3.6 In Vivo
Inflammatory phase is the phase where bleeding
occurs and freezing or cessation of bleeding due to
contraction of smooth muscle walls of blood vessels
that are open and blood clots by thrombin and fibrin.
The results showed that the inflammatory phase for
control (-), hydrogel samples and control (+) had
different healing phases. For control (-) takes more
than 7 days, the control treatment (+) takes 7 days to
heal but leaves a mark and not all mice tested
recovered, while the treatment for hydrogel samples
only takes 7 days to heal and does not leave made an
impression.
(a) (b)
Figure 5: (a) Heal (without trace), (b) Heal (with trace).
Making Hydrogel with Crosslinked Reactions between Chitosan and Dialdehyide Cellulose from Coconut Fiber as Wound Healers
209

4 CONCLUSIONS
From the results of the research that has been done,
it can be concluded as follows:
The synthesis of hydrogels from crosslinking
reactions between α-cellulose and chitosan tellah
was successfully carried out. The crosslinking
reaction forms an imine bridge (Schiff base) as a
change that links between chitosan compounds and
α-cellulose. This can be proven from the C = N
group formed which causes vibration on wavelength
absorption of 1643.35 and also has good water
absorption capability.
The results of the antibacterial activity of the
hydrogel showed good results for Gram-positive
bacteria Staphylococcus aureus and gram-negative
E. coli bacteria. This can be proven from the
formation of clear zones around the sample.
Hydrogels show better In vivo efficiency than
the healing drug Salticin Gentamicin Sulfate which
is within 7 days and leaves no trace.
REFERENCES
Carrijo, O. A., Liz, R. S., Makishima, N., 2002. Fiber of
Green Coconut shell as Agriculture substratum.
Brazilian Horticulture, 20, 533-535
Erizal. 2010. Synthesis and characterization of
superabsorbent hydrogels poly (acryl-amide-co-
acrylic acid) using radiation techniques. A Scientific
journal of application Isotopes and Radiation 6(2):
105-116.
Höglund E, 2015. Production of Dialdehyde Cellulose and
Periodate Regeneration: Towards Feasible Oxidation
Processes. Karlstads University
Kalia S, 2011. Cellulose Fibers: Bio- and Nano- Polymer
Composites. Springer-Verlag. London
Moenadjat Y, 2003. Burns Practical Clinic. Edition II.
Faculty of Medicine UI, Jakarta.
Pratama, A, 2018. Antibacterial Properties of Biofilm
Schiff Base Derived from Dialdehyde Cellulose and
Chitosan. Indones.J.Chem.
Wertz J, 2010. Cellulose Science and Technology. EPFL
Press. Italy
Zhao J, 2012. Chitosan-Based Gels For The Drug Delivery
System, in Yao K, Li J, Yao F, Yin F, (Ed), Chitosan-
Based Hydrogel; Functions And Applications, Taylor
&Francis Group, LLC,. New York.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
210
