
Rhizophora mucronata Leaf Litter Decomposition by Fungi on
Various Level of Salinity in Belawan
Yunasfi, Dinda Dwi Utami Sirait and Budi Utomo
Faculty of Forestry, Universitas Sumatera Utara. Jl. Tri Dharma Ujung No. 1, Campus USU, Medan 20155, North
Sumatra, Indonesia
Keywords: Decomposition, Fungi, Litter, Mangrove, Rhizophora mucronata.
Abstract: Rhizophora mucronata is one mangrove species that is quite dominant found in Belawan. R. mucronata litter
that falls to the forest floor will decomposed with soil microbial decomposers. Fungi are species that play an
important role in the decomposition process and can assist the process of plant growth. The purpose of this
study were to determine the frequency of colonization and the number of species and diversity index fungi in
leaf litter R. mucronata at different levels of salinity. The research using purposive sampling method by
determining the 3-point observation stations based on differences in salinity. The research showed that the
highest fungi population found in station 1 with salinity 0-10 ppt worth 4.04 × 102 cfu / ml. The highest
frequency of fungi colonization of litter decomposition process found in Trichoderma sp. The highest number
of fungi species was found in the level of salinity 0-10 ppt as many as 13 species of fungi. The index species
of fungi in Belawan waters show the same range that is currently illustrating that sufficient productivity,
ecosystem conditions fairly balanced, ecologically balanced pressure.
1 INTRODUCTION
Mangrove known as ecological services both in the
tropical and subtropical to provide niches (ecological
niche) for a variety of flora, fauna and microbes.
Endophytic fungus is one of the microbes that have
been found in nearly all plant families, including
mangroves. Community of endophytic fungi are
important components of a forest ecosystem and
contribute very real diversity and structure of the
vegetation (Giordano et al, 2009).
The coastal area includes parts of the land and sea.
Part mainland, both dry and submerged in water, still
influenced by the properties of the ocean such as tidal,
ocean breeze and salt water intrusion. Part of the
ocean is affected by a natural process that occurs in
the land, such as sedimentation and flow of fresh
water, as well as by human activities such as
deforestation and land pollution. The natural
processes affect the difference flooding resulting in
differences in salinity on growth and deployment
zone of mangrove areas. Zone grows in mangrove
areas reflect mangrove ecophysiological responses to
environmental degradation. This will determine the
adaptability of the species composition constituting a
mangrove forest (Jumiati, 2008).
Rhizosphere is an ideal area for the growth of soil
microorganisms that generally didominansi by
bacteria, aktinomicetes, and fungi. Rhizosphere rich
exudate released by plants through the roots secretion
process. The content of exudates include
carbohydrates, amino acids, organic acids, enzymes,
and other compounds. Microorganisms can take
advantage of exudate through the decomposition
process. Exudate decomposition by microorganisms
produce energy and precursor compound. These
precursor compounds can be utilized by
microorganisms and plants (Widiastutik and Nur,
2014).
Litter is fallen leaves that fall to the forest floor.
Litter decompose will donate organic material is a
source of food for many species of fish and biota, as
well as other organisms in the mangrove ecosystem.
Litter decomposition process conducted by
organisms such as crabs worms and microorganisms
are bacteria and fungi (Yunasfi, 2006).
Utilization of various fungi species are expected to
play a role in the decomposition of leaf litter mangove
is one business that can be used to exploit the
biological potential contained in the mangrove
ecosystem. Fungi are the primary decomposers in
decomposition of leaves of mangrove because it has
Yunasfi, ., Sirait, D. and Utomo, B.
Rhizophora mucronata Leaf Litter Decomposition by Fungi on Various Level of Salinity in Belawan.
DOI: 10.5220/0008553603090315
In Proceedings of the International Conference on Natural Resources and Technology (ICONART 2019), pages 309-315
ISBN: 978-989-758-404-6
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
309

the ability to degrade cellulose and lignin. Cellulose
and lignin together constitute a major component of
the cell wall constituent in the leaves (Yunasfi and
Suryanto, 2008).
The diversity of fungi influence on the rate of leaf
litter decomposition. Fungi are the main agents in the
decomposition process so as to produce nutrients.
Decomposition is closely related to bacteria and fungi
which is the main agent in the decomposition process.
