
Bioactivity and Phytochemical Constituents of Extract Etanol from
Stem Musa paradisiaca Linn
Mayang Sari
1,3
, Tamrin
2*
, Jamaran Kaban
2
and Zul Alfian
2
1
Postgraduate Chemistry Study Program, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Jl. Bioteknologi No. 1 Kampus USU, Medan, Indonesia
2
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Medan, 20155, Indonesia
3
Institut Kesehatan Helvetia , Jl. Kp. Sumarsono No.107, Medan-20124, Indonesia
Keywords: Phytochemical, Triterpenoid, DPPH, GC-MS.
Abstract: The search for active ingredients from plants that are secondary metabolites as a defense compound from
plants has been carried out. This study was conducted to investigate the phytochemical constituents of Musa
paradisiaca Linn’s pseudo-stem, such as alkaloids, flavonoids, steroids, terpenoids, and saponins. In this
study, we estimated the content of terpenoids and saponins and determined the activity of 1,1-diphenyl-2-
picrylhydrazyl (DPPH) scavenging. Ethanol extract of Musa paradisiaca Linn’s pseudo-stem, active as an
antioxidant (IC 50 = 494.2) with a comparison of Ascorbic acid. Chemical constituents of ethanol extract of
Musa paradisiaca Linn’s pseudo-stem are characterized by GC-MS, which shows that they contain
triterpenoid organic compounds, such as: Corticosterone, Stigmasterol, Obtusifoliol, Lupeol, and 9-
Cyclolanost-24-en-3-ol
1 INTRODUCTION
Indonesia's geographical location has a tropical
climate with high average rainfall throughout the
year so that Indonesia has very famous natural
resources. Various types of plants that can thrive
throughout the archipelago do not know the season.
The use of these plants as well as food ingredients is
also used as traditional medicine. Research on the
chemistry of natural materials is increasingly being
exploited today as a medicinal ingredient and for the
benefit of other fields. The chemical structure
diversity produced by these plants also reduces
abandoned and easily available side effects.
Banana plants, plants that are easy to breed in
tropical climates. One of the varieties known is
kepok banana plant (Musa paradisiaca Linn). Some
parts of this plant have benefits, one of which is as
an antioxidant.
The use of diphenylpicrylhydrazyl (DPPH) as a
stable free radical can estimate antioxidant activity
with IC50 parameters. As a good recommendation for
testing and evaluating data (Molyneux, 2003).
Dopamine and L-dopa compounds contained in
banana peels are significantly active as antioxidants.
The banana peel extract from the varieties (Cavendish
and Dream) had been analyzed by DPPH inhibitory
activity 26.55% to 52.66% (Fatemeh et al, 2012). The
compound content of banana peel extract (Musa
Cavendish) has been identified as Gallocatechin the
most, which is a strong antioxidant (158 mg / 100 g
dry weight) (Someya et al, 2002).
Extract of Banana peel (Musa acuminata Colla
AAA) from 4 types of banana peel has been ana-lyzed
has a high capacity to scavenge 2,2-diphenyl-1-
pikrillhidrazil (DPPH). DPPH scavenging activity of
acetone extract and methanol from banana peel
showed greater value than ethanol and water ex-tract
(Aboul-Enein, 2016). Peel extract from all nine
banana varieties showed significant antioxidant and
phytochemical activity. Antioxidant activity of fresh
green and yellow banana peel from fruit (Musa, cv.
Cavendish) was treated with 70% acetone, which was
partitioned with chloroform (CHCl
3
) and ethyl acetate
(EtOAc), which was evaluated (Mok-bel et al., 2005).
Pseudo-stem sap Banana has several special
properties related to various phenomena such as
browning of fruit after harvest, permanent coloring of
fabrics and fibers, antioxidant, antimicrobial and
antihemorrhagic properties. All aqueous pseudo-stem
Sari, M., Tamrin, ., Kaban, J. and Alfian, Z.
