
Modification and Characterization Natural Cycle Rubber
(Resipren-35) with Oleat Acid using Dicumyl Peroxide and
Divinilbenzena as Compatibility
Barita Aritonang
1,4
, Tamrin
2*
, Basuki Wirjosentono
2
and Eddiyanto
3
1
Postgraduate Chemistry Study Program, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Jl. Bioteknologi No. 1 Kampus USU, Medan, Indonesia
2
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara Jl. Bioteknologi
No.1 Kampus USU Padang Bulan, Medan, 20155
3
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Negeri Medan,
Medan, Indonesia
4
Faculty of Science, Technology and Information, University of Sari Mutiara Indonesia
Keywords: Cyclic Natural Rubber, Oleic Acid, Dicumil Peroxide, Divinyl Benzene, Grafting Copolymer.
Abstract: Modification of cyclic natural rubber with oleic acid using initiator diumil peroxide and divinilbenzene as a
compositer through the melting phase in the internal mixer using the copolymer grafting method at 160 oC
for 8 minutes at 80 rpm was carried out with the aim of increasing its compatibility as a paint binder. Varia-
tions in the concentration of oleic acid were used 3, 6 and 9 phr while the initiator concentrations of dicumil
peroxide 0.5 phr and divinilbenzena 1.0 phr. Determination of the degree of grafting was carried out by titra-
tion method and FT-IR spectra analysis to determine the presence of oleic acid grafting in the cyclic natural
rubber chain. The results showed that the process of transplanting oleic acid into the chain of cyclic natural
rubber molecules with the presence of initiators dicumyl peroxide and divinylbenzene was successfully car-
ried out marked the emergence of new absorption peaks at wave number 1705.07 cm-1 which is a typical
uptake of oleic acid indicating the presence of bond vibrations C=O carbonyl group asymmetry derived from
oleic acid. The maximum percentage grafting degree is 0.2630% at 9 phr oleic acid monomer concentration.
The degree of grafting increases with increasing concentration of oleic acid.The transition temperature of
cyclic natural rubber glass before being grafted with oleic acid is 102.86 0C but after the transplanting process
using the initiator diumil peroxide and divinylbenzene the glass transition temperature (Tg) decreases to 83.98
0C this proves that the process of transplanting oleic acid into the cyclic natural rubber molecule chain has
been successfully carried out with the formation of the new CNR-g-AO product.
1 INTRODUCTION
Natural rubber is a renewable polymer containing car-
bon atoms (C) and hydrogen atoms (H) obtained from
Hevea brasiliensis trees which have good tensile
strength and tear resistance, and have a fairly good
stickiness so that they can be widely used as adhe-
sives in the coating / coating industry (Khan et al.,
2011; Hirata et al., 2014).
However, as unsaturated polymers, natural rubber
has the limitation that it will experience gradual deg-
radation at high temperatures when exposed to oxy-
gen, ozone or ultraviolet, and its solubility in hydro-
carbon solvents thus affecting the quality of natural
rubber itself (Grassie et al., 1988; Wypych, 2015).
One of the efforts made to overcome the weakness
of natural rubber is chemical modification through
cyclization reaction using Lewis acid catalyst. The
aim of the chemical modification of natural rubber is
to improve the properties of natural rubber so that
new products are produced that are more useful, eco-
nomical and have superior properties, so that they can
be applied in various types of rubber products
(Tanaka et al., 2004; Saelao et al., 2005).
Cyclic natural rubber (CNR) is a chemical modifi-
cation of natural rubber through cyclization reaction
using Lewis acid catalyst, has considerable potential to
be applied as an adhesive, printing ink and paint indus-
try because it has abrasion resistance properties (fric-
tion), resistant to corrosion and has good adhesion to
Aritonang, B., Tamrin, ., Wirjosentono, B. and Eddiyanto, .
Modification and Characterization Natural Cycle Rubber (Resipren-35) with Oleat Acid using Dicumyl Peroxide and Divinilbenzena as Compatibility.
DOI: 10.5220/0008859701450151
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 145-151
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
145

metals, wood, rubber, leather, textiles and paper (Sire-
gar et al., 2014; Eddiyanto et al., 2015; Aritonang et al.,
2018; Ritonga et al ., 2018; Barita Aritonang et al.,
2019).
