
Modification and Characterization Starch Nanoparticles of
Mangrove Fruit using Chemical-mechanical Method and Application
as Basic Materials Making Hydrogel
Gimelliya Saragih
1,3
, Tamrin
2*
, Marpongahtun
2
and Darwin Yunus Nasution
2
1
Postgraduate Chemistry Study Program, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Jl. Bioteknologi No. 1 Kampus USU, Medan, Indonesia
2
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan, Indonesia
3
Department of Chemical Engineering, Politeknik Teknologi Kimia Industri, Medan, Indonesia
Keywords: Mangrove Starch, Chemical-mechanical Methods, Nanoparticles, Hydrogel, FTIR, PSA, SEM, XRD, DSC.
Abstract: Mangroves are plants that function as protectors of the land from ocean waves. Mangroves are a source of
starch that has not been explored. To expand the application, the starch needs to be modified. Natural starch
is made using wet extraction. Natural starch is synthesized into nanoparticle starch by chemical-mechanical
methods. Modified Mangrove Fruit Starch can be used as a base for making hydrogels. Characterization of
starch and starch nanoparticles includes proxy analysis, functional groups using the Fourier Transform
Infrared Spectroscopy (FTIR). Test the PSA (Partiicle size analyzer) to find out the particle size. Crystallinity
test of starch nanoparticles using X-Ray Diffraction (XRD). The morphological analysis of nanoparticles was
carried out using the Scanning Electron Microscopy (SEM) instrument. Thermal test using Differential
scanning calorimeter (DSC). The results showed that mangrove starch had a yield of 29.60% and particle size
of mangrove nanoparticles of 38.79 nm.
1 INTRODUCTION
Starch is a natural biopolymer used in the food,
chemical, pharmaceutical / biomedical, paper, textile
and so on industries. Starch is renewable, non-toxic,
edible and inexpensive and easy to obtain. Natural
starch has several disadvantages that need to be
modified so that it has the appropriate characteristics
as industrial ingredients.
Desirable important properties of modified starch
include higher brightness, lower viscosity, clearer gel
formed, easier starch granules to rupture, higher
gelatinization time and temperature (Koswara, 2009).
Modification of several types of starch namely
tapioca, sago can produce nanoparticle starch which
serves as a matrix binding to herbal active ingredients
and lactic acid bacteria (Sunarti, et al, 2015).
There are still many sources of starch that have not
been developed, among others, mangrove fruit starch
besides functioning as a protector of land from large
ocean waves (Irwanto, 2006), rhizophora mucronata
plants. also is one type of mangrove that can be used
as a new food source. This is because this species
contains high carbohydrates.
Nanotechnology has great potential to produce
new composites. One application is nanocomposite in
biomedicine. Nanocomposite can be done by
inserting nanoparticles into the matrix. The
nanoparticles commonly used for nanocomposites
include carbon nanotubes, cellulose, silica, and chitin.
Nanocomposite is generally used to improve
mechanical properties and packaging properties
(barrier properties) (Azeredo, 2009). Starch in the
form of nanoparticles has several advantages
including low suspension viscosity even though the
concentration is relatively high, and has a high
binding strength (Gularte and Rosell, 2011).
There are several previous studies that have been
carried out to isolate starch and synthesis of starch
nanoparticles. Among them are chemical methods of
hydrolysis with strong acids H
2
SO
4
(Wei, et al, 2014),
dissolution methods and non-solvent precipitation
(Saari, et al, 2017), ionic gelation methods (Yang, et
al, 2015). Enzymatic method of enzyme hydrolysis
using alpha amylase enzyme (Rahmawati and
Saragih, G., Tamrin, ., Marpongahtun, . and Nasution, D.
Modification and Characterization Starch Nanoparticles of Mangrove Fruit using Chemical-mechanical Method and Application as Basic Materials Making Hydrogel.
DOI: 10.5220/0008868501910196
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 191-196
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
191

Yunianta, 2014). Combined method of enzyme
hydrolysis and acid (LeCorre, et al, 2012). The
mechanical method uses ultrasonication (Haaj, et al,
2013). From several studies on starch making it is
known that chemical-mechanical methods have
proven effective for isolating starch and reducing its
size to starch nanoparticles (Kim, et al, 2013).