Inhibition of this process will result in the
accumulation of organic matter that can not be used
directly by the manufacturer (Bako et al., 2016).
2 MATERIALS AND METHOD
We strongly encourage authors to use this document
for the preparation of the camera-ready. Please follow
the instructions closely in order to make the volume
look as uniform as possible (Moore and Lopes, 1999).
The research was conducted in Belawan. The leaf
litter Rhizophora mucronata obtained and observed
in Belawan. Breeding and fungi identification carried
out in the Laboratory of Plant Pests and Diseases
Faculty of Agriculture, Universitas Sumatera Utara.
(A)
(B)
(C)
Figure 1: (a) Station 1 is at a salinity 0-10 ppt (b) Station 2 is at a salinity of 11-20 ppt (c) 3 stations namely at 21-30 ppt
salinity.
The tools used in this study is a refractometer,
Global Positioning System (GPS), bags of litter (litter
bag) size 30 × 40 cm is made of nylon, needles,
Erlenmeyer flask, glass beaker, burner, test tubes,
Petri dishes, Autoclave, ose needles, glass objects,
glass cover, oven, light microscopy, analytical
balance, mortar, micropipette, 1 ml pipette tip,
Bunsen, digital cameras, scissors, a ruler.
Materials used in this study was the leaf litter
Rhizophora mucronata, Seawater, markers
(stationery), rope, twine, alcohol, distilled water,
tissue paper, cotton, potato, dextrose, agar, mask,
cling wrap, stencil paper, aluminium foil, paper
labels, and methylene blue.
3 METHOD
3.1 Data Collection
The data collection is done in situ and laboratory
observations. Source data used are primary data. The
primary data used is the result of the transect
(sampling in the field) in the form of leaf litter R.
mucronata and data about the identity, the population
of each species, the diversity of species and frequency
of each type of fungal colonization.
Techniques of data retrieval by means purposive
sampling (Data retrieval through judgment) that
determines the third point of observation stations
based on differences in salinity. Station point
determination conducted by measuring the level of
salinity using a refractometer. Station 1 with salinity
0-10 ppt, station 2 with salinity 11-20 ppt, 3 stations
with salinity 21-30 ppt. Determination of the
coordinates of the station is done by using GPS
(Global Positioning System).
The data collection is done after a long period of
decomposition of litter placed on the ground with
various levels of salinity, over time as follows:
a. days - 15
b. days - 30
c. days - 45
d. days - 60
e. days - 75
For each time the survey was taken of the sample
in the form of litter in bags of up to 75 days, and each
time the survey is conducted three replications.
ICONART 2019 - International Conference on Natural Resources and Technology
310

3.2 Sampling
Mangrove leaves R. mucronata fallen collected and
bagged litter (litter bag) made of nylon, measuring 40
× 30 cm with a mesh of 1 × 1 mm by 50 g. The number
of bags that contain as many as 21 bags of litter
prepared at each station. Once inserted leaves, litter
bags sewn then provided with holes on both sides of
the right and left pockets that can be connected with
raffia. Then the litter bag tied tightly mangrove roots
so that when the tide of litter bags can not be
separated.
Identifikasian fungi was done by taking 3 bags
containing litter taken for each level of salinity once
in 15 days and taking the bag of litter do until the 90th
days after the litter is placed in the field.
3.3 Sterilization Equipment and
Materials
Sterilization is done by washing using soap cleaning
tool. Tools that have been rinsed with clean water
drained, to be wrapped in paper stencil. Sterilization
of tools and materials is done with wet sterilization
method, using autoclave at a pressure of 1.5 atm for
15 minutes. Then sterilized using oven dried at 121 °
C for 15 minutes to the farthest of unwanted
microbes.
3.4 Making the Media PDA
Making the Media PDA (Potato Dextrose Agar) is
done by boiling the potatoes that have been diced 250
grams using 1000 ml of distilled water. After boiling,
potato juice filtered into a glass beaker and added
dextrose and so each as much as 20 grams. The
solution was homogeneous and still liquid was poured
into 4 pieces of 250 ml Erlenmeyer flask, closed with
a sterile cotton, aluminum foil and sealed with cling
warp. Media put into an autoclave to be sterilized for
15 minutes at a pressure of 1.5 atm. Before doing the
casting media, added 0.1 gram chlorompenicol.
