
The Synthesis of Graphene from Coconut Shell Charcoal
Minto Supeno*, Rikson Siburian, Desi Natalia
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara
Keywords: charcoal, sp
2
orbital, graphene, activated carbon, pyrolysis, coconut shell
Abstract: The hybrid coconut shell charcoal is sp
3
, after being mixed with activated carbon and heated to 600 for 1
hour it produces sp
2
which shows the characterization of graphene. The process of making graphene in this
study, namely coconut shell dried under sunlight then hydrolyzed into charcoal then mixed with activated
carbon as a reducing agent at 600°C for 1 hour to produce graphene. The resulting graphene is characterized
by XRD, SEM-EDX, XRF and BET. The results of the XRD analysis showed that the resulting peaks were
not sharp and slightly broadened the diffraction peak at 24° and 44°. The results of SEM-EDX analysis at
4000x magnification showed smaller, thinner surface sizes and structural shapes and reduced buildup in the
graphene structure. XRF analysis results show that there are still organic impurities. The results of graphene
analysis with BET show the surface area of graphene 82.873 m
2
/ g with a pore volume of 0.116 cc / g.
1 INTRODUCTION
Coconut shell is a hard part on coconut which
has a thickness between 3-8 mm which consists
mostly of lignin, cellulose, and hemicellulose.
Coconut shells can be converted into charcoal or
activated carbon through the carbonization process.
Coconut shell can be used as a carbon source in
graphene synthesis (Liyanage and Pieris, 2015).
Now, the most popular method for producing
single-layered and multi- layered graphene is by
solving methods or known as mechanical and
chemical methods. For the mechanical method, the
graphene produced is single layered. However, the
cost needed in making graphene is very expensive,
and graphene is produced in small quantities.
Meanwhile, with the chemical method, graphene
produced in large quantities and the preparation of
graphene is very simple, but the graphene produced is
still not single-layered (Siburian, 2012).
In general, graphene is made through the Hummer
method and got from graphite mining which comes
from nature and is a non-renewable resource.
Previous researchers were done by Supeno and
Siburian (2018) using coconut shells which were
converted into graphite and graphene nano switching.
The conversion of coconut shell into graphene can be
seen from the comparison of Aluminum-vessel effect
and Pyrex-Glass effect on the cracking process. On
Pyrex-Glass vessels the process of pyrolysis at high
temperatures will less contribute in donating
electrons to the coconut shell compared to the
Aluminum-vessel vessel and then continued
pyrolysis at a temperature of 600°C. Previous
researchers assumed that coconut plants were an
abundant natural resource and the constituent carbon
was C-amorphous. Therefore, through this research
coconut shell was used as a carbon source in graphene
synthesis. Graphene synthesis starts from carbonize
coconut shell into charcoal. After the coconut shell
structure changed from physical structure to charcoal,
then the reducing agent is activated carbon is added
which functions to absorb the oxide present in the
charcoal with a temperature of 600°C which is
expected to synthesize graphene from coconut shell
charcoal. Based on the background described, it is
necessary to do some research with the title "The
Synthesis of Graphene from Coconut Shell
Charcoal".
Supeno, M., Siburian, R. and Natalia, D.
The Synthesis of Graphene from Coconut Shell Charcoal.
DOI: 10.5220/0008839600390044
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 39-44
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
39
2 MATERIALS AND METHODS
2.1 Tools
Tools used in this experiment: Glassware, Analytical
Balance, Tube Clamp, Watch Glass, Porcelain Saucer,
Filter Paper, Mortar, Funnel, Magnetic Bar,
Furnace, 100 mesh sieve, 150 mesh sieve, Oven,
Spatula, Electron Scanning Microscope, X-Ray
Diffraction, Brunauer–Emmet– Teller, Fluorescence
Spectroscopy X-ray.
2.2 Materials
Materials used in this experiment: coconut shell,
activated carbon, aquadest and KOH 1 N.
2.3 Procedure
2.3.1 Making of Charcoal
Coconut shells are dried in the sun until dry. Then, 1
kg of coconut shell is taken, then hydrolyzed in the
furnace in an oxygen free condition for 5 hours at
600°C until became charcoal. Smoothed using
mortar. Furthermore, it is shifted using a 100-mesh
size sieve. Then, it was characterized using XRD and
SEM.