Based on this background, the authors are
interested in conducting research on the modification
and characterization of starch from mangrove
nanoparticles with chemical-mechanical methods and
their application as a basis for making hydrogels
2 MATERIALS AND METHODS
2.1 Materials
The materials used in this study are Mangrove Fruit,
Aquadest, 2% Sodium Hydroxide (NaOH), Sulfuric
Acid (H
2
SO
4
) 98% (E.Merck), Na-metabisulfite
(E.Merck), Sodium Chloride (NaCl), Ethanol (E.
Merck).
2.2 Extraction of Mangrove Starch
Extraction of mangrove starch was carried out by
referring to the method developed by Wijayanti et al.
(Wijayanti, et al, 2010). Mangrove fruit obtained
from the coast in Aceh Tamiang Regency, mangrove
fruit weighed 1 kg and then peeled and then washed
into a 4% NaCl solution in 3 liters of water
(comparison 1: 3). To find out the net weight, the
peeled skin is weighed so that it gets clean weight.
Then soaked with 0.075% Na-metabisulfite with a
ratio of 1: 3. Then the tubers are washed with tap
water, shredded and filtered while being given water.
Then leave it to settle for 1 night, the filtrate is
removed and the sediment is taken. The precipitate is
dried at 40
o
C, then milled and sifted with a 100 mesh
sieve.
2.3 Characterization of Mangrove
Fruit Starch
2.3.1 Proximate Analysis
Mangrove starch obtained by proximate analysis of
the AOAC Method, 2006 (AOAC, 2006).
2.3.2 Fourier Transform Infrared
Spectroscopy (FTIR)
Analysis of mangrove starch functional groups using
the Fourier Transform Infrared Spectroscopy (FTIR)
tool. The FTIR spectrum of the sample was recorded
using the Bruker OPUS 7.5.18 infrared spectrometer
(Bruker, Germany) at wavelengths from 400 to 4000
cm
-1
at a speed of 20 cm
-1
.
2.4 Isolation of Nanoparticles of
Mangrove Starch
Starch nanoparticles are prepared using a
modification of the procedure described by Angellier,
et al (2004). Briefly, mangrove fruit starch (44.07 g,
dry solid) was dispersed in an acidic solution of
H
2
SO
4
(3.16 M, 300 ml), and the dispersion was
stirred with a magnetic stirrer (200 rpm) at 40 ° C.
After various periods of hydrolysis, the sample taken
and neutralized with NaOH (1 M) to neutral pH and
centrifuged at 3500 rpm for 10 minutes. Added
deionized water (300 ml) into the precipitate, and the
mixture stirred for 30 minutes at room temperature.
This washing process is repeated twice to remove the
remaining salt. Next, the starch was deposited, and
the suspension was centrifuged at 500 rpm for 10
minutes to separate the solids in the supernatant. Then
it was ultrasonified at the highest amplitude (90%) for
15-30 minutes. Then freeze drying was used to obtain
starch nanoparticle powder.
2.5 Characterization of Mangrove
Starch Nanoparticles
2.5.1 Proximate Analysis
Particle size was characterized using Particle Size
Analyzer (PSA) nanoq cordouan v2.0.0.1
2.5.2 X-Ray Diffraction (XRD) Analysis
Analysis of crystallinity of mangrove starch using X-
Ray Diffraction (XRD) was operated at 40 kV and
current of 30 mA of electricity using Cu Kα radiation
at 1.5418 A wavelength and scanned from 0.0050 (2
Ɵ / s).
2.5.3 Scanning Electron Microscopy (SEM)
Analysis
The surface morphology of mangrove starch
nanoparticles was characterized using SEM (DX
EVO MA 10 Carl Zeiss, Germany).
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
192
2.5.4 Differential Scanning Calorimeter
(DSC) Analysis
Thermal properties of nanoparticle starch using
Differential scanning calorimeter (Shimadzu). This
analysis is carried out to measure the energy absorbed
or emitted by a sample which gives measurements of
calorimetry and transition energy at a certain
temperature.
3 RESULTS AND DISCUSSION
3.1 Extraction of Mangrove Starch
Making mangrove starch is done by wet extraction.
The yield of mangrove fruit starch was 29.60%. The
high rendeman of large-potential mangrove fruit
starch is developed into a starch source and
alternatively becomes a new food source (Kardiman,
et al, 2017). The resulting mangrove fruit is brownish
white. The results of extracting mangrove fruit can be
seen in Figure 1.
Figure 1: Mangrove Fruit Starch.