However, cyclic natural rubber has a very weak
adhesion to the polar surface. This is because cyclic
natural rubber is a nonpolar polymer which is very
vulnerable to oxidation degradation reactions by
ozone, oxygen or utraviolet (uv) compounds and has
a double bond in the polymer chain and its surface
energy is very weak, causing interface interactions
and the adhesion is weak when mixed with polar pol-
ymers. To overcome this problem, it is necessary to
do chemical modifications to the structure of cyclic
natural rubber using the method of grafting (copoly-
mer grafting). An effective method for modifying cy-
clic natural rubber is the copolymer grafting tech-
nique, so that cyclic natural rubber can be functional-
ized according to the desired properties without af-
fecting the basic structure of cyclic natural rubber.
Oleic acid is a polar unsaturated fatty acid that can
be used as a monomer to increase CNR compatibility,
reactivity and adhesive strength because the double
bonds in AO provide an opportunity to be able to
modify the CASH structure through a copolymer
grafting process and the same acid composition can
react with hydroxyl groups. This has been proven by
Zhou et al. (2002), that through oleic acid monomer
polymerization (AO) grafting process successfully
grafted the main acrylonitrile-butadiene-styrene
(ABS) chain using benzoyl peroxide (BPO) initiator
in 1.2 solution -dichloroethane. The result is that AO
can increase the flexibility, elasticity and stability of
polymers against heat and ultraviolet radiation.
Preliminary research that has modified cyclic natu-
ral rubber using the copolymer grafting method, Sire-
gar Said et al (2014) modified cyclic natural rubber us-
ing monomer maleic anhydride in the presence of ben-
zoyl peroxide through melting techniques in mixer in-
ternal mixers using the copolymer grafting method.
The results of the study obtained AM-g-CNR products.
The higher the concentration of maleic anhydride, the
more maleic anhydride groups are grafted on the CNR
polymer chain. Transplant products have physical
properties that do not experience significant changes
except the glass transition temperature, where there is
an increase. Nasution et al. (2015) modified cyclic nat-
ural rubber using monomer methyl methacrylate with
the presence of the initiator diumil peroxide.
The results of the study obtained CNR-g-MMA
products, which are characterized by the appearance
of wave number absorption peaks in the area of 1731
cm
-1
(absorption of carbonyl groups) typical for car-
bonyl (C = O) from metal methacrylate.
2 MATERIALS AND METHODS
The materials used consisted of Acetone, ethanol
(C
2
H
5
OH), commercial cyclic natural rubber (CNR)
production of PTPN-3, dicumyl peroxide (DKP),
oleic acid (OA), divinil benzene (DVB), hydrochloric
acid (HCl), xylene. Glassware, Mettler Toledo Ana-
lytical Balance, Memmert Oven, Thermo haake pol-
ydrive mixer Shimadzu FT-IR Spectrophotometer,
Shimadzu Differential Scanning Calorimetry (DSC).
Grafting of oleic acid in cyclic natural rubber with
heat initiation, without dicumil peroxide As many as
33 grams of cyclic natural rubber are put into the
chamber slowly and left for about 4 minutes until all
of them melt perfectly. Then add 6 phr oleic acid into
the chamber so that it mixes and undergoes a copoly-
merization reaction. After 8 minutes, the process is
stopped by pressing the STOP button. Furthermore,
in rapid heat conditions the copolymerization product
is removed from the chamber. After the cold is made
in the form of pellets / granules.
2.1 Grafting Oleic Acid in Cyclic
Natural Rubber using Dicumyl
Peroxide
Cyclic natural rubber is weighed as much as 33 grams
(100 phr), after which the Thermo mixer internal
mixer temperature is set haake polydrive at 160
o
C,
then slowly cyclic natural rubber is inserted into the
internal chamber mixer and allowed to last for ap-
proximately 4 minutes until cyclic natural rubber
melts perfectly, then added with oleic acid as much as
6 phr, followed by the addition of initiator dicumyl
peroxide as much as 0.5 phr until mixed homogene-
ously. The mixing process is carried out for 8 minutes
to experience the grafting reaction. After 8 minutes,
the process is stopped. In hot conditions quickly the
reaction product is removed from the chamber. The
products produced are then chilled and made in the
form of granules / pellets. Variations in the concen-
tration of oleic acid used 3, 6 and 9 phr.
