
Pyrolisis of Kapok (Ceiba pentandra) Pods Wastes as Sources of
Potassium Oxide (K
2
O)
Lilis Sukeksi
1,2
, Chandra Sitorus
1
and Andy Junianto Sidabutar
1
1
Department of Chemical Engineering, Faculty of Engineering, Universitas Sumatera Utara, Jl. Almamater Kampus USU,
Medan 20155, Indonesia
2
Natural Produk Base of Technology, Centre of Excellent, Universitas Sumatera Utara, Jl. Dr. T. Mansur No. 9 Kampus
USU, Medan 20155, Indonesia
Keywords: Waste, Utilized, Kapok Pods, Burned, Potassium Oxide.
Abstract: Nowadays, various combinations of agricultural waste and others material have been successfully made into
commercial products. Kapok pods can be processed into a source of potassium oxide which may have an
impact on the reduction of environment pollution caused by industrial kapok. The purposes of this works were
to determine the best of combustion time and temperature to produced potassium oxide from ash that made
from kapok pods. The kapok pods were dried at 110˚C for 24 hours to obtain the dry of kapok pods. Those
kapok pods were burned at 500˚C, 550˚C, 600˚C, and 650˚C by muffle furnace, with a burning time of 3, 4,
5, and 6 hours. The potassium that contained in ash had been extracted by distilled water for 24 hours. The
highest content of Potassium Oxide (K
2
O) resulting from the 3 hours of combustion at 500˚C was 35.91%.
Atomic Absorption Spectroscopy (AAS) was used to analyse the Potassium Oxide (K2O).
1 INTRODUCTION
Kapok tree (Ceiba pentandra) is a tropical tree comes
from South America and has spread to the rain forests
of West Africa and Southeast Asia from the Malay
Peninsula to the Indonesian archipelago (Mojica,
2002), (Chaiarrekij, 2011).
Kapok tree has many uses for humans, for
example wood is light and porous, so good for
carving, casket and canoeing. Delicate fibers can be
used as mattresses and pillows, and the seeds can be
used as bio diesel (Putri, 2012) (Bates, 2004), (Orwa,
2009). Other parts of the tree can also be used as a
drug for example by drinking a decoction of cured
skin can be useful as a diuretic, aphrodisiac, and to
treat headaches, as well as type II diabetes (rainforest-
alliance.org, 2018), (Wikipedia, Ceiba Pentandra,
2017).
Kapok plantations are common in Indonesia,
spreading from the region Lebak Wangi, Bandung,
Pati, Kudus, Jepara, in west and center of Java.
Meanwhile in East of Java are Tulung Agung, Blitar,
Pasuruan, and Banyuwangi area. According to the
estate agency, Pati is the most spacious area of kapok,
ranges about 15,020 hectares, meanwhile Kudus
reach about 4,000 hectares. Total area for the territory
development of Kapok in region Centre of Java
covering area of 95,107.17 hectares. This area can
produce kapok fiber 340 kg, seed 220 kg, pods or
shell 540 kg (Wibowo, 2012). Research conducted by
Anigo and others also says that the content of the
kapok pods is 57.87% of the total weight of the fruit
of the kapok. Obviously, it can be concluded that
industry generates waste kapok pods in bulk that can
pollute the environment. Therefore, it is necessary to
explore the potential of waste kapok pods can be
different types of products that have economic value.
In addition to economic value, the kapok tree can also
be used as a buffer against erosion, flood control, as
well as green plants that can also be used to conserve
natural resources. Indonesia is the largest producer of
kapok before World War I in the world, where the
biggest kapok production is from the island of Java.
Kapok plantations in Indonesia are owned by, private
and government plantation estates.
Agricultural waste is the result of agricultural
activities, may be in the form of chaff, straw, cob or
fiber (Abba, 2013). In recent time, industry must
move towards a green industry and sustainable
development, global warming and the depletion of
natural resources has occurred and will continue.
Kapok pods is kapok industrial waste that still
Sukeksi, L., Sitorus, C. and Sidabutar, A.
Pyrolisis of Kapok (Ceiba pentandra) Pods Wastes as Sources of Potassium Oxide (K2O).
DOI: 10.5220/0008552202290234
In Proceedings of the International Conference on Natural Resources and Technology (ICONART 2019), pages 229-234
ISBN: 978-989-758-404-6
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
229

containing potassium 20% -25% (Purbasari, 2008).
The potassium compounds contained in the kapok
pods, can be manufactured into useful products, such
as raw materials for the manufacture of soap and
shampoo (Chekuboyina, 2012).