3.2 Characterization of Mangrove
Starch
3.2.1 Analysis Proximate
For the results of a preliminary analysis of mangrove
starch following the procedure of the Association of
Official Analytical Chemist (AOAC), 2006, it can be
seen in Table 1 below. Proximate analysis result
showed that starch mangrove fruit is a surce of high
carbohydrate.
Table 1: Test Results Mangrove Fruit Starch Proximate.
Proximate Components
Content (%)
Protein
4.26
Crude fiber
36.98
Starch
56.61
Crude fat
0.76
3.2.2 Analysis Fourier Transform Infrared
Spectroscopy (FTIR)
FTIR spectrum from mangrove starch nanoparticles
can be seen in Figure 2.
Figure 2: FTIR spectrum of mangrove starch.
The shape of the widening peak is seen in the absorption
area of 3000 - 3600 cm
-1
. This vibration shows the vibration
of the stretching region of hydrogen with O-H bonds
(carboxylic acids). The vibrational peak in the area of 2850
- 2960 cm
-1
and vibration in the area of 1340 - 1470 cm
-1
,
this stretch shows the vibration of the aliphatic C-H bond.
In the absorption area with wave number 1636 cm
-1
, this
vibration shows the vibration of the C = C bond. This peak
shows the vibration of the area which is cyclic or aromatic
ring and in the absorption area of 1050 - 1300 cm
-1
This
vibration shows the vibration of the stretching region of
hydrogen with the C-O bond). The results of FTIR
spectroscopic analysis showed that the mangrove starch
provides a spectrum that describes the structure of starch.
3.3 Synthetic Mangroveti Fruit Starch
Nanoparticles
The making of mangrove starch nanoparticles
consists of several stages, namely: the acid hydrolysis
stage, the neutralization stage of the mechanical stage
and the phase of separation or often called the
chemical-mechanical method.
In this study, the hydrolysis of mangrove starch
was dispersed in an acid solution of 3.16 M H
2
SO
4
,
the use of sulfuric acid was too concentrated to
convert starch to glucose and the dispersion was
stirred with a magnetic stirrer (200 rpm) at 40 ° C.
After various periods of hydrolysis, samples were
taken and neutralized with NaOH (1 M) to neutral pH
and centrifuged at 3500 rpm for 10 minutes. Added
deionized water (300 ml) into the precipitate, and the
mixture stirred for 30 minutes at room temperature.
This washing process is repeated twice to remove the
remaining salt. Next, the starch was deposited, and
the suspension was centrifuged at 500 rpm for 10
Modification and Characterization Starch Nanoparticles of Mangrove Fruit using Chemical-mechanical Method and Application as Basic
Materials Making Hydrogel
193
minutes to separate the solids in the supernatant. Then
it was ultrasonified at the highest amplitude (90%) for
15-30 minutes. Then freeze drying was used to obtain
starch nanoparticle powder.
The point of Hee-Young Kim et al., (2013) the
chemical treatment aims to degrade amorphous
regions in starch granules. Mechanical treatment is
carried out such as homogenization by ultrasonication
in starch that has undergone chemical treatment
before. During the hydrolysis process, starch
nanoparticles continue to be produced because starch
granules are fragmented by acid but producing starch
nanoparticles may tend to form aggregates that are
easily deposited as microparticles. To inhibit
aggregation or to separate nanoparticles, ultrasonic
treatment is applied to starch dispersion, and changes
in the size distribution of starch particles are
examined by dynamic light scattering. Mild acid
hydrolysis combined with ultrasonication can
effectively produce starch nanoparticles.
Ultrasonication plays an important role in separating
the aggregates of nanoparticles that can form during
hydrolysis, thus effectively increasing the yield of
starch nanoparticles. However, starch nanoparticles
which are processed by ultrasonication can reduce the
crystallinity of starch.
3.4 Characterization of Starch
Nanoparticles
3.4.1 Results of Particle Size Analyzer (PSA)
Analysis
Particle Size Analyzer (PSA) is a characterization that
can be used to determine particle size and distribution
in a solution.
This method uses the principle of light scattering.
In this study wet method was used for the particle size
testing process. In the wet method using dispersion
media to disperse the test material. So that the starch
nanoparticles to be tested are dissolved in distilled
water for 4 hours at room temperature while the
stirring process is carried out so that the particles do
not agglomerate (clump) each other. Thus the
measured particle size is the size of a single particle.