2.3.2 Synthesis of Graphene
Charcoal from the coconut shell is in chip form, then
weighed as much as 15 g and mixed with activated
carbon powder. Then it was heated at 600°C for 1
hour. Then sifted using 150 mesh sieves. Coconut
shells are washed with distilled water until clean, and
dried in an oven at 70°C. Furthermore, it was
characterized using XRD, XRF, SEM-EDX, and
BET.
2.3.3 Effect of Addition of KOH 1 N Solution
on Surface Area and Graphene Pore
Size
Weighed 15 g of graphene and then soaked with KOH
1 N for 2 hours. Then precipitated in the oven for 1 h
until dry. Furthermore, the graphene is heated in a
furnace at 600°C for 1 hour. Furthermore, it was
characterized by using XRD and BET.
3 RESULTS AND DISCUSSIONS
3.1 Making of Charcoal
Coconut shells are cleaned then the coconut
shell is burned at 600°C for 5 hours until it turns black
and turns into charcoal. According to Liyanage and
Pieris (2015) the heating process in the coconut shell
will produce gradual changes. Coconut shell charcoal
produced, mashed with a 100-mesh sieve. The
coconut shell charcoal powder produced was
characterized using XRD and SEM-EDX.
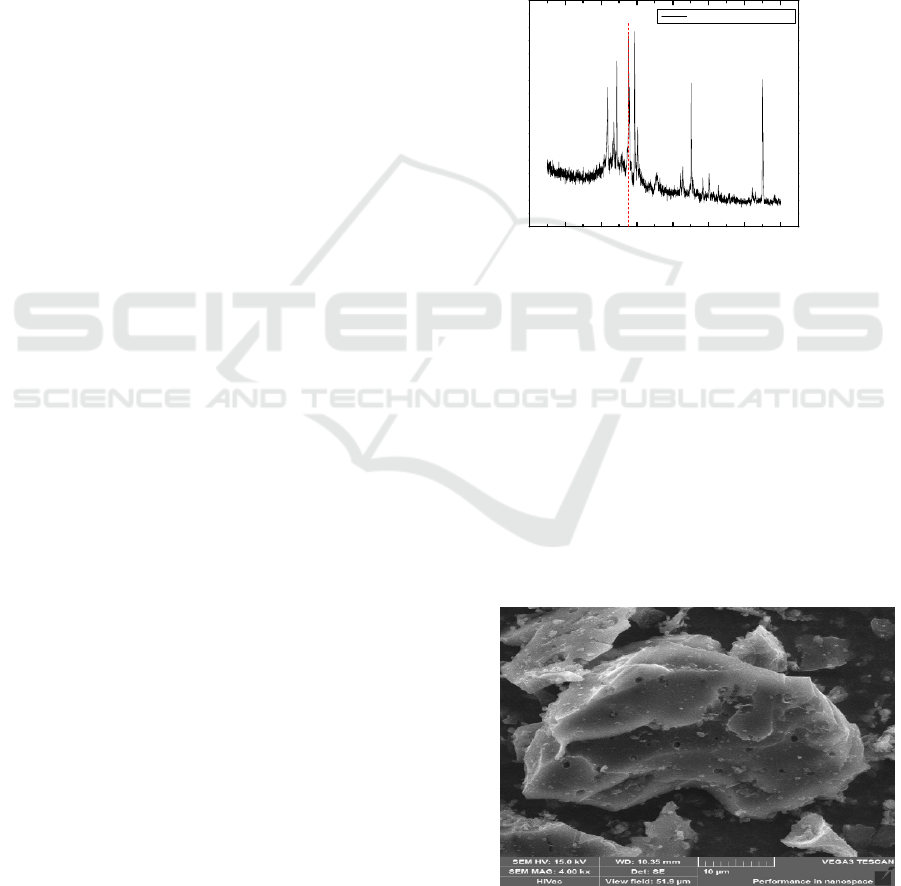
Figure 1: XRD diffractogram coconut shell charcoal
powder
The diffractogram showed by Figure.1, which is
XRD analysis shows that there are sharp peaks and
densities in several regions 2θ starting from 28°
diffraction angle which indicating that the phase
formed is the crystalline phase. The data obtained is
characteristic of the crystal structure of graphite
(Chen and Yan, 2009).
The results of the surface morphology scale
analysis by Scanning Electron Microscope (SEM) on
coconut shell charcoal powder with 4000x
magnification can be seen in Figure 2.
