
Lead (II) Nitrate Catalyzed Esterification Palmitic Acid with Alcohols
Nimpan Bangun
1*
, Justaman Karo-Karo
2
, Hamonangan Nainggolan
1
, Slamet Silaban
1
,
Nia Erisa Tarigan
1
, Rahmad Ramadan
1
1
Department of Chemistry, Universitas Sumatera Utara, Jl. Bioteknologi No. 1 Kampus USU, Medan,
North Sumatera, Indonesia
2
BARISTAND, Medan, Indonesia
niaerisa7@gmil.com, rahmadramadhan32@gmail.com
Keywords: Lead Nitrate, Fatty Acid, Alcohols, Esters.
Abstract: Esters has been long known in some applications that has been attempted to prepare in many fashion
methods. Several catalysts used based on acid either solid or liquid has been known popular. A transition
salt also has been intensively reported catalyzes esterification. Very few catalysts from Group A metal
shown activities esterification catalysis. Lead (II) nitrate shown a good catalyst performance in esterification
long chain fatty acid reacts with primary and secondary alcohol. Palmitic acid reacts with alcohols, glycerol,
1,2-propane diol as well as stearyl alcohol gives high yield ester respectively. All ester has been
characterized in FT-IR, 1H NMR and 13C NMR spectrophotometer shown the yield was 90-97%.
1 INTRODUCTION
Oleochemical industry is still growing sustainable
due to the product is needed in daily living. Esters
for example have high demand as fuel, plasticizers,
fragrance, adhesive and also as lubricants (Joseph,
2005; Mbaraka, 2006; Krause 2009; Martinez 2009).
Catalysis esterification reactions can be performed
in several manner catalyst such as, homogeneous
acid include AlCl
3
, HF, H2SO4. Although those
catalysts bring high environmental risk and the cost
inefficiency, this is still used in production industrial
esters (Ziarani, 2013). Part of research to improve
the environmental risk, then the heterogeneous
catalyst has been developed. This catalyst system
can be allowed to recovery, recycle and then reused
(Gupta, 2007). Parallels to the chemical process
environmentally, sulfonates acid functional was
modified, by grafting with silica gel (Davison, 2008)
anchoring to silica mesoporous (Keppelera, 2011),
inorganic solid supported (Vijaykumar, 2012) that
can be less toxic. Attempt to apply in chemical
process esterification long chain acid, fatty acid with
glycerol (alcohol multivalent), a sulfonate catalyst
was used in a high condition (240
0
C) (Sari, 2017).
In a relative new esterification catalyst
development such as Barnstead acid salt, Sn (II) and
Sn (IV) performs reaction long chain acid with
alcohols to be esters. The key reaction is a forming
bonding Sn-OR (Cardoso, 2009; da Silva, 2011;
Ferreira, 2012). However the report is still limited
on a simple alcohols. Due to Lead has a very similar
chemistry to Tin, then it has been shown catalytic
activity in esterification palmitic acid toward stearyl
alcohol, diol and also triol as reported here.
2 MATERIALS AND METHODS
2.1 Materials
Materials used in this research Pb(NO
3
)
2
, 1,2-
propane diol, 1, glycerol, octadecyl alcohol (stearyl
alcohol), palmitic acid and m-xylene were purchased
from Merck and used without pretreatment.
2.2 Analysis Method
1
H NMR and
13
C NMR spectra were recorded at 500
MHz on Agilent spectrometer. Wave number of
carbonyl were recorded on spectrometer FT IR.
Bangun, N., Karo-Karo, J., Nainggolan, H., Silaban, S., Tarigan, N. and Ramadan, R.
Lead (II) Nitrate Catalyzed Esterification Palmitic Acid with Alcohols.
DOI: 10.5220/0008857901330135
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 133-135
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
133
2.3 Esterification Reaction
Into a round bottle flask provided a hotplate stirrer, a
bar magnetic and a condensor, a mixture of xylene
30 ml; glycerol (1.84 g; 0.02 mol), palmitic acid
(15.384 g; 0.06 mol) and lead (II) nitrate (0.02 g;
6x10
-5
mol). The system was heated at 140
0
C for 8
hours. The product mixture was distilled to free
xylene, then extracted with n-hexane 2x 50 mL. The
solution was washed with water 50 mL followed by
addition with ethanol- water 30 mL. Finally the n-
hexane fraction was dry with Na
2
SO
4
anhydrous 5 g.
After filtration, then the solution was evaporated and
dried in vacuum, gave a solid was 14.526 g; 90%
yield. Glyceryl tripalmitate was characterized with
spectroscopy FT-IR and
1
H NMR (CDCl
3
).
A similar work was done by change the alcohol,
1,2-propane diol (1.58 g ; 0.02 mol and stearyl
alcohol (5.41 g; 0.02 mol) reacts toward palmitic
acid (10.256 g; 0.04 mol) and (5.12g; 0.02 mol)
respectively producing 1,2-propanyl dipalmitate
(11.244 g; 95%) and stearyl palmitate (10.21 g;
97%).