Chlorompenicol homogenized in liquid media and wa
ready to be poured into the Petri dish.
3.5 Identification of Fungi
Rejuvenate fungi in pure culture on PDA and then
incubated for 5-7 days at room temperature. Isolates
of fungi have grown on the media, grossly identified
by looking at the nature of the growing hyphae,
colony colour and diameter of the colony. Fungal
isolates were also grown on glass objects (object-
glass), that is by putting the pieces in order of 4 x 4 x
2 mm on a glass slide, then stroked fungi loopful on
the PDA media. Then covered with a glass cover
(cover glass). Isolates on glass objects are placed in a
petri dish which has been given in the form of wet
cotton moisturizing. Isolates of fungi on a glass slide
left for several days at room conditions to isolate the
fungi grow sufficiently developed. When isolates of
fungi have evolved removal of the cover glass that has
been overgrown fungi carefully with the aim to
dispose of the pieces in order. Next on the cut so that
a few drops of 1 drop of methylene blue solution.
Glass cover that has been overgrown with fungi
subsequently placed on a solution of methylene blue
on glass objects. Glass culture is observed using a
light microscope to determine the characteristics of
microscopic fungi that is characteristic of hyphae,
hyphae whether there is a bulkhead on,
conidiophores, as well as the characteristics of
conidia or spores (form and sequence). The
characteristics obtained later in the match with fungi
identification key books according to Barnet and
Barry (1987), Gandjar et al (1999), Watanabe (1937)
This activity is carried on a litter every time retrieval
from the field during the decomposition process.
3.6 Data Analysis
To analyse the data the diversity of fungi, used
formula Shannon-Wiener diversity index (Krebs,
1985).
H' : Diversity Index
s : The number of overall sample
i : the data to-i
ni : The number of i-th
N : Total number of species
Diversity index has a range of values as follows:
H' < 1 : lower Diversity
1 < H '<3 : Diversity is being
H '> 3 : High Diversity
Rhizophora mucronata Leaf Litter Decomposition by Fungi on Various Level of Salinity in Belawan
311
4 RESULT AND DISCUSSION
4.1 Fungi That are Kind to the Leaf
Litter Decomposition Process R.
mucronata in Salinity 0-10 pp
The results showed there were 13 species of fungi
decomposers that can be isolated from leaf litter
decomposition process R. mucronata which in
salinity 0-10 ppt. The average number of colony
highest Trichoderma sp. with the average number of
colonies of 0.99 × 10
2
cfu / ml. The average number
of colonies can be seen in Table 1.
The level indicates that the emergence of a type of
fungi Trichoderma able to compete with other fungi
in the uptake of nutrients (nutrients) during
decomposition. It accordance with the statement of
Harman et al. (2004) which states thatTrichoderma
can be found in almost all soil types and in a variety
of habitats, these fungi can multiply rapidly in the
root zone.
In addition Trichoderma has the ability to
compete with soil pathogens especially in getting
Nitogen and Carbon. So this type in inoculation into
the ground to suppress the disease that attacks the
plant nursery, this is because in Trichoderma are
toxins that can control the plant.
Table 1: The average number of colonies × (10
2
cfu / ml) of each species of fungi within 15 days and the frequency of
colonization in the process of leaf litter decomposition R.mucronata for 75 days at a salinity 0-10 ppt.
No.
Species of fungi
The average number of colony (days)
The average number of
colony × (10
2
cfu/ml)
CF (%)
a
15
30
45
60
75
1
Aspergillus flavus
0,33
0,66
0
0
0
0,198
33,33
2
Epicoccum nigrum
0,33
0
0
0
0
0,066
16,66
3
Acremonium sp
0,66
0,33
0
0
0
0,198
33,33
4
Trichoderma sp
0
1
1,66
1,33
1
0,998
66,66
5
Aspergillus sp
0
0,33
0,33
2,33
0,33
0,664
66,66
6
Trichoderma sp. 1
0
0
0,66
1
0,66
0,464
50
7
Rhizopus stolonifer
0
0
0,33
0
0
0,066
16,66
8
Trichoderma sp. 2
0
0
1,66
1,33
0,66
0,73
50
9
Trichoderma sp. 3
0
0
0,66
0
0,33
0,198
33,33
10
Rhizoctonia sp
0
0
0
0,66
0
0,132
16,66
11
Curvularia sp
0
0
0
0,33
0,33
0,132
33,33
12
Fusarium sp
0
0
0
0
0,33
0,066
16,66
13
Penicilium sp
0
0
0
0
0,66
0,132
16,66
4.2 Fungi that are Kind to the Leaf
Litter Decomposition Process R.