Figure 2: Surface morphology scale by scanning electron
microscope (SEM) on coconut shell charcoal powder with
4000x magnification
0 10203040506070
Intensity (a.u)
2 (
)
Coconut Shell Charcoal
C (002)
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
40
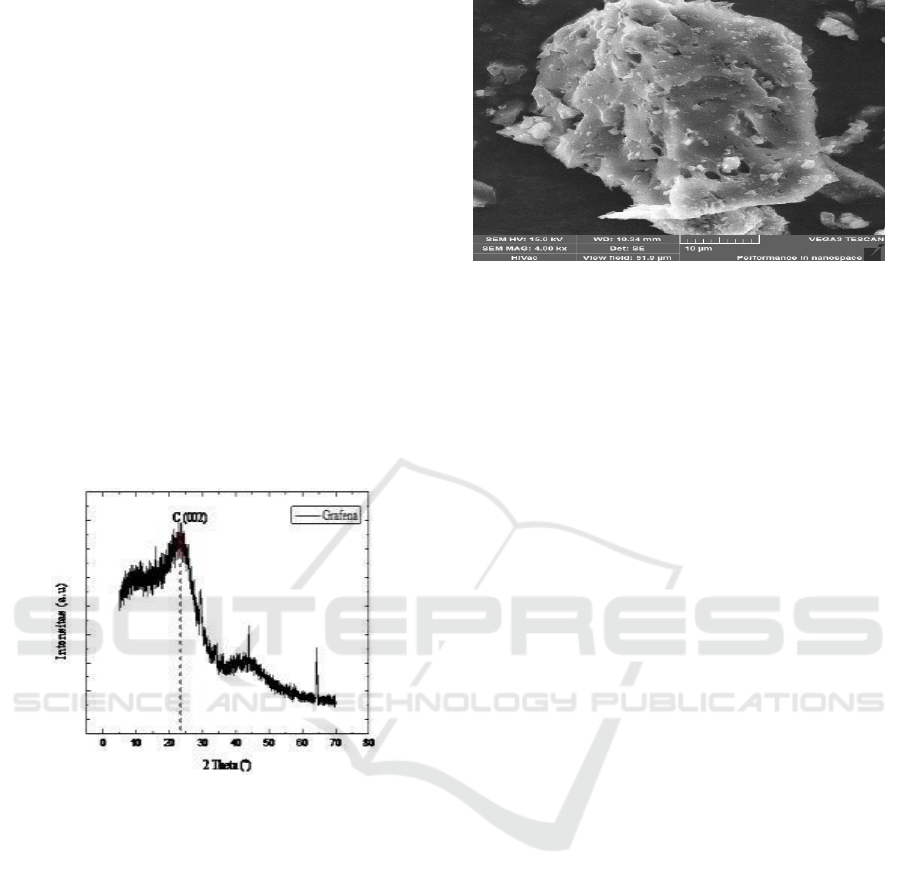
SEM testing aimed to observe the surface
morphology and particle shape of the sample. At
4000x magnification, the coconut shell charcoal
powder in the form of piles showed that the coconut
shell charcoal powder had a layer structure.
3.2 Analysis of Graphene Powder
Structure from Coconut Shell
Charcoal
Graphene powder from coconut shell charcoal in this
experiment was produced from the pyrolysis of old
coconut shell by mixing activated carbon as a
reducing agent with a temperature of 600°C in the
furnace for 1 hour. The structure and phase analysis
of graphene from coconut shell charcoal used X-Ray
Diffraction (XRD), SEM-EDX, XRF and BET.
3.2.1 XRD Data Graphene Powder from
Coconut Shell Charcoal
Figure 3: XRD diffratogram graphene powder from
coconut shell charcoal
Diffraction peaks resulted are weaker and wider
indicating a reduction in some functional groups.
3.2.2 SEM-EDX Data Graphene Powder
from Coconut Shell Charcoal
The results of the surface morphology scale analysis
by Scanning Electron Microscope (SEM) analysis of
graphene powder from coconut shell charcoal with
4000x magnification can be seen in Figure 4.
Figure 4: Surface morphology scale by sem of graphene
powder from coconut shell charcoal with 4000x
magnification
Based on the results of SEM analysis, it is seen that
the formation of graphene layers piled on the 4000x
scale (Supeno and Siburian, 2018) was formed due to
the reduction of carbon on the surface of coconut shell
charcoal.