2.2 Purification Process of Cyclic
Grafted Natural Rubber Products
Oleic Acid
Oleic acid which has been grafted on cyclic natural
rubber (CNR-g-OA) is weighed as much as 1 gram
and refluxed with 100 ml of xylene with a series of
reflux devices namely heating, boiling pumpkin and
liebig condenser at 110
o
C until dissolved, 100 ml
added after dissolving ethanol to form deposits. The
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
146
precipitate formed is filtered with Whatman filter pa-
per No.42 which is connected with a vacuum pump
and washed with ethanol repeatedly with the aim of
dissolving the remaining acidic reactions. The precip-
itate formed was dried in an oven at a temperature of
120
o
C for 24 hours, then determined percent degree
of grafting. Determination of the degree of grafting is
done using the following formula:
(%) DG =
𝑁 (𝑒𝑞/𝐿) [(𝑉𝑜−𝑉) 𝑚𝑙] 𝐵𝑀𝐴𝑂 𝑔/𝑒𝑞
𝐵𝑆 𝑥 1000 𝑚𝑔/1𝑔
x 100 (1)
Where,
N = KOH concentration (0.05 N)
V = ml of KOH titration used on CNR-g-AO
samples modified
V0 = ml of KOH titration used on he CNR sample
blank is not modified
BM oleic acid = 282.47 g / mol
BS = sample weight
1000 = Conversion factor of carboxylic groups
from oleic acid molecules
3 RESULTS AND DISCUSSIONS
Analysis of CNR-g-AO FT-IR without dicumyl Per-
oxide Initiator To determine the success or failure of
the oleic acid grafting process into the cyclic natural
rubber molecule chain with and without the presence
of the initiator dicumyl peroxide after being purified,
it can be seen from the analysis of the spectroscopic
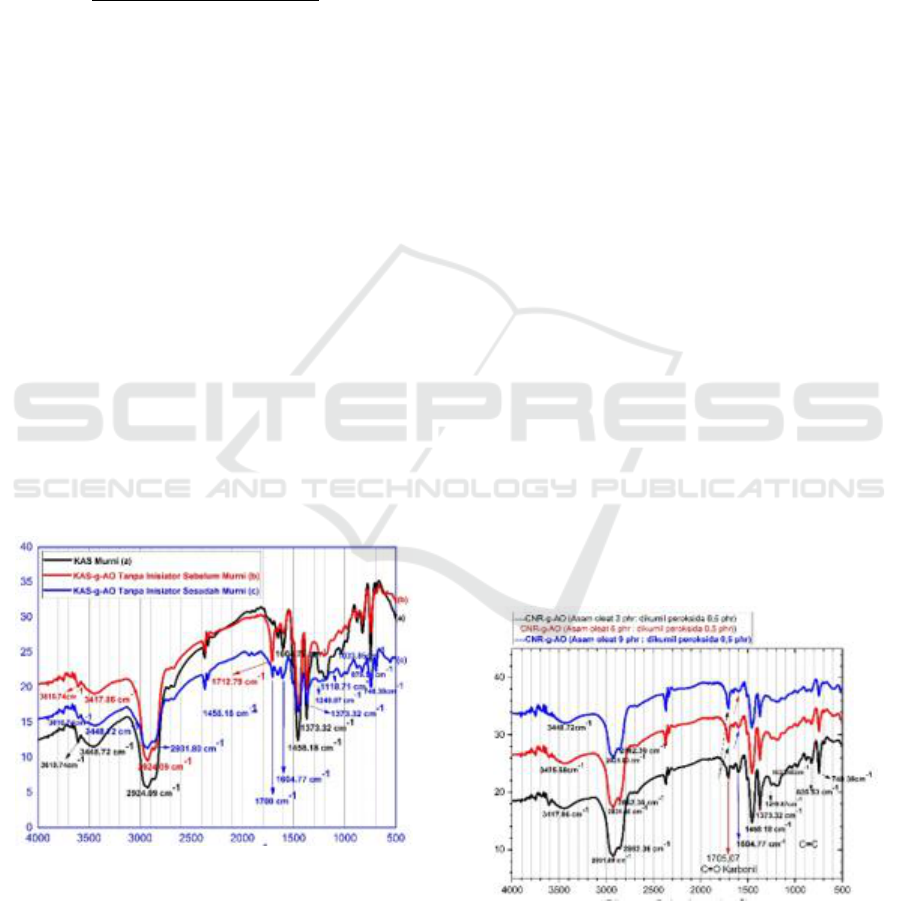
Fourier Transformed-Infra Red shown in Figure 1.