mucronata on Salinity 11-20 pp
The results showed there are 11 species of fungi
decomposers that can be isolated from leaf litter
decomposition process R. mucronata which in
salinity 11-20 ppt. The average number of colony
highest Aspergillus sp. and Trichoderma sp. 2. the
average colony count of 0.66 × 102 cfu / ml. The
average number of colonies can be seen in Table 2.
Aspergillus is one type of fungi that are
cosmopolitan and easily isolated. This is consistent
with the statement Mizanaet al. (2016) which states
that the Aspergillus is a microorganism eukaryotes, is
now recognized as one of the few living creatures that
have spread area the most widespread and abundant
in nature, than that of the mold is also a common
contaminants on various substrates in tropical and
subtropical regions.
Trichoderma is one type of fungi which has a role as
decomposers of litter in the leaves of plants
Mangrove called decomposers. This is consistent
with the statement Susanto (2013) which states that
Trichoderma sp. an antagonist fungus species
commonly found in the soil, especially in organic soil
and is often used in biological control. Species of
Trichoderma sp. in addition as decomposers
organisms, can also function as a biological agent.
Biological control agents is one option of controlling
plant pathogens promising because it is inexpensive,
readily available, and safe for the environment.
ICONART 2019 - International Conference on Natural Resources and Technology
312
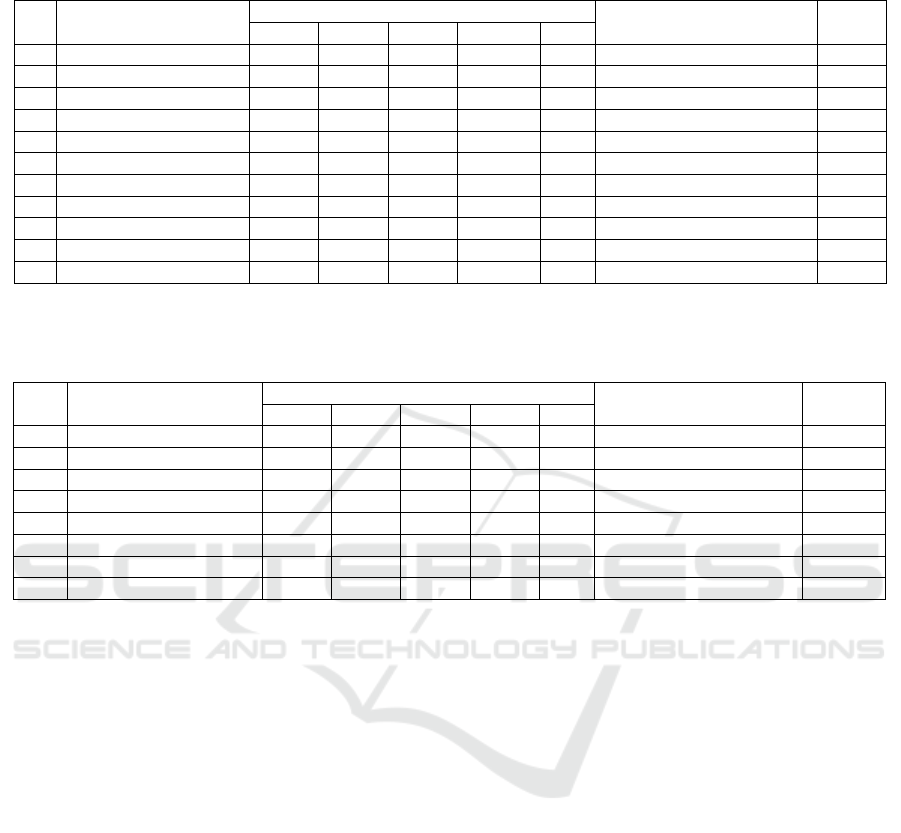
Table 2: The average number of colonies × (102 cfu / ml) of each species of fungi within 15 days and the frequency of
colonization in the process of leaf litter decomposition R.mucronata for 75 days at a salinity of 11-20 ppt.