Bioactivity and Phytochemical Constituents of Extract Etanol from Musa paradisiaca Linn.
DOI: 10.5220/0008855100890095
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 89-95
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
89

extracts, methanol and ethanol have been found to
contain good amounts of antioxidants along with
different phytochemical compounds such as
carbohydrates, proteins and phenolic compounds
(Kumar et al., 2014).
This research has been carried out phytochemical
screening test and determined the compounds
contained in the ethanol extract of kepok banana
stem (Musa paradisiaca Linn) as well as knowing
the antioxidant activity of the crude extract.
2 MATERIALS AND METHODS
2.1 Collection and Processing of Kepok
Banana Pseudo-stem (Musa
Paradisiaca Linn)
Kepok Banana pseudo-stems were collected from
the Kotamadya Medan which has a stem diameter of
about 5-10 cm collected in February 2019. Sample
was washed with tap water and dried in drying
cabinet at 500 C for 3 days, crushed with a blender
into powder. And preparations were immediately
made for crude extract ethanol.
2.2 Extraction Procedures
Kepok banana stem powder (250 gr) was extracted
with ethanol 96% immersion for 72 hours and
occasionally stirring. Filtration was carried out, the
filtrate was collected and the precipitate was soaked
with ethanol 96% for 48 hours and filtered again.
The first and second filtrates were combined and
then concentrated at 70
o
C using a rotary flash
evaporator. The crude extract obtained is stored in
the dark at 4ºC for further testing.
2.3 Phytochemical Tests
Introduction Phytochemical analysis is carried out to
determine the presence of various phytochemicals.
2.3.1 Phenolic Compounds
The extract (500 mg) was dissolved in 5 ml of dis-
tilled water. For this, a few drops of neutral 5%
ferric chloride solution is added. Dark green
indicates the presence of phenolic compounds
(Ingonga et al., 2015).
2.3.2 Terpenoids and Steroids
Steroids (Liebermann-Burchard reaction). The 200
mg extract material was added in 10 mL of chloro-
form. Acetic anhydride is added in a 1: 1 ratio which
results in a blue-green ring formation pointing to-
wards the presence of steroids.
Terpenoid (Salkowski test). For 200 mg of ex-
tract material, 2 mL of chloroform (CHCl
3
) and 3
mL of concentrated sulfuric acid (H
2
SO
4
) were
added carefully. Reddish brown marks the presence
of terpenoids (Ingonga et al., 2015).
2.3.3 Phytochemical Tests
200 mg of extract is diluted to 10 mL with
Methanol, boiled and filtered. For 5 mL of filtrate, 2
mL of dilute ammonia is added. 5 mL Chloroform is
add-ed and gently shaken the alkaloid base extract.
The chloroform layer was extracted with 1 mL of
acetic acid. This is divided into two parts. Mayer
reagent was added to one part and Draggendorff
reagent to the other. The formation of cream (with
Mayer reagent) or reddish-brown precipitate (with
Draggendorff reagent) is considered positive for the
presence of alkaloids (Abdallah, 2016).
Mayer reagent & Wagner reagent confirmed the
presence of alkaloids in the extract. Plant extracts
are heated with 2% H
2
SO
4
for two minutes. It is
filtered and a few drops of reagent are added
separately. With a few drops of Reagent Mayer
creamy white precipitation appears a positive result.
Indi-cates the presence of alkaloid compounds.
Wagner's reagent, the precipitate of reddish brown
appeared which also confirmed the presence of
alkaloids in the extract (Rdhia et al, 2018).
2.3.4 Saponins
Crude extract (2 g) boiled in 20 mL of distilled
water in a water bath and filtered. The filtrate was
shaken violently for stable froth which was
considered positive for the presence of saponins
(Moubayed et al., 2017).