Figure 1: Spectrum of CNR-g-AO FT-IR without DKP
Initiator.
Seen in Figure 1 (b) the FT-IR spectrum of CNR-
g-AO before being purified new functional groups ap-
pear with sharp absorption peaks and high intensity
wave numbers 1712.79 cm
-1
, after purification there
is a shift in peak absorption of wave numbers and
changes in intensity shown in Figure 1 (c) FT-IR
spectrum CNR-g-AO, obtained a weak absorption
peak with low intensity wave number 1700 cm
-1
shows the existence of strain vibration C = O carbonyl
group derived from oleic acid. This is in accordance
with the results of previous studies which explained
that absorption peaks with spectral regions 1760-
1665 cm
-1
showed the presence of a group C = O car-
bonyl derived from oleic acid (Liu Mingzhu et al.,
2003; Zhou et al., 2002; Boonyod et al., 2017; Wirjo-
sentono et al., 2018; Barita Aritonang et al., 2019).
The emergence of new functional groups in CNR-
g-AO samples after being grafted in the mixer inter-
nal mixer through heat initiation without using an in-
itiator before and after being purified at the peak wave
number 1700 cm
-1
and 1712.79 cm
-1
indicates the
presence of carbonyl groups (C = O) which is a typi-
cal absorption derived from oleic acid (AO), has
proven that the process of transplanting oleic acid in
cyclic natural rubber in the mixer internal mixer has
been successfully carried out.
3.1 Analysis of CNR-g-AO FT-IR with
Dicumyl Peroxide Initiator
The FT-IR analysis test was carried out on CN-g-AO
samples without initiator in bulk peroxide by compar-
ing CNR-g- [AO / DKP] samples with the presence
of initiators in wax peroxide after being purified by
looking at the absorption peaks in the CNR spectrum
and CNR spectrum- g- [AO / DKP] especially in the
area of wave number which is an indication of the ex-
istence of C = O and C-O bonds from oleic acid. The
FT-IR CNR-g- [AO / DKP] spectrum with the di-
cumyl peroxide initiator is shown in Figure 2.
Figure 2: CNR-g-AO FT-IR Spectrum Using DKP Initiator.
Figure 2 analysis of CNR-g-AO FT-IR spectra
shows that there has been a chemical interaction be-
tween CNR, AO and DKP, this can be proven from
Modification and Characterization Natural Cycle Rubber (Resipren-35) with Oleat Acid using Dicumyl Peroxide and Divinilbenzena as
Compatibility
147

the results of the copolymer grafting of oleic acid into
cyclic natural rubber with a new absorption peak at
wave number 1705,07 cm
-1
which is a typical uptake
of AO indicates a vibration of the C = O carbonyl
group asymmetry which originates from oleic acid,
and is also supported by the presence of CO bonds at
wave number 1257.59 cm
-1
. At 3448.72 cm
-1
wave
number obtained the peak of the widening and strong
absorption band with high intensity shows the pres-
ence of a hydroxyl group O-H vibration vibration
originating from CNR. The typical strong and sharp
absorption peak with high intensity derived from
CNR appears at wave number 2931.80 cm
-1
which is
a stretching vibration of C-H methyl group, at wave
number 2862.36 cm
-1
is stretching vibration of CH
3
methylene group. At the peak of wave number
1458.18 cm
-1
shows the vibration of CH
2
methylene
group strain and at the peak of wave number absorp-
tion band 1373.32 cm
-1
shows the presence of methyl-
CH
3
group amplified by vibrations of swing group -
CH
2
methylene at peak wave number 719 cm
-1
. At the
peak of absorption wave number 1257.59 cm
-1
with a
spectrum area of 1080-1300 cm
-1
indicates the pres-
ence of vibrations of C-O bonds. Based on the FT-IR
spectrum analysis data described above it can be con-
cluded that the copolymerization process of grafting
AO into the CNR molecular chain has been success-
fully carried out.