Pyrolysis is a process of thermo chemical that can
be done on the conversion of biomass which has a low
density or organic materials into the biomass has a
high energy. Pyrolysis involves the heating of organic
material to temperatures over 400 ° C in the absence
of oxygen. At this temperature, organic material
normally decompose generating steam phase and
phase residual solid (bio char). On cooling the steam
pyrolysis, polar compounds with high molecular
weight condensed as a liquid (bio-oil) while the
molecular weight volatile compounds remains low in
the gas phase (Laird D. A., 2009).
The consider factors in conducting pyrolysis:
a. Temperature pyrolysis, which affect the resulting
product, it is caused the increase of the
temperature then the process will be more perfect.
b. Time of pyrolysis, the longer the pyrolysis time
will improve the results of liquid and gas, whereas
the solid of results generated will decrease.
c. Moisture content of materials, if the moisture
content higher cause the time used will be longer
so that more energy is needed.
d. The size of the material, when the bigger size of
material, the equipment used is greater.
The steps that must be done to get the minerals
that formed in the ash during combustion is still
unclear, but for obvious reasons it is assumed that the
conversion of mineral changed based on the
temperature of combustion. Carbonate is formed at
low temperatures while the ashes will be formed at
high temperatures in an atmosphere containing
oxygen, which is the main form of metal oxides. At
high temperatures, potassium oxides formed will
react with other elements to form chemical bonds, in
the same situation would occur dissociation of
sodium and potassium oxide compounds and will
undergo rapid vaporization. While at low
temperatures, the heat will move to the surface of
KOH, will then form K2CO3.
Soda Q is extracts combustion results from the
Kapok pods. There are several stages of process to
manufacture Soda Q maximum. The stages are
extraction, evaporation, and crystallization. Soda Q
generally contains 50.78% K
2
CO
3
, 26.27% Na
2
CO
3
,
and 4.37% NaOH (Wibowo, 2012).
This study will evaluate the effect of temperature
and time pirolis on the quality and amount of K
2
O
produced. Temperatures used in the pyrolysis process
are 500, 550, 600 and 650 C, while the burning time
are 3, 4 and 5 hours.
2 METHODOLOGY
2.1 Raw Materials and Equipment’s
The materials used in this research are the skin or
pods of the fruit of the kapok (Ceiba Pentandra),
aquadest, acetic acid (CH
3
COOH), and
phenolphthalein indicator.
The instrument used was a muffle furnace as
burner sample, oven as a drying to calculate the
moisture content, porcelain bowls as a sample
container while burning, analytical balance to
measure the mass of the material, a beaker glass as a
container to extract the ashes, a measuring cup to
measure the volume of the solution.
2.2 Procedure of Making the Ashes
In the process of making the ashes, first step, the
kapok pods was inserted into the oven at a
temperature of 110˚C for 24 hours in order to reduce
the moisture content. Then the kapok pods was
weighed as much as 20 g, and was inserted into the
muffle furnace combustion, with temperature and
time that have been determined.
2.3 Determination of Normality
To determine the normality of aqueous alkaline
extracts by the method of titration of acid base. 1 gr
ash dissolved in 30 ml aquadest and soaked for 24
hours, the solution is then filtered using filter paper
and the filtrate accommodated in an erlenmeyer flask.
Then do a titration using acetic acid (CH
3
COOH) 0.1
N by adding phenolphthalein as an indicator. The
normality from the extract can be calculated from the
total volume of titration.
2.4 Determination of pH
1 gr ash dissolved in 30 ml of aquadest and soaked for
20 minutes, the solution is then filtered using filter
paper and the filtrate was accommodated in a beaker
glass. Then the pH of each extract was measured
using a pH meter.
2.5 Determination of Ash Yield
In the process of making the ashes, kapok pods were
inserted into the oven at a temperature of 110˚C over
ICONART 2019 - International Conference on Natural Resources and Technology
230
the past 24 hours to reduce the moisture content. Then
the kapok pods weighed as much as 20 g, inserted into
the muffle furnace with the time and temperature
which has been specified. Results of the combustion
ash was weighed and ash yield can be calculated by
the equation:
Ash Yield = ( M2 / M1) × 100 % ………… .(1)
Where:
M1 : weight of the initial sample (g)
M2 : weight of the end sample or ash (g)
Then the ash yield as a sampling was taken as 1
gr and it would be analyzed to determine the levels of
K
2
O using an Atomic Absorption Spectrophotometer
(AAS).
3 RESULT AND DISCUSSIONS
3.1 Moisture Content of Kapok Pods
Utilization of kapok pods as alkaline source can be
done in 2 stages, the first step was process reduction
moisture content of the kapok pods and the second
was process pyrolysis kapok pods using the muffle
furnace. On the process of reduction of moisture
content, the kapok pods are dried in the oven until the
kapok pods become dry and until the fixed weight.