No
Species of fungi
The average number of colony (days)
The average number of
colony × (10
2
cfu/ml)
CF
%
(a)
15
30
45
60
75
1
Aspergillus sp
1,33
0,33
0
0,33
1,33
0,664
66,66
2
Cladosporium herbarum
0,33
0
0
0
0,33
0,132
33,33
3
Tidak Teridentifikasi
0,33
0
0
0
0
0,066
16,66
4
Aspergillus sp. 2
0
0,33
0
0,66
0,33
0,264
50
5
Rhizoctonia sp
0
0,33
0
0,33
0
0,132
33,33
6
Scytalidium sp
0
0
0,66
0
0,33
0,198
33,33
7
Rhizoctonia sp. 1
0
0
0,33
0,33
0
0,132
33,33
8
Trichoderma sp. 2
0
0
2,33
0,66
0,33
0,664
50
9
Trichoderma sp. 4
0
0
1,33
0,66
0
0,398
33,33
10
Trichoderma sp. 3
0
0
0
0,33
0
0,066
16,66
11
Mucor sp
0
0
0
0
0,66
0,132
16,66
Table 3: The average number of colonies × (102 cfu / ml) of each species of fungi within 15 days and the frequency of
colonization in the process of leaf litter decomposition of R. mucronatafor 75 days at a salinity of 21-30 ppt.
No.
Species of fungi
The average number of colony (days)
The average number of
colony × (10
2
cfu/ml)
CF
(%)
a
15
30
45
60
75
1.
Trichoderma sp. 2
1,33
2,66
2,33
0,66
0,33
1,462
83,33
2.
Aspergillus fumigatus
1
0,66
0
0
1
0,532
50
3.
Curvularia sp
0,66
0,33
0
0,33
0,66
0,39
66,66
4.
Mucor sp
0
0,33
0
0
0,33
0,132
33,33
5.
Trichoderma sp. 4
0
0,66
0,33
0,66
1
0,53
66,66
6.
Mycocladus sp
0
0
0,33
0,33
0
0,132
33,33
7.
Humicola fuscoatra
0
0
0,66
0
0,33
0,198
33,33
8.
Scytalidium lignicola
0
0
0
0,66
0,33
0,198
33,33
4.3 Fungi that are Kind to the Leaf
Litter Decomposition Process R.
mucronata on Salinity 21-30 ppt
The results showed there were 8 species of fungi
decomposers that can be isolated from leaf litter
decomposition process R. mucronata which in
salinity 21-30 ppt. The average number of colony
highest Trichoderma sp. 2 with an average number of
colonies of 1.46 × 102 cfu / ml. The average number
of colonies can be seen in Table 3.
Besides being a very common type of fungus
found in the soil and is a fungus that is antagonistic to
other fungi. According to a statement from Herlina
(2009) states that Trichodermasp. in promoting
hormone / plant growth stimulators. In addition,
application of Trichoderma sp. can optimize the core
crop production.
However, Trichoderma sp. Having the ability to
control fungal pathogens different. In
accordance with the statement of Chet (1987)
which states that the ability of each species of
Trichoderma sp. in controlling different
fungal pathogens, This is because the
morphology and physiology are different. For
example, Trichoderma harzianum and
Trichodrma hamate produce glucanase and
chitinase enzymes that can cause eksolisis
host hyphae.