3.2 Determination of Grafting of
CNR-g-AO Products
The purpose of refining CNR-g-AO products is to
find out how much oleic acid is grafted on a cyclic
natural rubber polymer chain. Determination of the
degree of grafting of CNR-g-AO products after puri-
fication was determined by the acid-base titration
method. Based on the results of the research that has
been done, the percentage of grafting for CNR-g-AO
products before being purified can be seen in table 1.
(%) DG =
0,05 (𝑒𝑞/𝐿)
[(
0,42 𝑚𝑙−0,29 𝑚𝑙
)]
282,47𝑔/𝑒𝑞
1,0162 𝑥 1000 𝑚𝑔/1𝑔
=
1,8361
1016,2
= 0.1807
Table 1: Effect of Oleic Acid Concentration on Grafting
Degrees.
CNR
phr
AO phr
DKP
phr
Grafting degree
(%)
CNR-g-OA
100
3
0,50
0,1807
100
6
0,50
0,2074
100
9
0,50
0,2630
The effect of monomer concentration on percent
degree of grafting is shown in Figure 3.
Figure 3: Graph of the Effect of Oleic Acid Concentration
on Degrees of Grafting.
Figure 3 clearly shows that percent degree of
grafting, in this case the amount of oleic acid that is
grafted (attached / grafted) to CNR increases with in-
creasing concentration of oleic acid monomers. Per-
centage of minimum grafting degree was obtained at
0.1807% at 3 phr oleic acid monomer concentration,
at 6 phr oleic acid monomer concentration obtained
percent degree of grafting at 0.2074%, for percent
grafting maximum degree obtained at 0.2630% at
acid monomer concentration oleat 9 phr, this shows
that percent degree of grafting is increasing gradually
with increasing concentration of oleic acid monomers
used, even though the increase is not too significant.
Even though the percentage of grafting obtained is
very small, but can change the surface of non-polar
CNR to be polar hydrophilic, this proves that oleic
acid has been successfully grafted into the molecular
chain of CNR. This data is supported based on the re-
sults of research conducted by Kang and Liaw (1999)
reporting that even though the percent of grafting ob-
tained is very small, it has been able to change the
nonpolar surface of the polymer to be polar.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
148

3.3 Analysis of Thermal Properties of
Pure CNR before and after
Grafting with Oleic Acid using
Differential Scanning Calorimetry
(DSC)
Differential Scanning Calorimeter (DSC) analysis is
one of the tools of the Thermal Analyzer that is used
to study phase transitions, such as melting tempera-
ture (Tm), glass transition temperature (Tg), or exo-
thermic decomposition, and to analyze the stability of
oxidation and heat capacity of a material. Glass tran-
sition temperature (Tg) is the temperature when the
polymer changes from the glass state to rubber.
Where when the outside temperature approaches the
glass transition temperature, a polymer undergoes a
change from a rigid hard state to a soft rubber. Melt-
ing temperature (Tm) is the temperature at which sol-
ids turn into liquids at a pressure of one atmosphere,
in other words, the melting temperature is the temper-
ature when the solid and liquid phases are equally in
equilibrium. Based on the results of the research that
has been done while the glass transition temperature
(Tg) of pure CNR before copolymerized grafts (co-
polymer grafting) can be seen in Figure 4.
Figure 4: DSC Pure Cyclic Natural Rubber Thermogram.
Based on Figure 4 the results of the DSC thermo-
gram of pure CASH samples before copolymer graft-
ing had a temperature of glass transition temperature
(Tg) 102.86 0C and enthalpy change (ΔH) of 0.188 J
/ g 0C. The higher the glass transition temperature
(Tg) shows the polymer is increasingly brittle.
3.4 Analysis of Thermal Properties of
Grafted Differential Scanning
Calorimetry CNR with Dicumyl
Peroxide Initiator
The product formed from the oleic acid grafting reac-
tion into the CNR molecular chain using the dicumil
peroxide initiator (DKP) in the mixer internal mixer
is CNR-g-AO. The CNR-g-AO product formed was
then characterized by Differential Scanning Calorim-
etry (DSC) to determine the glass transition tempera-
ture (Tg) and melting temperature (Tm). The results
of the analysis of thermal properties in the form of
DSC thermograms of CNR-g-AO products that have
been grafted using dicumil peroxide initiator (DKP)
after purification can be seen in Figure 5.