Moisture content of the kapok pods down to 90%
from the initial weight, with drying time 24 hours in
the oven.
Some researcher have examined about the
moisture content of kapok pods and they said that
moisture content significant influence the nature of
biomass that would serve as a source of energy
especially its influence the heat generated value. The
higher moisture content resulting in the more low
value biomass heat. This is due to more heat is needed
to remove the water in the biomass to be steam so the
remaining energy will be smaller in the fuel
(Haygreen and Bowyer 1996). Good biomass for
energy is to have a low moisture content, its because
at the time of the burning they not a lot of smoke
(Hendra dan Wirnani 2003). Putra has reported that
kapok pods moisture content at dry air around of
9.54-14.04% (Putra, 2014; Hendra D, 2003;
Haygreen, 1996).
3.2 Influence of Combustion
Temperature of Dry Kapok Pods to
the Normality (N)
The results obtained from the combustion
experiments, was extracted using aquadest as a
solvent, and soaking for 24 hours, then filtered with
filter paper and the filtrate was analyzed using
titration method by acid to determine the
concentration of base that conceived from the results
of the combustion. The influence of the temperature
of combustion versus normality extract from the
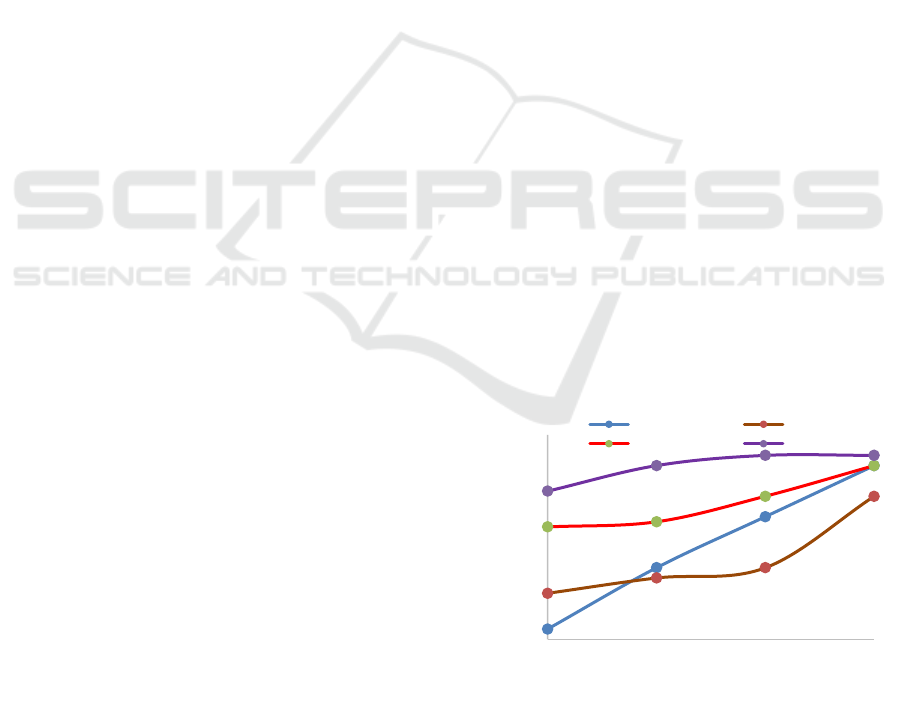
ashes can be seen in Figure 1.
From Figure 1, it can be seen that at the same of
extraction time, increasing the temperature of
combustion will increase the normality of extracts
from the ash. Similarly, with increasing of extraction
time, normality obtained is also increasing. Extract
lye from ashes are alkaline hydroxide, this can be
explained as K
2
O or Na
2
O is formed on the burning
of material a plant and dissolves in the water during
extraction, become a hydroxide. It can be said that the
order of K
2
O or Na
2
O formed by the combustion of
pure metals (K or Na) in the air, where K or Na in
plant material bound in his organic matrix (Babayemi
J. O., 2010).
In addition, the ability of ash to dissolves is a
function amount of alkali metal compounds and
others salts can dissolve more (such as chloride and
sulphate of K and Na) that contained in the ashes, its
depend on the type of plants that burned. The
components that are not soluble in the ash contain
silicates and other metals are insoluble in water.
When the ash dissolved in water, only the carbonate
and sulfate and chloride may be of
Figure 1: Influence of Time and Temperature of
Combustion (˚C) versus Normality (N).