4.4 Comparison of type of Fungi in
Different Levels of Salinity
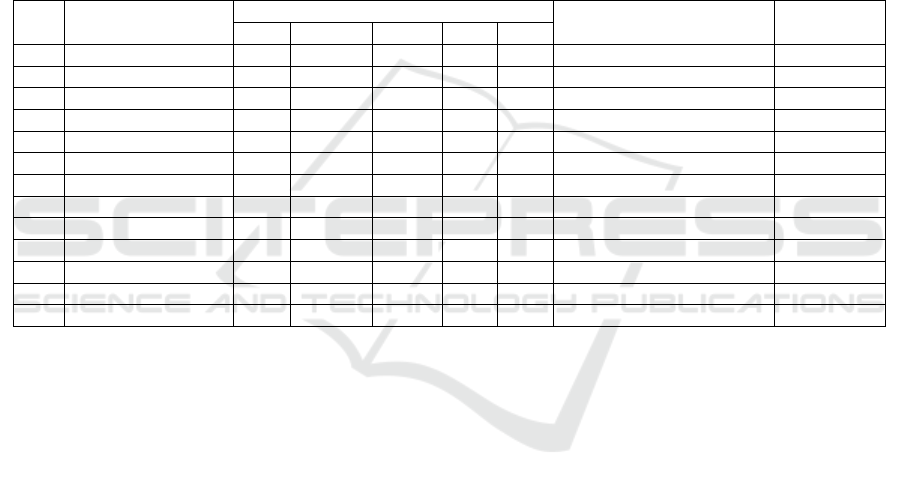
The number of species of fungi found in leaf litter R.
mucronata that which has undergone a process of
decomposition at the level of salinity 0-10 ppt, 11-20
ppt, 21-30 are presented in Figure 2.
At the level of salinity found the number of
different fungi. The higher the salinity level of the
less number of fungi in it, it is consistent with the
statement Yunasfi (2006) conditions were similar to
those freshwater (brackish) is good enough for the
growth and development of various types of fungi
compared to conditions at higher salinity levels. This
is in line with the statement of Silva and Fay (2012)
which states that the fungus was reported more
sensitive to osmotic stress than bacteria. There was a
decrease in the total number of fungi in soil watered
Rhizophora mucronata Leaf Litter Decomposition by Fungi on Various Level of Salinity in Belawan
313
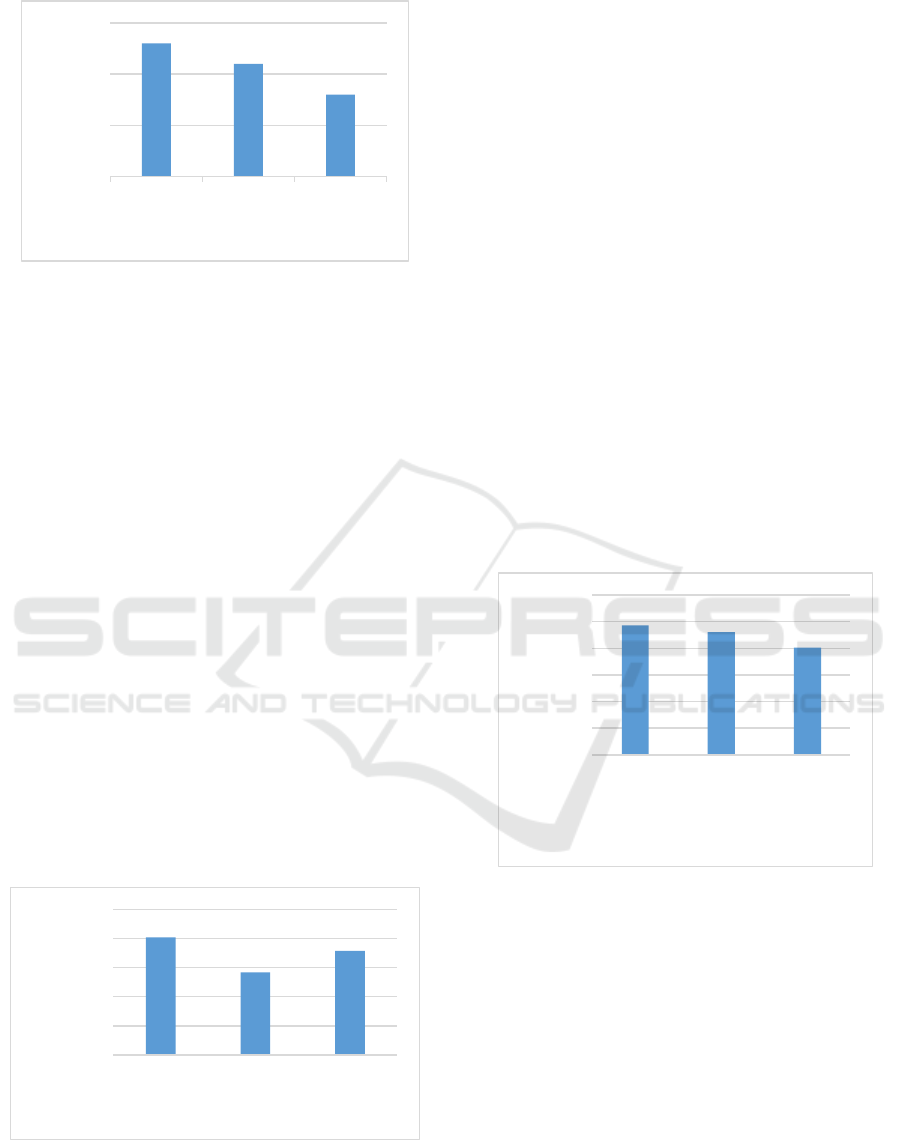
Figure 2: Comparison of Total Type Fungi in Different
Levels of Salinity.