3.2.3 Analysis of Composite Graphene
Powder from Coconut Shell Charcoal
To analyze impurity phase and to determine the
composition of the elements contained in the material
used X- Ray Fluorescence (XRF) which can analyze
elements of Sodium to Uranium. However, testing
using XRF has not been able to measure the
percentage of the main content of graphene in the
form of C, H, O, N, and S because, it has a lower
atomic number than Sodium.
Coconut shell has a lot of lignin, so it is common
to find a lot of potassium content from the resulting
graphene powder. Other impurities such as Sulfur and
Phosphorus are natural materials which are also found
in natural materials such as coconut shells (Campbell,
2006).
3.2.4 Adsorption-Desorption Nitrogen
Isotherm Analysis of Graphene
Powder from Coconut Shell Charcoal
To measure porosity of mesopore graphene material
and pore size distribution, isotherm adsorption-
desorption of nitrogen is carried out. Graphic curve
adsorption-desorption of graphene powder with the
BJH method can be seen in Figure 5.
The Synthesis of Graphene from Coconut Shell Charcoal
41
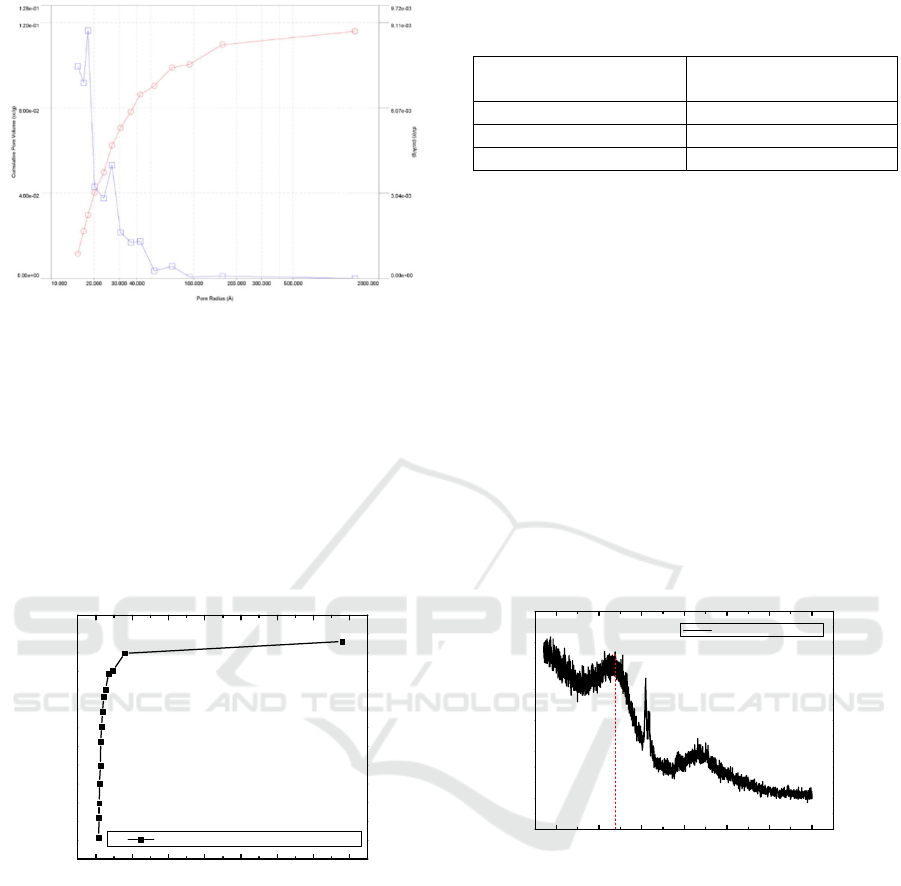
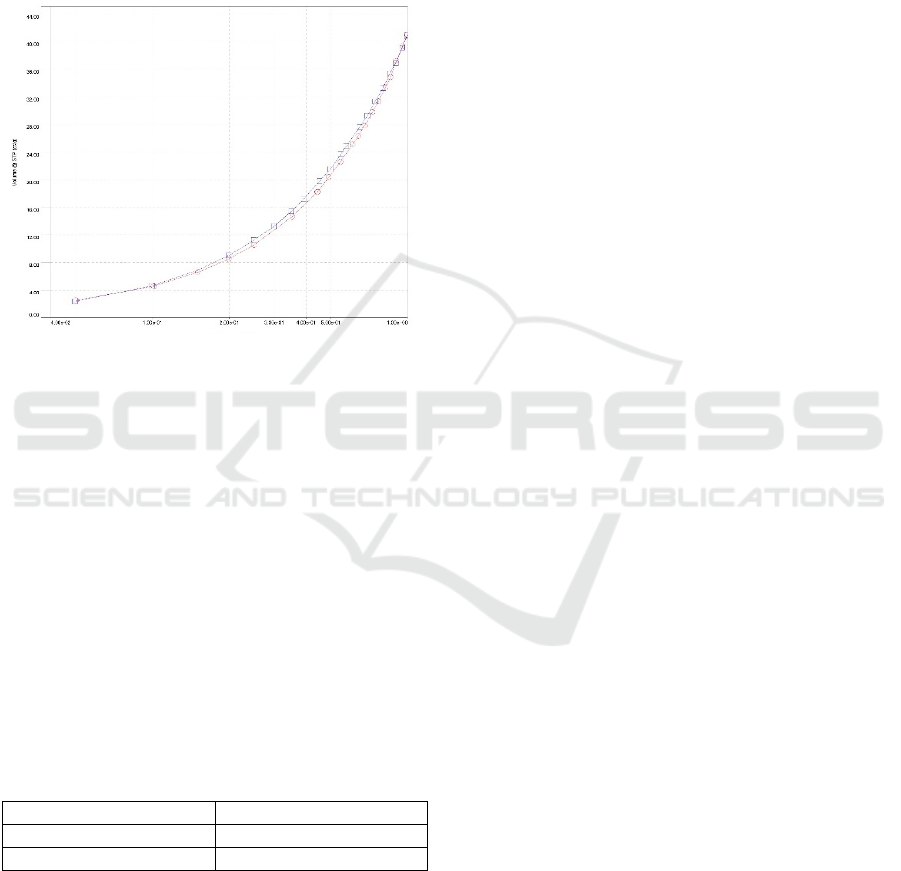
Figure 5: Adsorption-desorption curve of graphene powder
by BJH method
Figure 5 showed type VI adsorption isotherm
according to the IUPAC classification. The non-
uniform surface of graphene to produce Type VI is a
very homogeneous and non-porous characteristic of
two-dimensional solids. To find out the graph of the