2.3.4 Flavonoids
In the aqueous filtrate, 5 mL of dilute ammonia
solution is added, followed by concentrated H
2
SO
4
.
yellow staining indicates the presence of flavonoids
(Ingonga et al., 2015).
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
90

2.4 Gas Chromatography and Mass
The analysis was carried out using Shimadzu
GCMS-QP2010S with Detector: 0.85 kV + 0.00 kV.
Electron Energy: 10 to 200 eV with helium at 1.51
ml for 1 minute as a carrier gas. The mass spec-
trometer is operated in electron (El) impact mode at
70 eV in the scanning range 50-500 m / z. Column
Flow: 1.03 mL / min Separation ratio is adjusted to
1:10. The injector temperature is 200◦C, and the
oven temperature is maintained at 70◦C for 3
minutes, rising to 200◦C. Identification of peak
extracts of raw banana plant extracts was carried out
by comparison with standard retention times, and
mass spectra obtained compared to those available in
the NIST library (NIST 14 - MassSpectral Library,
2014 version).
2.5 Activities for DPPH Radical
Scavengers
Scavenging 1,1-diphenyl-2-pikrillhidrazil (DPPH)
Radicals by the sample were monitored according to
the modified Yen and Chen (1995) method. Briefly,
2 mL aliquots of the test sample were added to 1 mL
of DPPH 0.4 mM methanol solution. Mix the vortex
for 1 minute and then leave the room temperature for
30 minutes in dark conditions, and the absorbance is
read at 517 nm (Moubayed et al., 2017). Synthetic
antioxidant ascorbic acid is used as a positive
control.
The ability of test samples to scavenge DPPH
radicals is calculated using the following equation
(Wu el al., 2009):
Inhibition percent = [(AB - AA) / AB] x 100 (1)
Where: AB = absorbance value of blank sample, AA
= absorbance value of test sample
3 RESULTS AND DISCUSSION
3.1 Phytochemical Screening
Important phytochemicals, such as alkaloids,
triterpenoids, steroids, phenolics, flavonoids and
saponins for their presence and are presented in
Table 1.
From the results of the phytochemical tests
carried out, it is clear that the high content of the
banana plant stems is terpenoids, steroids and
saponins. Further testing of crude extracts in GC-MS
was carried out to determine the compounds present
in the plant extract.
Table 1: Phytochemical test results of crude ethanol
extract of Kepok Banana pseudo-stem (Musa paradisiaca
Linn).
Secondary metabolite
Ethanol extract
Phenolic
-
Terpenoids
++
Steroids
++
Alkaloids
-
Saponins
++
Flavonoids
-
+ : The presence of secondary metabolite
- : The absence of secondary metabolite
3.2 GC-MS of Crude Extract
Table 2: Chemical constituents of ethanolic crude extract
from Musa paradisiaca Linn pseudo-stem.
Compound’s name
RT
Peak
area
(%)
1,3,5-triazine-2,4,6-triamine
12.49
0.17
Trihloroacetic acid, tridecyl
ester
13.31
0.08
Phytol
19.07
0.04
Corticosterone
22.27
0.19
Heptasiloxane tetradecamethyl
28.29
54.05
Corticosterone 21-acetate
28.78
10.95
Stigmasterol
29.05
4.63
Obtusifoliol
29.33
8.47
Butyl-4-
[(trimethylsilyl)amino]benzoate
29.77
3.93
Propionic acid, 3-
(benzol[1,3])dioxol-5-yl)-3-(4-
methylbenzoylamino)
29.89
1.67
Sebacic acid, 4-bromo-2,6-
difluorobenzyl isobutyl ester
30.02
1.59
Lupeol
30.37
4.14
9,19-Cyclolanost-24-en-3-ol,
(3-beta)-
30.63
3.69
9,19-Cyclolanost-3-ol, 24-
methylene-,
31.22
5.49
Pentasiloxane,
1,1,3,3,5,5,7,7,9,9-decamethyl
31.65
0.22
Trimethylsilyl-2-(5H-
chromenol[2,3-b]pyridine
31.96
0.33
Ethanethioic acid, S-[8-
(diethylphosphono)octyl
33.69
0.34
The consequences associated with GC-MS
investigations led to the recognition of many
Bioactivity and Phytochemical Constituents of Extract Etanol from Musa paradisiaca Linn
91
compounds from GC from the ethanol extract of the
Musa paradisiaca Linn. This compound is
recognized through the mass spectrum assembled
with GC. The active principle with their retention
time [RT], molecular formula (MF), molecular
weight (MW) and concentration (%) can be accessed
in Table 2.