with different concentrations of sodium Cloride. In
the long-term decline in the genetic diversity of the
fungus to the influence of hydrostatic and osmotic
pressure rise that can alter the physiology of the
fungus.
At each station there are differences in the number
of species is found, it is due to several factors. Among
them is the salinity, because of a higher level of saline
in an area the less the population of fungi in it, this is
in accordance with the statement Damanik (2010) that
the microorganisms contained in water is influenced
by physical factors and chemicals such as hydrostatic
pressure, light, pH, salinity and temperature. One
response to salinity microorganism is intolerant and
will die in conditions of high salinity
4.5 Comparison Fungi Population in
Different Levels of Salinity
Comparison population of fungi in leaf litter R.
mucronata has undergone a process of decomposition
at a rate of 0-10 ppt salinity, 11-20 ppt, 21-30 ppt
presented in Figure 3.
Figure 3: Comparison of Population of the fungi at
Different Levels of Salinity.
The highest average opulasi fungi found in leaf
litter R. mucronata that the process of decomposition
at 0-10 ppt salinity level is 4.04 × 102 cfu / ml (Figure
3). The number of fungal population showed that the
nutritional needs of fungi at station 1 (0-10 ppt) is
fulfilled, thus adding to the fungal population.
Differences in the number of population of fungi
were obtained from each station depending on the
resilience of the fungus can survive in the soil,
nutrients for their life cycle, it is consistent with the
statement of Hasyimi (2008) that the difference fungi
to survive in different levels of salinity indicates that
the need fungi are to fulfilled life on the salinity and
able to withstand the salinity conditions. Fungi as
other microorganisms, for life requires organic matter
as an energy source.
4.6 Fungi Diversity Index at Different
Levels of Salinity
Fungal diversity index contained in the leaf litter R.
mucronata that which has undergone a process of
decomposition at the level of salinity 0-10 ppt, 11-20
ppt, 21-30 are presented in Figure 4.
Figure 4: Graph Fungi Species Diversity Index at Different
Levels of Salinity.
Fungal diversity index calculation results at a salinity
0-10 ppt, ppt 11-20, 21-30 ppt is 2.43; 2.3; 2.01
(Figure 4). The average value of Shannon index -
Wiener's diversity of fungi in leaf litterR. mucronata
which plays a role in the decomposition process with
different salinity showed the same range that was.
Diversity of fungi can affect litter decomposition
process, many fungi were found to indicate the high
value of the rate of decomposition of organic matter
from the litter. This is certainly good, especially for
the plant because of the decomposition process will
produce nutrient ready for use by the plant.
0
5
10
15
0-10 ppt 11-20 ppt 21-30 ppt
Total Type Fungi
Levels of Salinity
0
1
2
3
4
5
0-10 ppt 11-20 ppt 21-30 ppt
Fungi Population
×
(10
2
cfu/ml)
Levels of Salinity
0
0,5
1
1,5
2
2,5
3
0-10 ppt 11-20 ppt 21-30 ppt
Species Diversity Index
Levels of Salinity
ICONART 2019 - International Conference on Natural Resources and Technology
314

The highest diversity index value that is at 0-10
ppt salinity, it is stated that salinity affects the
diversity of fungi. The lower the salinity level then
the higher the species diversity of fungi, while the
higher the salinity level, the lower the diversity of
fungi in it. This is consistent with the statement of
Yunasfi and Suryanto (2008) stated below 10 ppt
salinity level is more suitable environmental
conditions for survival, growth and development of
various types of fungi in litter higher in the
salinity.Salinity level influence can be seen by the
number of species of fungi are present in the process
of decomposition of litter.