Besides the measurement results in the form of
distribution, the measurement results can be assumed
to have described the overall condition of the sample.
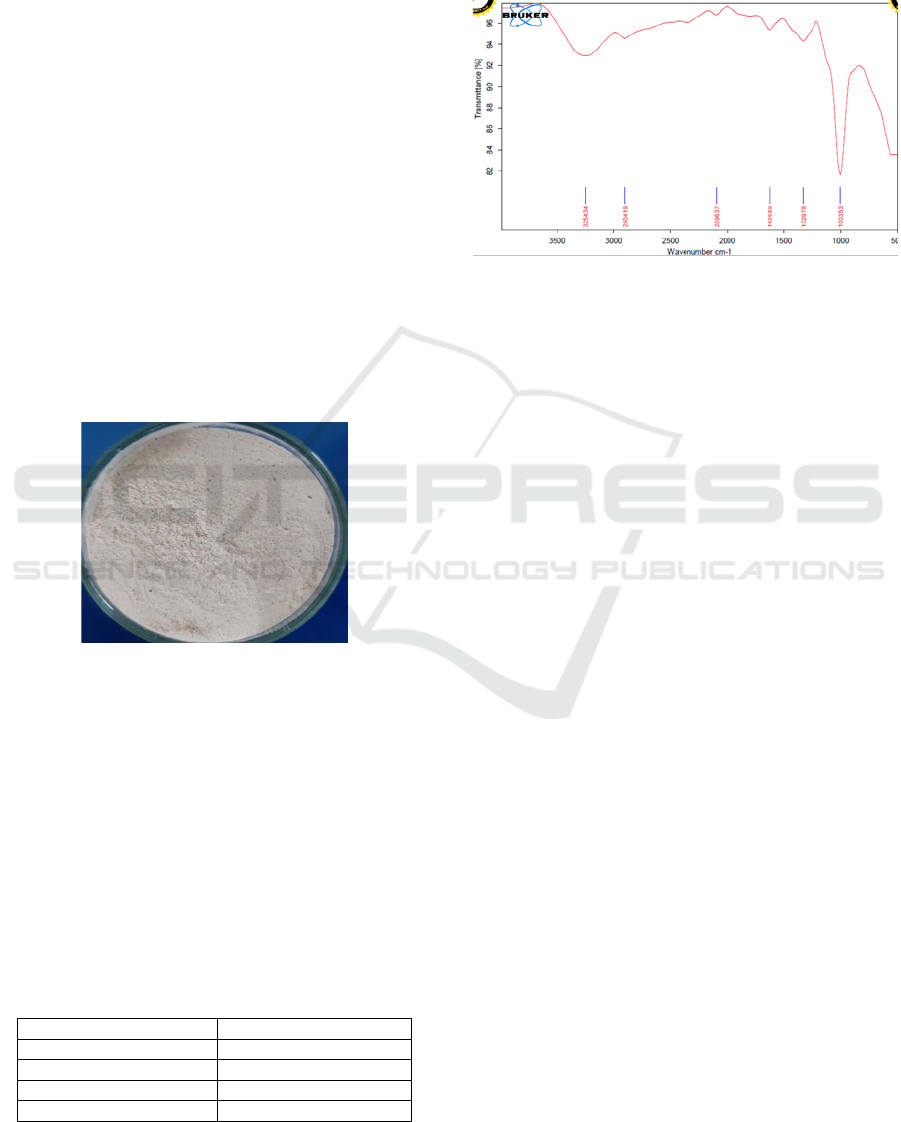
Based on Figure 3, there is a symmetrical peak
which indicates the particle size distribution is evenly
distributed in all parts. The peak appears in an area
with an average particle size of 38.79 nm. The size
and distribution of the particles produced reflects or
represents the size of the particle diameter in its bulk
state.
Figure 3: Distribution curve for the size of the Mangrove
Starch Nanoparticles.