Seventeen chemical compounds identified from
ethanol extract from the stem of Musa paradisiaca
Linn by GC-MS analysis. The occurrence of various
bioactive compounds confirms the application of the
stem of Musa paradisiaca Linn to various diseases,
by traditional practitioners, a number of compounds
previously reported from a number of other plant
species.
From the data above (Table 2), there are eight of
the most chemical compounds: Heptasiloxane,
1,1,3,3,5,5,7,7,9,9,11,11,13,13-tetradecamethyl ( RT
28.29; 54.05%); Corticosterone 21-acetate (RT
28.78; 10.95%); Obtusifoliol (RT 29.33; 8.47%);
9,19-Cyclolanostan-3-ol, 24-methylene -, (RT 31.22;
5.49%); Stigmasterol (RT 29.05; 4.63%); Lupeol
(RT 30.37; 4.14%); Butyl 4 - [(trimethylsilyl)
amino] benzoate (RT 29.77; 3.93%); 9,19-
Cyclolanost-24-en-3-ol, (3.beta.) - (RT 30.63;
3.69%). And the bioactive compounds in the extract
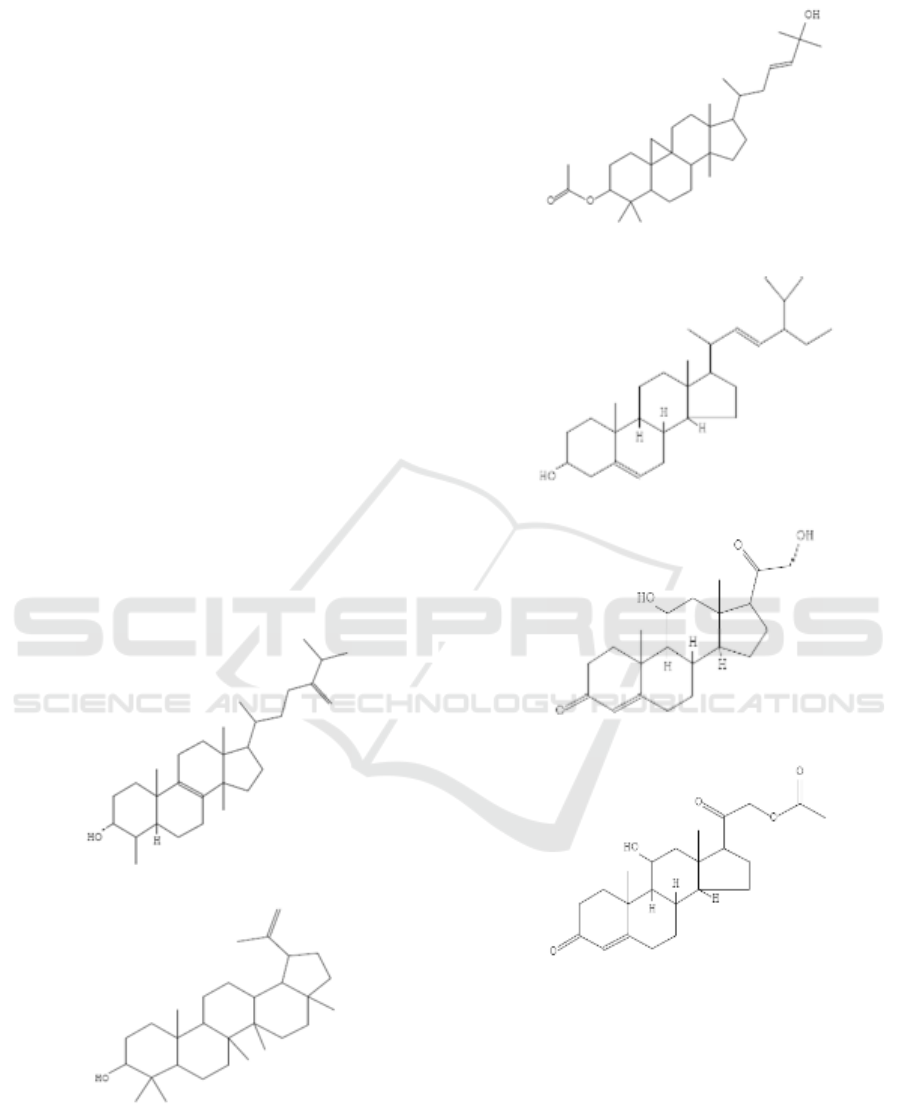
have the following chemical structures:
(a)
(b)
(c)
(d)
(e)
(f)
Figure 1: Chemical structures of (a) Obtusifoliol (b)
Lupeol (c) Stigmasterol (d)9,19-Cyclolanostan-3-ol, 24-
methylene- (3β. (e) Corticosterone (f) Corticosterone
acetate.
The obtusifoliol content of 8.47%, the type of
steroid contained in stem ethanol extract is very
potential as a compound that can inhibit the
proliferation of MCF-7 and MDA-MB231 breast
cancer cells through the cell cycle which is stopped
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
92
the development and induction of apoptosis.
(Aghaei et al., 2016)
Lupeol obtained from GC-MS results as much as
4.14%. Lupeol is a pharmacologically active penta-
cyclic triterpenoid found in several medicinal plants
throughout the world. This compound shows a