Figure 5: CNR-g-AO DSC Thermogram Using Dicumyl
Peroxide Initiator.
Based on Figure 5 the results of research that have
been carried out from the DSC thermogram curve ob-
tained two glass transition temperatures (Tg) which
were observed at temperatures of 83.98
0
C and 107.67
0
C. Based on the results of the DSC thermogram
curve analysis in Figure 5, there has been a decrease
in glass transition temperature (Tg) in cyclic natural
rubber before being grafted with oleic acid. The tran-
sition temperature of cyclic natural rubber glass be-
fore being grafted with oleic acid is 102.86
0
C but af-
ter the transplanting process using the initiator diumil
peroxide and divinylbenzene the glass transition tem-
perature (Tg) decreases to 83.98
0
C this proves that
the process of transplanting oleic acid into the cyclic
natural rubber molecule chain has been successfully
carried out with the formation of the new CNR-g-AO
product. According to Yang et al, (2003) the main
Modification and Characterization Natural Cycle Rubber (Resipren-35) with Oleat Acid using Dicumyl Peroxide and Divinilbenzena as
Compatibility
149

trigger for transplantation reactions (copolymeriza-
tion grafting) is when the initiator decomposes into
free radicals and there are one or more unpaired elec-
trons. The oleic acid (AO) grafting process (AO) into
the cyclic natural rubber molecule chain can occur
when AO monomers attach and form covalently
branching to the main chain of cyclic natural rubber
molecules when the polymer becomes radical by the
presence of an initiator. In the early stages of initiator
dicumyl peroxide (DKP) will form free radicals that
will attack the CNR polymer chain, so that cyclic nat-
ural rubber free radicals will be formed, then the cy-
clic natural rubber free radicals that form will initiate
the process of transplanting oleic acid into the natural
rubber molecule chain cyclical.
4 CONCLUSIONS
Based on the results of the research that has been
done, a conclusion can be drawn as follows:
a) Modification of cyclic natural rubber with oleic
acid using dicumyl peroxide and divinylbenzene
in an internal mixer with a temperature of 160 oC
and a rotor speed of 80 rpm using the graft copol-
ymer method has been successfully carried out.
This can be proven from the results of FT-IR spec-
tra analysis of emerging new absorption peaks at
wave number 1705.07 cm-1 which is a typical ab-
sorption of oleic acid indicating the presence of vi-
brations of the C=O bond of asymmetric carbonyl
groups originating from oleic acid.
b) The amount of oleic acid grafted on cyclic natu-
ral rubber increases with increasing concentra-
tion of oleic acid monomers in the presence of
dicumyl peroxide initiators and the addition of
divinylbenzene comonomers. The maximum
percent degree of grafting is 0.2630 % at the con-
centration of oleic acid monomers 9 phr.
c) The transition temperature of cyclic natural rubber
glass before grafted with oleic acid was 102.86
o
C
but after the graft process was carried out using the
initiator dicumyl peroxide and divinylbenzena the
glass transition temperature decreased to 83.98
o
C
this proves that the graft process of oleic acid into
the chain of cyclic natural rubber molecules has
been successfully carried out with the formation of
a new product CNR-g-AO
REFERENCES
Aritonang, B., Tamrin, T., Wirjosentono, B., & Eddiyanto,
E. (2018). Functionalization of cyclic natural rubber
(CNR) with oleic acid and divinylbenzene as compati-
bilizer in variation of dicumylperoxide. In AIP Confer-
ence Proceedings (Vol. 2049). https://doi.org/
10.1063/1.5082465
Aritonang, B., Tamrin, T., Wirjosentono, B., & Eddiyanto, E.
(2019). Grafting of Oleic Acid on Cyclic Natural Rubber
Resiprene-35 Using Dicumyl Peroxide Initiator and
Divinylbenzene Compatibilizers for Paint Binder in Pol-
yamide Thermoplastics. Oriental Journal of Chemistry,
35 (1), 173–179. https://doi.org/10.13005/ojc/350119
Boonyod, S., & Vudjung, C. (2017). Effect of Oleic Acid
on Properties of Natural Rubber Filled Bacterial Cellu-
lose. In Key Engineering Materials (Vol. 744, pp. 295-
299). Trans Tech Publ.