3 RESULT AND DISCUSSION
3.1 Esterification Process
Reaction alcohols to palmitic acid catalyzed by lead
(II) nitrate has been successfully to form esters.
However the reaction took place in a consuming
time. This is may be needed the evaluation catalyst
amount to increase amount. Normally, the catalyst
amount is proportional to speed of reaction. The end
of the reaction shown that the catalyst turn to black
powder more soluble in ethanol rather the product
that allowable easy to separated from the ester
product in the reaction. The black powder is a future
challenge for investigated.
Based on the ratio product to catalyst, turn over
frequency catalyst found 300- 323 per second.
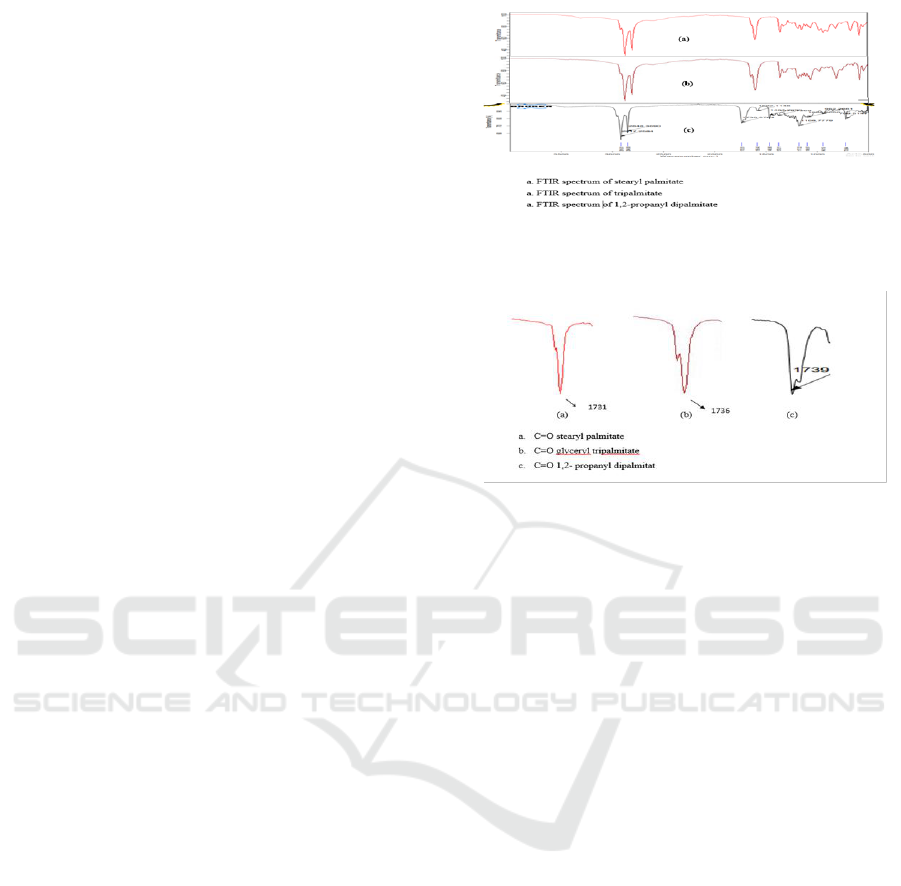
3.2 Spectroscopy FT- IR
Product glyceryl tripalmitate shows wave number (ν
C=Ocm-1) 1731, 1,2-propanyl dipalmitate 1739 and
strearyl palmitate 1736. Figure 1 shows feature of
FT IR spectrum of the esters.
Figure 1: FT-IR Spectrum of the Esters.
Figure 2: Carbonyl fashion on FT IR.
Spectrum FT IR shows on stearyl palmitate
perform a single band C=O) at 1731 cm
-1
with trace
band at 1735 cm
-1
(unknown) while in glyceryl
tripalmitate shows two bands C=O, 1740 cm
-1
(shoulder) and 1736 cm
-1
(strong band) may be
indicated two types of ester resulted 1 and 3 carbon
atom which are equal contribution to IR spectra and
the other one resulted from carbon atom 2 . The
similar mode has also been indicated on 1,2-
propanyl dipalmitate.
Analysis on
1
H NMR (500MHz, CDCl3) and
13
C
NMR (125 MHz, CDCl
3
). The data collected as
below:
a) Stearyl palmitate: 1H NMR δ (ppm) 0.86 -
0.90 (br m, 6H), δ 1.26 - 1.30 (br s, 54H), δ
1.57-1.65 (br m, 4H), δ 2.29 (t, 2H), δ 4.05
(t, 2H)
13
C NMR( 125 MHz, CDC
13
) δ (ppm)
13.98, 22.13, 24.53, 25.54, 28.57, 28.73,
28.77, 28.93, 29.00, 29.05, 31.32, 32.57,
33.70, 60.76, 174.53.