hepatoprotective effect on Aflotoxin B1-induced
damage in mice. In addition, lupeol has a hepato-
protective effect on CCl
4
poisoning. The protective
effect of the Lupeol compound will improve kidney
injury associated with hypercholesterolemia, namely
the presence of cardioprotective which can be
beneficial in the condition of hypercholesterolemia
because it minimizes lipid abnormalities and ab-
normal biochemical changes caused by cholesterol
and mice fed colic acid (Mbaveng et al., 2014).
Stigmasterol in the crude extract of ethanol stem
as much as 4.63%. The presence of cholesterol-
lowering activities by Stigmasterol, other
bioactivities are ascribed to plant sterol compounds,
one of which has the potential to cause anti-
inflammatory effects. To investigate the effects of
stigmasterol, plant sterols, on inflammatory
mediators and metalloproteinases produced by
chondrocytes (Gabay et al., 2010).
9,19-Cyclolanostan-3-ol, 24-methylene- (3β.) In
this extract contained 9.08%, were triterpenes and n-
Hexadecanoic acid, have been reported to have
antimicrobial activity. It has also been reported that
plant sterols are a good therapeutic choice for the
management of hypercholesterolemia (Ameachi and
Chijioke, 2018).
Some antibacterial compounds, such as 24, 24-
dimethyl-9,19-cyclolanostan-3 b-ol, daucosterol,
allantoin, and D-mannitol have been reported in
aerial parts (Phthalides et al., 2018). 9.19-
cyclolanostan-3-ol.24-methylene-3.beta acting as an
anti-HIV compound, used to prevent the HIV virus.
(Arora and Kumar, 2017). Corticosterone is a
compound that can act as a metabolism (low to
moderate levels) and stress hormones (high levels)
and, can affect reproductive (Apfelbeck et al., 2017).