distribution of pore size and surface area adsorption,
the Barrett-Joyner-Halenda (BJH) method can be
seen in Figure 6.
Figure 6: Distribution of pore size and surface area
adsorption by Barret-Joyner-Halenda method
Based on Figure 6 graphene powder from coconut
shell charcoal absorbs nitrogen gas (adsorption
isotherm) on the surface of the sample at low
pressure. The results of adsorption- desorption
analysis of nitrogen powder gas graphene BJH
method produce the surface area and pore size shown
in Table 4.1
Table 1: The results of adsorption- desorption analysis of
nitrogen powder gas graphene BJH method
Adsorption-desorption
data
Graphene
Surface area 82.873 m
2
g
Pore volume 0.116 cc/g
Pore radius 1.8098 nm
3.3 Structure Analysis of Graphene
Powder from Coconut Shell
Charcoal by Adding KOH 1 N
Solution
Graphene powder from coconut shell charcoal with
the addition of 1 N. KOH solution. Analysis of the
structure and phase of graphene from coconut shell
charcoal using X-Ray Diffraction (XRD) and BET.
3.3.1 Analysis of XRD Graphene Powder
from Coconut Shell Charcoal by
Adding KOH 1 N Solution
The results of XRD diffraction analysis of graphene
powder from coconut shell charcoal can be seen in
Figure 7.
Figure 7: XRD from graphene powder with adding KOH 1
N from coconut shell charcoal
The resulting diffraction peak is at 24°. The resulting
diffraction peak is weak and wide which indicates
formation of graphene. The effect of adding KOH to
activate graphene produces a large surface area and
pore size.