5 CONCLUSIONS
The highest number of fungi populations found in
station 1 with salinity 0-10 ppt worth 4.04 × 10
2
cfu /
ml. And the frequency of fungal colonization of the
highest in litter decomposition process at different
levels of salinity that is Trichoderma sp. 2.
The index value multifaceted types of fungi in
Belawan showed the same range that is moderate,
illustrating the diversity of being, productivity
sufficiently, fairly balanced ecosystem conditions,
and the pressure of ecological balance.
ACKNOWLEDGEMENTS
The research was funded by Ministry of Research and
Technology Contract Number: 93 / UN5.2.3.1/ PPM
/ KP-DRPM/2018.
REFERENCES
Bako, S., Yunasfi., Leidonald, R. 2016. Leaf Litter
Decomposition Avicennia marina in the waters of Pulau
Sembilan Pangkalan Susu Langkat District of North
Sumatra Province. University of Northern Sumatra.
Field.
Chet, I. 1987. Innovative Approaches to Plant Disease
Control. John Wiley and Sons, A Wiley-Interscience
Publication, USA. Pp. 11-201.
Damanik, A. Y 2010. Types of Fungi which Berasosisasi
the Leaf Litter Decomposition Process Avicenia marina
(Forsk) vierh after application Fungi Aspergillus sp,
Curvullaria sp, Penicillium sp, at Different Levels of
Salinity in the village Sicanang Belawan. [Thesis]
Faculty of Mathematics and Natural Sciences. USU.
Field
Silva, C. M. M. S., Fay, E. F. 2012. Effect of Salinity on
Soil Microorganisms. Embrapa Meio Ambiente-
Capítulo em livro científico (ALICE). Brazil.
Giordano, L., Gonthier, P., Varese, G. C., Miserere, L., &
Nicolotti, G. 2009. Mycobiota inhabiting sapwood of
healthy and declining Scots pine (Pinus sylvestris L.)
trees in the Alps. Fungal Diversity, 38(69), 83.
Harman, G. E., Howell, C. R., Viterbo, A., Chet, L., &
Lorito, M. 2004. Trichoderma species opportunistic,
avirulent plant symbionts (en línea). Nature Reviews 2
(1): 43-56. Consultado 03 ene 2014.Hasyimi, MH 2010.
Microbiology and Parasitology. Trans Media Info.
Jakarta.
Herliana L. 2009. The potential of Trichoderma harzianum
As Biofungisida on Tomato Plants. Department of
Biology, State University of Semarang. Semarang.
Jumiati, E. 2008. Growth Rhizophora mucronata and R.
apiculata in Berlantung Region Growth of Rhizophora
mucronata and R. apiculata in Oil Polluted Area.
Department of Forestry, Faculty of Agriculture,
University of Borneo, Tarakan.
Mizana, D. K., Suharti, N., Amir, A. 2016. Identification of
Fungi growth of Aspergillus sp. Sold in Padang Based
Temperature and Long Storage. Leading Medical
Journal, 5(2): 355-360.
Susanto L. 2013. Introduction to Biological Control of Plant
Diseases. Issue 2. Rajawali Pers. Jakarta. 456 p.
Widiastutik, N., Nur, H, A. 2014. Isolation and
Identification of the Yeast of the rhizosphere
Rhizophora mucronata Wonorejo. Department of
Biology, Faculty of Mathematics and Natural Sciences,
Institute of Technology. Department of Biology,
Faculty of Mathematics and Natural Sciences, the
Institute of Technology (ITS). Surabaya.
Yunasfi. 2006. Leaf Litter Decomposition Avicennia
marina by bacteria and fungi at Different Levels of
Salinity. [Dissertation]. Bogor Agricultural Institute.
Bogor.
Yunasfi, Suryanto, D. 2008. Type of Fungi Involved in Leaf
Litter Decomposition Process Avicennia marina at
various level of salinity. Research Journal of
Mathematics.
Rhizophora mucronata Leaf Litter Decomposition by Fungi on Various Level of Salinity in Belawan
315