3.4.2 Results of Particle Size Analyzer (PSA)
Analysis
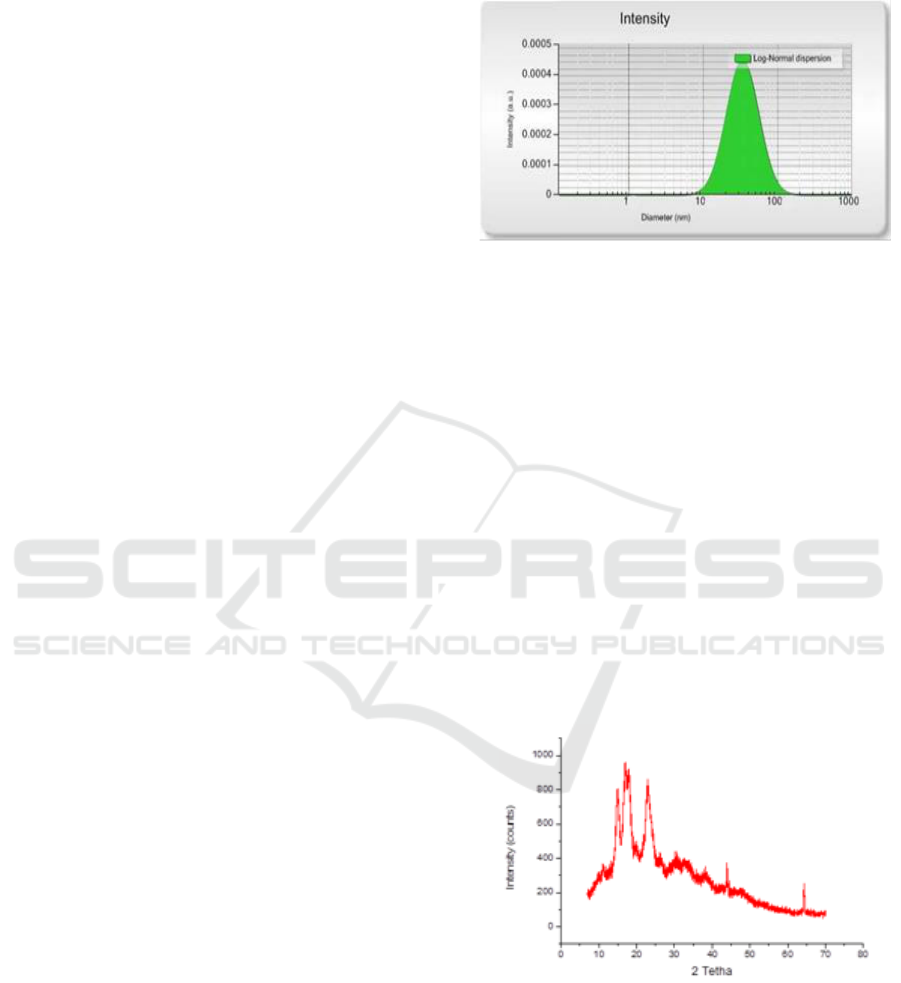
Determination of the crystallinity of mangrove fruit
nanoparticles was done by XRD, which is by placing
samples of mangrove fruit nanoparticles in a place so
that they can rotate on one axis. Then irradiate the
sample with X-rays, so the field devices in the crystal
reflect the X-ray beam. Then the beam is received by
the detector, so that the diffractogram is obtained. The
diffractogram of the polymer samples produced
contained crystalline and amorphous regions which
mixed randomly. The diffractogram of X-ray
crystalline polymers has a sharp peak, while
amorphous polymers have a wide peak. For the XRD
analysis of mangrove fruit starch nanoparticles can be
seen in Figure 4 below.
Figure 4: Distribution curve for the size of the Mangrove
Starch Nanoparticles.