3.3 Antioxidant Test
Effect of ethanol extract of Musa parasiaca Linn
containing active compounds reacting with DPPH
free radicals will change to 1,1-diphenyl-2-
picrylhydrazine which is non-radical. Look at the
color changes that occur from DPPH which is purple
to yellow and proven by the smaller absorbance
value (Molyneux, 2003). The decrease in absorbance
along with the increase in plant ethanol extract was
added. So that we can determine% inhibition of each
concentration and we can determine the linear
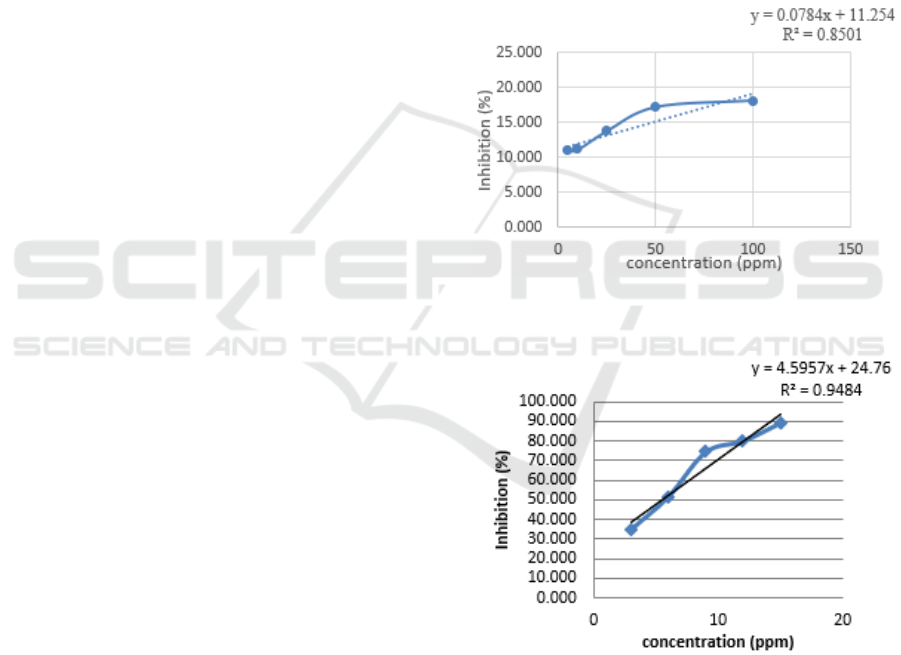
regression equation in the Figure 2.
Based on Figure 2, the linear line equation is
obtained and from this equation is used to calculate
the IC50 value of the ethanol extract of the sample.
The results of calculation of IC50 values were
obtained 494.209. And from Figure 3 as a
comparison is ascorbic acid with an IC50 value of
5.492.
The ability of extracts to ward off DPPH is
thought to be the presence of Lupeol compounds.
The statement that Lupeol as a natural active
constituent is well-known for its anti-inflammatory,
antioxidant and neuroprotective activities (Kaundal,
2017).
Figure 2: Curve of % inhibition of Musa paradisiaca Linn
extract.
Figure 3: Curve of % inhibition of ascorbic acid.
Besides lupeol, there is also the presence of
Stigmasterol, also known as Stigmasterin or the
Wulzen anti-stiffness factor, a non-saturated plant
sterol found in various medicinal plants.
Stigmasterol is used in a number of chemical
processes designed to produce various synthetic and
semi-synthetic compounds for the pharmaceutical
industry. It acts as a precursor in the synthesis of
Bioactivity and Phytochemical Constituents of Extract Etanol from Musa paradisiaca Linn
93

progesterone and acts as an intermediary in
androgen biosynthesis, estrogen, corticoids 1 and in
the synthesis of vitamin D and Stigmasterol has also
been investigated for its pharmacological prospects
such as antiosteoarthritis, antihypercholestrolemic,
cytotoxic, antitumor, hypoglycemic, antimutagenic,
antioxidant, and anti-inflammatory (Chaudhary et
al., 2011).