3.3.2 Adsorption-desorption Nitrogen
Isotherm Analysis of Graphene
Powder from Coconut Shell Charcoal
byA KOH 1 N
To measure the porosity of mesopore graphene
material by adding KOH 1 N solution and pore size
0 200 400 600 800 1000 1200 1400
Pore Volume (cc/g)
Radius (Å)
Distribution of Pore Size and Surface Area Adsorption
10 20 30 40 50 60 70
Intensity (a.u)
Graphene+KOH 1N
C (002)
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
42
distribution, adsorption-desorption nitrogen isotherm
analysis carried out. Potassium hydroxide as an
activator solution played an important role in yield
results. The presence of KOH during activation
results in degradation of the material that will form
the pore. Graphic curve adsorption-desorption of
graphene powder with the addition of KOH 1 N with
the BJH method can be seen in Figure 8.
Figure 8: Graphic curve adsorption-desorption of graphene
powder with the addition of KOH 1 N with the BJH
method
From Figure 8 showed type IV adsorption
isotherm according to IUPAC classification. From
the graph of adsorption-desorption of nitrogen
isotherm from graphene with the addition of KOH 1
N. The results showed that the porous material
subjected to nitrogen gas was included in the
mesoporous category. The results of gas
adsorption-desorption analysis nitrogen graphene
powder with the addition of KOH 1 N with the BJH
method produced the surface area and pore size
shown in Table 2
Table 2: The results of gas adsorption-desorption analysis
nitrogen graphene powder with the addition of KOH 1 N
with BJH method produced surface area and pore size
Adsorption-desorption Graphene
Surface area 40.494 m
2
g
Pore volume 0.060 cc/g
From Table 2, it can be concluded, in this
activation process carbon will react with KOH so
that carbon will be eroded (forming holes) resulting
in the formation of pores. In this experiment the pore
size and surface area were smaller than before the
addition of KOH 1 N, graphene surface area 82.873
m
2
/ g and the graphene pore size 1.8098 nm. The
researcher thinks this is because the concentration of
the activating solution is low and between the
substances reacting between the mixed substances
do not touch each other so that it produces a small
surface area and pore size (Erliana, 2015).
4 CONCLUSIONS
Based on the results of the experiment conducted, it
can be concluded as follows: Graphene can be
synthesized from coconut shell charcoal using
activated carbon as a reducing agent. The results of
characterization by XRD analysis showed
diffraction peaks at 24°. The results of SEM-EDX
analysis at 4000x magnification showed smaller,
thinner surface sizes and structural shapes and
reduced build-up in the graphene structure. XRF
analysis results showed that there are still organic
impurities. The results of graphene analysis with
BET showed the surface area of graphene 82.873
m
2
/g with a pore volume 0.116 cc/ g. Activated
carbon can reduce graphene oxide to graphene.
REFERENCES
Allen, M. J., Tung, V . C., Kaner, R.B, 2010,
Honeycomb carbon : a review of graphene,
Chem. Rev., 110,132.
Anggraeni,D.S., 2008. Analisa SEM (Scanning
Electron Microscopy) dalam Pemantauan Proses
Oksida Magnetite Menjadi Hematite. Institut
Teknologi Nasional. Bandung.
Campbell, NA, 2006. Biology : concepts and
connections, 11th ed. Benjamin/Cummings,
USA.
Chen, W. dan Yan. 2009. Preparation Of Graphene
By A Low-Temperature Thermal Reduction At
Atmosphere Pressure. Nanoscale.2010.559-563.
Chen Dong Xiaon, at al., 2012. Materials Science and
Engineering: An Introduction. 6th edition. John
Wiley and Sons. Inc.
Choi, S. M., Seo, M. H., Kim, W . B. (2011),
Synthesis of graphene and their application to
methanol electro oxidation, Carbon, 49,904-909.
Du, Xiaoming., Kalfeng Zheng, Fengguo Liu (2017).
Synthesis and Characterization of Graphene
Nanosheets/Magnesium Composite Processed
Through Metallurgy, 5, 967-971. Shenyang
Ligong University : China.
Erliana, Umiatin, Budi Esmar, 2015. Pengaruh
Larutan Konsentrasi KOH pada Karbon Aktif
Tempurung Kelapa untuk Adsorpsi Logam Cu,
volume IV, MIPA : UNJ
The Synthesis of Graphene from Coconut Shell Charcoal
43

John, A., Alexanda, S. and Larry, A. 2001.
Approaching a Universal Sample Preparation
Method for XRF Analysis of Powder Materials.
International Center for Diffraction Data 2001.
Advances in X – Ray Analysis. 44: 368-370.
Kanellopoulus N, 2011. Nanoporous Materials:
Advanced Techinques for Characterization,
Modeling, and Processing. CRC Press Taylor &
Francis Group. New York.