The highest mangrove starch nanoparticle peaks were
formed at 150 and 230, indicating that mangrove fruit
nanoparticles were successfully synthesized.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
194
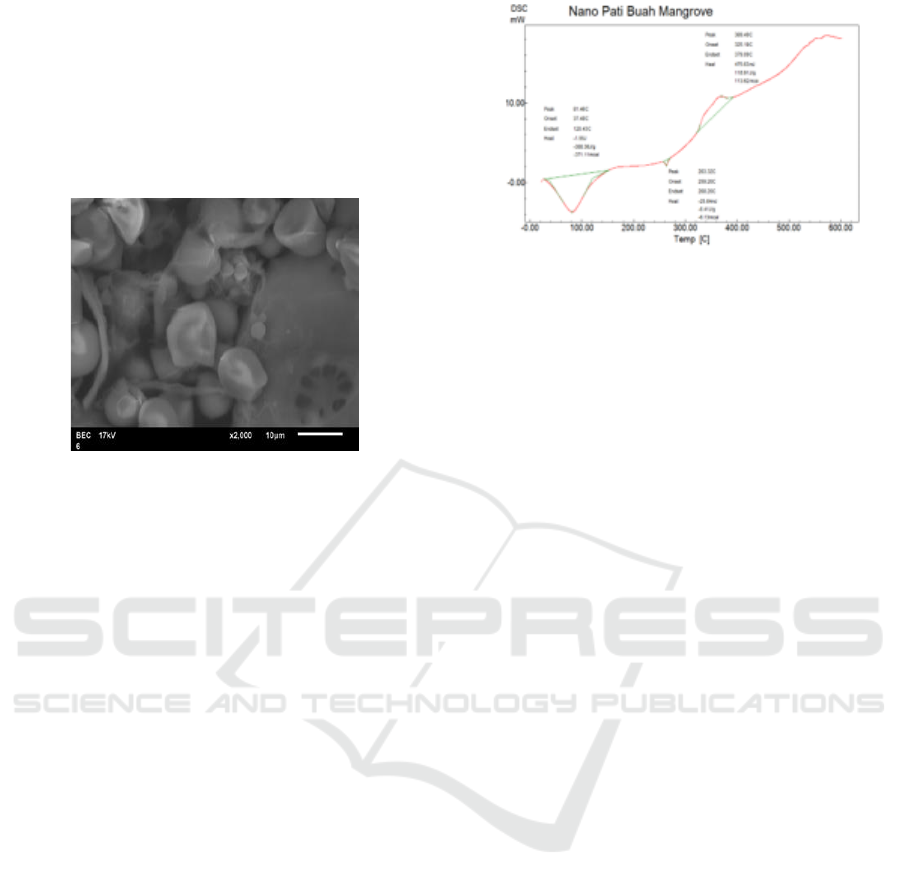
3.4.3 Results of Scanning Electron
Microscopy (SEM) Analysis
Morphological analysis was carried out to see the
pore size of the hydrogel with variations in the
addition of mangrove starch nanoparticles in this case
using the Scanning Electron Microscopy (SEM) tool.
For SEM photos can be seen in Figure 5 below.
Figure 5: Results of SEM of Mangrove Fruit Starch
Nanoparticles.
The treatment of acidic and mechanical hydrolysis
during the precipitation process can cause the
formation of smaller particles when starch is
degraded. This mechanical treatment causes the
cutting of bonds between amylose and amylopectin
molecules when starch is degraded so that the shape
and size of the particles of starch do not return to their
original conditions, this indicates that the structure of
starch has been modified. Based on morpholytic
analysis of starch using SEM, it was seen that the
starch particles after precipitation were still not
completely separated and still combined to form
pores. This porous structure can affect the functional
characteristics of nanocrystalline starch.
3.4.4 Results of Analysis of Mangrove
Starch DSC Analysis
Differential Scanning Calorimetry (DSC) is a thermal
property analysis technique where the function is
measured. DSC is used to study thermal properties
and phase changes in calorimetry of a material. DSC
analysis has been conducted on mangrove starch
samples which can be seen in Figure 6.
Figure 6: Graph of Analysis Results of DSC Nanoparticles
of Mangrove Starch.
In Graph 5 shows changes in endothermic reactions
and exothermic reactions of mangrove starch
nanoparticles. At temperatures of 81.46
o
C showed the
temperature of the endothermic reaction (heat
absorbing), at temperatures of 369.49
o
C showed the
exothermic reaction temperature, which stated that
the material had been degraded (damaged), this meant
that mangrove starch nanoparticles could be used
below the degraded temperature which was below
369.49
o
C.
4 CONCLUSIONS
Mangrove starch nanoparticles were successfully
isolated by chemical-mechanical methods, through
three stages of using acid hydrolysis stage, H2SO4
3.16 M, mechanical phase neutralizing with NaOH
(1M) using centrifugation at 500 rpm for 10 minutes
and ultrasonication at highest amplitude (90% ) for
15-30 minutes. FTIR Characterization Results of
mangrove starch nanoparticles provide a spectrum
that describes the structure of starch, the highest peak
of mangrove starch nanoparticles formed at 150 and
230, This shows that mangrove fruit nanoparticles
were successfully synthesized and the size of starch
nanoparticles obtained was 38.79 nm.
ACKNOWLEDGEMENTS
The authors are grateful to the Pusdiklat Industri
Kementerian Perindustrian who has supported the
funding of this research, Politeknik Teknologi Kimia
Industri medan, Department of Chemistry, University
of Sumatera Utara, Medan for its support in the use of
laboratories, and not forgetting my promoters who
have provide useful guidance and advice in
conducting this research.