4 CONCLUSIONS
Crude ethanol extract of Musa paradisiaca Linn
from 250 g of dried powder phytochemical
screening test was carried out and continued with
compound analysis by GC-MS. Some active
compounds are obtained which are thought to be
very beneficial for biological activities. The
presence of antioxidant activity extracts has been
evaluated. As far as we know, this research is the
first report on the bioactivity of plant ethanol extract
Musa Paradisiaca Linn Further phytochemical
research is needed to identify the active principle
responsible for activity antioxidant. This
investigation presents sufficient data about
phytochemical constituents in one polar solvent.
Bioactive compounds found in the stem of Musa
paradisiaca Linn plant hope to be applied as natural
antioxidants and can be extrapolated for clinical
studies.
ACKNOWLEDGEMENTS
We are grateful to Doctoral Department of Chemis-
try Universitas Sumatera Utara, and LPDP for
funding my research under the scholarship number
20161141021370.
REFERENCES
Abdallah, Emad M. 2016. “iMedPub Journals Preliminary
Phytochemical and Antibacterial Screening of
Methanolic Leaf Extract of Citrus Aurantifolia
Abstract.” (Figure 1): 1–5.
Aboul-Enein, Ahmed M et al. 2016. “Identification of
Phenolic Compounds from Banana Peel (Musa
Paradaisica L.) as Antioxidant and Antimicrobial
Agents.” Journal of Chemical and Pharmaceutical
Research 8(4): 46–55. http://www.jocpr.com/articles/
identification-of-phenolic-compounds-from-banana-
peel-musa-paradaisica-l-as-antioxidant-and-
antimicrobial-agents.pdf.
Aghaei, Mahmoud, Zeinab Yazdiniapour, and Mustafa
Ghanadian. 2016. “Obtusifoliol Related Steroids from
Euphorbia Sogdiana with Cell Growth Inhibitory
Activity and Apoptotic Effects on Breast Cancer Cells
(MCF-7 and MDA-MB231).” Steroids.
http://dx.doi.org/10.1016/j.steroids.2016.07.008.
Ameachi, Nuria Chinonyerem, and Chinemerem Linda
Chijioke. 2018. “Evaluation Of Bioactive Compounds
In Pseudarenthemum Tunicatum Leaves Using Gas
Chromatography- Mass Spectrometry.” 18(1): 53–60.
Apfelbeck, Beate et al. 2017. “Baseline and Stress-
Induced Levels of Corticosterone in Male and Female
Afrotropical and European Temperate Stonechats
during Breeding”: 1–16.
Arora, Sunita, and Ganesh Kumar. 2017. “Gas
Chromatography-Mass Spectrometry (GC-MS)
Determination of Bioactive Constituents from the
Methanolic and Ethyl Acetate Extract of Cenchrus
Setigerus Vahl ( Poaceae ).” 6(11): 635–40.
Baskar, Ramakrishnan et al. 2011. “Antioxidant Potential
of Peel Extracts of Banana Varieties
(<i>Musa
Sapientum</i>).” Food and Nutrition
Sciences 2(10): 1128–33.
http://www.scirp.org/journal/doi.aspx?DOI=10.4236/f
ns.2011.210151.
Chaudhary, Jasmine, Akash Jain, Navpreet Kaur, and Lalit
Kishore. 2011. “Stigmasterol : A Comprehensive
Review.” (January).
Fatemeh, S. R., R. Saifullah, F. M.A. Abbas, and M. E.
Azhar. 2012. “Total Phenolics, Flavonoids and
Antioxidant Activity of Banana Pulp and Peel Flours:
Influence of Variety and Stage of Ripeness.”
International Food Research Journal 19(3): 1041–46.