Keenan CW, 1984. General College Chemistry. Sixth
Edition. Harper and Row, Publishers, Inc. Inggris
317, 328.
Liani. 2012. Pengaruh Temperatur Terhadap
Struktur Kristal dan Morfologi Lapisan TiCl4
pada Pelapisan Logam dengan Menggunakan
Metode Sol-g el. Medan. UNIMED.
Liyanage, C.D., Pieris, M., 2015. A Physico-
Chemical Analysis of Coconut Shell Powder.
Procedia Chem. 16, 222–228.
Loryuenyong, V., Totepvimarn,K., Eimburanapravat,
P., Boonchompoo, W., Buasri, A.,
2013. Preparation and Characterization of Reduced
Graphene Oxide Sheets via Water- Based
Exfoliation and Reduction Methods.
Lotya, M., Hernandez, King, Smith, Nicolosi,
Karlsson, Blighe, De, Wang. McGovern.
Duesberg. Jonathan. Coleman. 2009. Liquid
Phase Production of Graphene by Exfoliation of
Graphite in
Surfactant/Water Solutions. J. Am. Chem. Soc. 131.
3611–3620.
Martinez, M. 2010. Sebuah Pemahaman Dasar
Scanning Electron Microscopy (SEM) and
Mikroskop Elektron (SEM) dan Energy
Dispersive X-ray Detection (EDX
Novoselov, K. S., Geim, A. K., Morozov, S.V., Jiang,
D., Zhang, Y., Dubonos, S. V.,Grigorieva, I. V.
A., 2004. Electric field effect in atomically thin
carbon films, Science,306,666-669.
Nugraheni, A.Y., Nasrullah, M., Prasetya, F.A.,
Astuti, F., Darminto, 2015 Study
on Phase, Molecular Bonding, and Bandgap of
Reduced Graphene Oxide Prepared by Heating
Coconut Shell. Mater. Sci. Forum 827, 285–289.
Pari Gustan., Adi Santoso, Djeni Hendra. 2006,
Pembuatan dan Pemanfaatan Arang Aktif
sebagai Reduktor Emisi Formaldehida Kayu
Lapis, Vol 24, 5,
Jurnal Penelitian Hasil Hutan: Bogor. Prasodjo
Prolessara, 2010. Studi Kapasitas, 18-20, FT
UI : Jakarta.
Rianto,S, dkk., 2012. Pembuatan Sistem Perangkat
Lunak Alat Surface Area Meter Sorptomatic
1800. Prosiding Seminar. Yogyakarta.
Sharma, S. dan Pollet, B. G. 2012. A Review of
Apllication of Carbon Nanotubes for Lithium Ion
Batteray Anode Material. Power Sources.Sharma,
S., Pollet, B.G,2012. A Review of Application of
Carbon Nanotubes for Lithium ion battery anode
material J. Power Sources, 208, 96-119.
Siburian, R., Nakamura, J 2012, Formation Process
of Pt Sunbnano-Clusters on Graphene
Nanosheets, Journal of Physical Chemistry C,
116, 22947-22953, University of Tsukuba : Japan
Siburian, R., Kondo T., Nakamura J 2013, Size
Control to a Sub-Nanometer Scale in Platinum
Catalysts on Graphene, Journal of Physical
Chemistry C, 117,3635-3645. University of
Tsukuba : Japan
Syakir, Norman., Rhesti Nurlina, 2015, Kajian
Pembuatan Oksida Grafit untuk Produksi Oksida
Grafena dalam Jumlah Besar, Vol XIX, edisi
November,55. Departemen Fisika Universitas
Padjajaran : Bandung.
Taqiyah, R., 2012. Perbandingan Struktur Kristal dan
Morfologi Lapisan Tipis Barium Titanat (BT) dan
Barium Zirkonium Titanat (BZT) yang
ditumbuhkan dengan Metode Sol-Gel. Surakarta:
Skripsi, Fisika FMIPA Universitas Sebelas
Maret.
Zhang, J. Z., Wang, Z. –lin., Liu. J., Chen And Liu,
G.-yu., 2003. Self- Assembled Nanostructure.
Kluwer Academic Publisher. New York.
Zhao, Q, et al., 2011. Stability and Textural
Properties of Cobalt Incoporated MCM-48
Mesoporous Molecular Sieve. Applaid surface
Science, 257, 2436-2442.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
44