Modification and Characterization Starch Nanoparticles of Mangrove Fruit using Chemical-mechanical Method and Application as Basic
Materials Making Hydrogel
195

REFERENCES
Angellier, H., Choisnard, L., Molina-Boisseau, S., Ozil, P.,
& Dufresne, A., 2004. Optimization of the preparation
of aqueous suspensions of waxy maize starch
nanocrystals using a response surface methodology.
Biomacromolecules, 5(4), 1545-1551.
AOAC Association of Official Analytical Chemists, 2006.
Official Methods of Analysis of The Association of
Official Agriculture Chemists 16th edition.
Azeredo, H. M., 2009. Betalains: properties, sources,
applications, and stability–a review. International
journal of food science & technology, 44(12), 2365-
2376.
Gularte, M. A., & Rosell, C. M., 2011. Physicochemical
properties and enzymatic hydrolysis of different
starches in the presence of hydrocolloids. Carbohydrate
Polymers, 85(1), 237-244.
Haaj, S. B., Magnin, A., Pétrier, C., & Boufi, S., 2013.
Starch nanoparticles formation via high power
ultrasonication. Carbohydrate polymers, 92(2), 1625-
1632.
Irwanto, 2006. Keanekaragaman Fauna Pada Habitat
mangrove. Yogyakarta www.irwantoshut.com. [20
Desember 2009]
Kardiman, K., Ridhwan, M., & Armi, A., 2017. Buah
Lindur (Bruguera gymnorrhyza) sebagai Makanan.
Serambi Saintia: Jurnal Sains dan Aplikasi, 5(2).
Kim, H. Y., Han, J. A., Kweon, D. K., Park, J. D., & Lim,
S. T., 2013. Effect of ultrasonic treatments on
nanoparticle preparation of acid-hydrolyzed waxy
maize starch. Carbohydrate polymers, 93(2), 582-588.
Kim, H. Y., Park, D. J., Kim, J. Y., & Lim, S. T., 2013.
Preparation of crystalline starch nanoparticles using
cold acid hydrolysis and ultrasonication. Carbohydrate
polymers, 98(1), 295-301.
Koswara, S., 2009. Teknologi modifikasi pati. Teknol.
Pangan, 1-32.
LeCorre, D., Bras, J., & Dufresne, A., 2012. Influence of
native starch's properties on starch nanocrystals thermal
properties. Carbohydrate Polymers, 87(1), 658-666.
Rahmawati, A., & Yunianta, Y., 2014. Hidrolisis Enzimatis
Pati Jahe Emprit (Zingiber officinale Var. Rubrum)
Dengan Enzim Αlfa Amilase (Kajian Pengaruh
Konsentrasi Enzim Dan Lama Inkubasi Terhadap Sifat
Fisik Dan Kimia Dekstrin) [In Press Juli 2015]. Jurnal
Pangan dan Agroindustri, 3(3).
Saari, H., Fuentes, C., Sjöö, M., Rayner, M., & Wahlgren,
M., 2017. Production of starch nanoparticles by
dissolution and non-solvent precipitation for use in
food-grade Pickering emulsions. Carbohydrate
polymers, 157, 558-566.
Sunarti, T.C., Mangunwidjaja, D., dan Richana, N., 2015.
Potensi Dan Aplikasi Pati Termodifikasi Sebagai
Bahan Matriks Enkapulasi Senyawa Bioaktif Herbal.
Balai Besar Penelitian dan Pengembangan Pascapanen
Pertanian, Bogor.
Wei, B., Xu, X., Jin, Z., & Tian, Y., 2014. Surface chemical
compositions and dispersity of starch nanocrystals
formed by sulfuric and hydrochloric acid hydrolysis.
PloS one, 9(2), e86024.
Wijayanti, R. E., Mulyati, A. H., & Winarti, C., 2010.
Modifikasi Pati Umbi Gembili (Dioscorea esculenta L.)
dan Gembolo (Dioscorea bulbifera) Sebagai Pati
Nanopartikel Melalui Hidrolisis Asam. Universitas
Pakuan. Bogor.
Yang, J., Han, S., Zheng, H., Dong, H., & Liu, J., 2015.
Preparation and application of micro/nanoparticles
based on natural polysaccharides. Carbohydrate
polymers, 123, 53-66.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
196