b) Glyceryl tripalmitate: 1H NMR δ (ppm)
0.86 - 0.90 (br m, 2H), δ 1.26 - 1.30 (br s,
78H), δ 2.32 (t, 2H), δ 4.32 (t, 1H), δ 5.25
(t, 2H)
13
C NMR( 125 MHz, CDCl3) δ 14.26,
22.85, 24.84, 29.22, 29.28, 29.40, 29.44,
29.52, 29.59, 29.64, 29.75, 29.76, 29.80,
29.81, 29.83, 29.84, 29.85, 32.08, 34.21,
34.36, 62.15, 68.93, 180.21.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
134

c) 1,2-propanyl dipalmitate:
1
H NMR δ(ppm)
0.86 - 0.90 (t, 3H), δ 1.19- 1.30 (br s, 27H),
δ 1.57-1.65 (br m, 2H), δ 2.32-3.94 (t, 4H),
δ 4.11 (t, 2H), δ 4.96 (m, 2H), δ 5.14 (m,
1H)
(a)
(b)
(c)
a. Structure of stearyl palmitate
b. Structure of glyceryl tripalmitate
c. Structure of 1,2-propanyl dipalmitate
Figure 3: Chemical Structure of a,b and c.
3.3 Yield of Reaction
Influence of Alcohols with palmitic acid catalyzed
by Pb(NO
3
)
2
.
Stearyl palmitate (1-OH) 97%
Gyceryl tripalmitate ( 3-OH) 90%
1,2- propanyl dipalmitate (2-OH) 95%
The reaction alcohols in a same mole (0.02 mol)
mono, diol and triol with palmitic acid at
stoichiometric reaction gives a range of yield 97%,
95% and 90%. The alcohol might have steric effect
on the reaction that shown in triol less yield of ester
then the two others.
4 CONCLUSIONS
Lead (II) nitrate has a good catalyst esterification for
long chain acid with long chain, multivalent
alcohols. In future work, we need to show the
catalyst performance a simple acid esterfied with a
series alcohol chain.
REFERENCES
Cardoso, A. L; Neves, S C G; and da Silva, M. J., 2009.
Kinetic study of alcoholysis of the fatty acids
catalyzed by tin (II) chloride: an alternative catalyst
for biodiesel production,”Energy and Fuels. 23 (3):
1718–1722.
Da Silva, M J; Goncalves, C E and Laier, L O., 2011,
Novel esterification of glycerol catalysed by tin
chloride (II): a recyclable and less corrosive process
for production of bioadditives,” Catalysis Letters.
141(8): 1111–1117.
Ferreira,A B; Cardoso, A L and daSilva, M J., 2012. Tin-
Catalyzed Esterification and Transesterification
Reactions: A Review
G.M. Ziarani, A. Badiei, Z. Dashtianeh, P. Gholamzadeh,
N.H. Mohtasham., 2013. Application of SiO2–Pr–
SO3H as an efficient catalyst in the Ritter reaction.
Gupta, R; Paul, S; Gupta, R.,c2007. Covalently anchored
sulfonic acid onto silica as an efficient and recoverable
interphase catalyst for the synthesis of 3,4-
dihydropyrimidinones/thiones. Res. Chem. Intermed.
39, 3157– 3163.
J. Mol. Catal. A Chem. 266, 50–54.
Joseph, T., S. Sahoo and S. B. Halligudi (2005) Brönsted
acidic ionic liquids: A green, efficient and reusable
catalyst system and reaction medium for Fischer
esterification. Journal of Molecular Catalysis A:
Chemical, 234, 107-110, ISSN 13811169.
Krause, P., L. Hilterhaus, G. Fieg, A. Liese & U.
Bornscheuer., 2009. Chemically and enzymatically
catalyzed synthesis of C6-C10alkyl benzoates.
European Journal of Lipid Science and Technology,
111, 194-201, ISSN 14387697 14389312.
Martnez, M., R. Oliveros & J. Aracil., 2011. Synthesis of
Biosurfactants: Enzymatic Esterification of Diglycerol
and Oleic Acid. 1. Kinetic Modeling. Industrial &
Engineering Chemistry Research, 50, 6609-6614,
ISSN 0888-5885 1520-5045.
Mbaraka, I. K. & B. H. Shanks., 2006. Conversion of oils
and fats using advanced mesoporous heterogeneous
catalysts. Journal of the American Oil Chemists'
Society, 83, 79-91, ISSN 0003-021X.
Sari, V I; Hambali, E; Suryani, A and Permadi, P., 2017.
Esterification Reaction of Glycerol and Palm Oil Oleic
Acid Using Methyl Ester Sulfonate Acid Catalyst as
Drilling Fluid Formulation. IOP Conference Series:
Materials Science and Engineering, Volume 172, Issue
1, pp. 012062
Vijakumar, B; Mahadevaiah, N; Nagendrappa, G and
Prakash, J., 2012. Esterification of stearic acid with p-
cresol over modified Indian bentonite clay catalyst. J.
Porous Mater. 19: 201–210
C
15
H
31
C O C
17
H
35
O
C
15
H
31
C O CH
2
CH
CH
2
OCC
15
H
31
OCC
15
H
31
O
O
O
C
15
H
31
C O
H
C
H
CHO
CH
3
CC
15
H
31
O
O
Lead (II) Nitrate Catalyzed Esterification Palmitic Acid with Alcohols
135