Fitokimia, Kandungan et al. 2012. “Phytochemical
Constituents and In Vitro Bioactivity of Ethanolic
Aromatic Herb Extracts.” 41(11): 1437–44.
Gabay, O et al. 2010. “Stigmasterol : A Phytosterol with
Potential Anti-Osteoarthritic Properties.”
Osteoarthritis and Cartilage 18(1): 106–16.
http://dx.doi.org/10.1016/j.joca.2009.08.019.
Ingonga, Johnstone, Albert Kimutai, and Lydia Bonareri
Nyamwamu. 2015. “Phytochemical Constituents Of
Senna Didymobotrya Fresen Irwin Roots Used As A
Traditional Medicinal Plant In Kenya.” 3(6): 431–42.
Kaundal, Madhu. 2017. “iMedPub Journals Lupeol
Isolated from Betula Alnoides Ameliorates Amyloid
Beta Induced Neuronal Damage via Targeting Various
Pathological Events and Alteration in
Neurotransmitter Levels in Rat’ S Brain Abstract.”: 3–
10.
Mbaveng, Armelle T, Rebecca Hamm, and Victo Kuete.
2014. Toxicological Survey of African Medicinal
Plants 19 Harmful and Protective Effects of
Terpenoids from African Medicinal Plants. Elsevier
Inc. http://dx.doi.org/10.1016/B978-0-12-800018-
2.00019-4.
Mokbel, Matook Saif, and Fumio Hashinaga. 2005.
“Antibacterial and Antioxidant Activities of Banana
(Musa, AAA Cv. Cavendish) Fruits Peel.” American
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
94

Journal of Biochemistry and Biotechnology 1(3): 125–
31.
http://www.thescipub.com/abstract/?doi=ajbbsp.2005.
125.131.
Molyneux, Philip. “The Use of the Stable Free Radical
Diphenylpicryl- Hydrazyl (DPPH) for Estimating
Antioxidant Activity.” 50(December 2003).
Moubayed, Nadine M S et al. 2017. “Antimicrobial,
Antioxidant Properties and Chemical Composition of
Seaweeds Collected from Saudi Arabia (Red Sea and
Arabian Gulf).” Saudi Journal of Biological Sciences
24(1): 162–69.
http://dx.doi.org/10.1016/j.sjbs.2016.05.018.
Phthalides, Bioactive, Chengke Zhao, Yuan Jia, and
Fachuang Lu. 2018. “Angelica Stem: A Potential
Low-Cost Source of.”
Radhia, Abdelkebir, Najjaa Hanen, Ben Arfa Abdelkarim,
and Neffati Mohamed. 2018. “iMedPub Journals
Phytochemical Screening, Antioxidant and
Antimicrobial Activities of Erodium Glaucophyllum
(L .) L’ Hérit.”: 1–7.
Ranjan Kumar, Priya et al. 2278. “Study of Antioxidant
and Antimicrobial Properties, Phytochemical
Screening and Analysis of Sap Extracted from Banana
(Musa Acuminata) Pseudostem.” International Journal
of Advanced Biotechnology and Research 5: 976–
2612. http://www.bipublication.com.
Sheel, Rimjhim, Kumari Nisha, and Prof Jainendra
Kumar. 2014. “Preliminary Phytochemical Screening
of Methanolic Extract Of Clerodendron Infortunatum.”
7(1): 10–13.
Someya, Shinichi, Yumiko Yoshiki, and Kazuyoshi
Okubo. 2002. “Antioxidant Compounds from Bananas
(Musa Cavendish).” Food Chemistry 79(3): 351–54.
Wu, Nan et al. 2009. “Antioxidant Activities of Extracts
and Main Components of Pigeonpea [Cajanus Cajan
(L.) Millsp.] Leaves”: 1032–43.
Bioactivity and Phytochemical Constituents of Extract Etanol from Musa paradisiaca Linn
95