Eddiyanto, Ardina, Y. W., & Siregar, M. S. (2015). Modi-
fication of the Process of Making Cyclic Natural Rub-
ber through Chain Scission and Cyclization. AGRIUM:
Journal of Agricultural Sciences, 18 (1).
Grassie, N., & Scott, G. (1988). Polymer degradation and
stabilization. CUP Archive.
Hirata, Y., Kondo, H., & Ozawa, Y. (2014). Chemistry,
Manufacture and Applications of Natural Rubber.
Woodhead / Elsevier, Amsterdam, 325–352.
Kang, E. T., Neoh, K. G., Shi, J. L., Tan, K. L., & Liaw, D.
J. (1999). Surface modification of polymers for adhe-
sion enhancement. Polymers for Advanced Technolo-
gies, 10 (1-2), 20-29.
Khan, I., & Poh, B. T. (2011). Natural rubber-based pres-
sure-sensitive adhesives: a review. Journal of Polymers
and the Environment, 19 (3), 793.
Liu, M., Liu, Z., Ding, S., Li, S., & Zhang, L. (2003). Graft
copolymerization of oleic acid onto low-density poly-
ethylene in the molten state. Journal of Applied Poly-
mer Science, 90 (12), 3299–3304.
Nasution, A. S., Said, E., & Siregar, M. S. (2015). Methyl
Methylacating in Natural Cycle Rubber with Dicumyl
Peroxide Initiator: Monomer Concentration Effects.
Agrium: Journal of Agricultural Sciences, 19 (1).
Ritonga, A. H., Aritonang, B., & Zai, L. I. P. (2018). Mod-
ification of Natural Cycle Rubber Oleat Acid Coololy-
mer Using Benzoil Peroxide Initiator and Materials
Filling Trimethyl Ammonium Bromida Bentonits.
Journal of Chemistry of Mulawarman, 16 (1), 45–51.
Saelao, J., & Phinyocheep, P. (2005). Influence of styrene
on grafting efficiency of maleic anhydride onto natural
rubber. Journal of Applied Polymer Science, 95 (1), 28–
38.
Siregar, M. S., Thamrin, B. W. S., & Eddiyanto, J. A.
(2014). Grafting of Maleic Anhydride on Natural Cy-
clized Reactive Processing: the Effect of Maleic Anhy-
dride Concentrations. Chem. Mater. Res., 6 (11), 15–
20.
Siregar, M. S., Thamrin, Basuki, Eddiyanto, & Mendez, J.
A. (2014). Grafting of Maleic Anhydride to Natural Cy-
clized Rubber: The Effect of Maleic Anhydride Con-
centrations. Chemistry and Material Research, 6 (11),
15–21.
Tanaka, Y., Ichikawa, N., Sakaki, T., Hioki, Y., & Hayashi,
M. (2004, September 28). Modified natural rubber.
Google Patents.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
150

Wirjosentono, B., Siregar, A. H., Nasution, T. I., Da-
limunthe, K. Z., & Nasution, D. A. (2018). Compatibil-
itation of cyclic natural rubber (Resiprene-35) with pol-
ypropylene in the presence of oleic acid and benzoyl
peroxide. In the Journal of Physics: Conference Series
(Vol. 1116, p. 42043). IOP Publishing.
Wypych, G. (2015). Handbook of UV degradation and sta-
bilization. Elsevier.
Yang, L., Zhang, F., Endo, T., & Hirotsu, T. (2003). Micro-
structure of maleic anhydride grafted polyethylene by
high-resolution solution-state NMR and FTIR spectros-
copy. Macromolecules, 36 (13), 4709–4718.
Zhou, Z. F., Huang, H., & Liu, N. C. (2002). Kinetic and
terpolymer mechanism of crotonic acid onto acryloni-
trile-butadiene-styrene. Journal of Applied Polymer Sci-
ence, 85 (4), 726–733. https://doi.org/10.1002/app.10621
Modification and Characterization Natural Cycle Rubber (Resipren-35) with Oleat Acid using Dicumyl Peroxide and Divinilbenzena as
Compatibility
151