0,045
0,047
0,049
0,051
0,053
0,055
0,057
0,059
0,061
0,063
0,065
500 550 600 650
3 hours 4 hours
5 hours 6 hours
Temperature of Combustion (˚C)
Normality
(N)
Pyrolisis of Kapok (Ceiba pentandra) Pods Wastes as Sources of Potassium Oxide (K2O)
231
In addition, the ability of ash to dissolves is a
function amount of alkali metal compounds and
others salts can dissolve more (such as chloride and
sulphate of K and Na) that contained in the ashes, its
depend on the type of plants that burned. The
components that are not soluble in the ash contain
silicates and other metals are insoluble in water.
When the ash dissolved in water, only the carbonate
and sulfate and chloride may be of alkali metals in
solution, includes a small portion of other metals that
insoluble or a little soluble (Babayemi J. O., 2010).
3.3 Influence of Time and Temperature
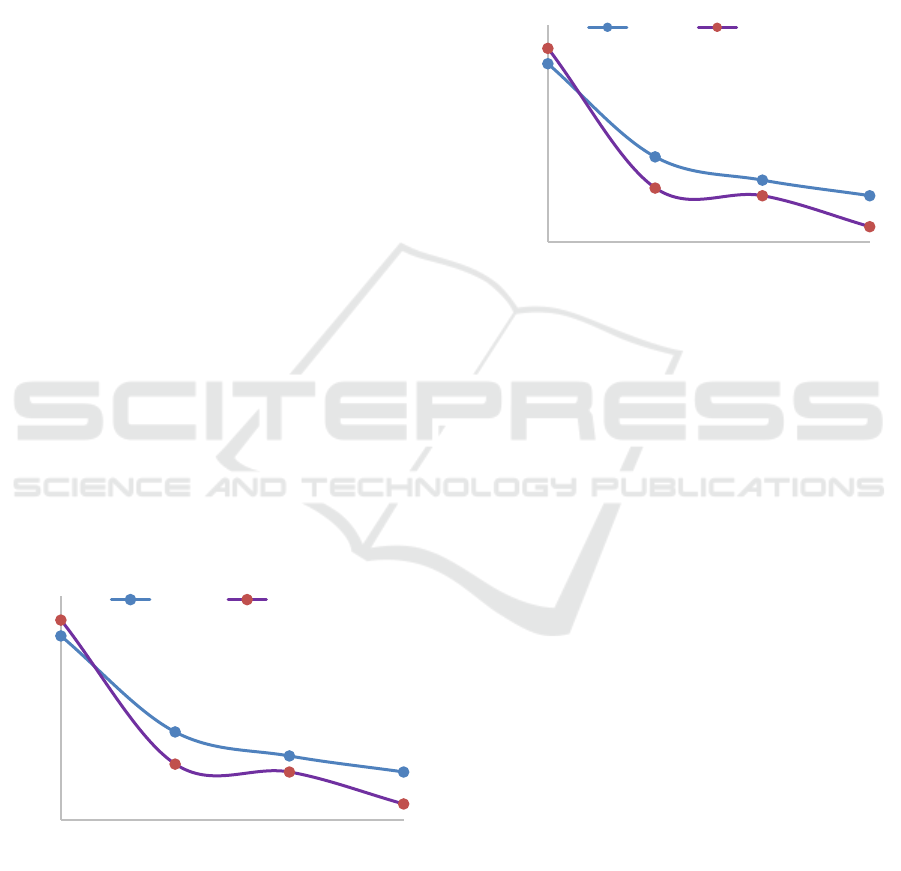
of Combustion to the pH of Ash
The results obtained from the pyrolysis experiment
were extracted using aquadest as a solvent, and then
the filtrate was filtered and analyzed to measure the
pH of ash.The influence of burning temperature
versus the pH of ash can be seen in Figure 2.
From the Figure 2 it can be seen that the increase
combustion temperature generate an increasing of
pH. Carbonate formed at low temperatures, while ash
formed at high temperature in the state of atmospheric
oxygen which is the main form of metal oxides. With
the formation of alkaline carbonate compound as well
as alkali oxides in ash water added, the mixture will
become the alkaline solution (Misra, 1993).
Similarly, by increasing the burning time will also
increase the pH of the resulting solution. This is due
to the longer burning time, will increase the amount
of alkali is formed, thereby increasing the pH of the
solution.
Figure 2: Influence of Time and Temperature Combustion
versus the pH of ash.
3.4 Influence of Extraction Time and
Temperature Combustion to the
Ash Yield
The influence of extraction time and temperature
combustion can be seen in Figure 3 below. Ash
combustion results were weighted, to calculate the
yield or rendemen of ash by using the Formula 1
Figure 3: Influence of Time and Temperature Combustion
Versus the Ash Yield.
From Figure 3 it can be seen that the addition of
the combustion temperature generate reduce yield of
ash, so was against the addition of the combustion
time generate yield ash decrease. The cause of the low
yield in this ash because of the reaction of carbon with
water vapor increasing with increasing temperature
and the length time of the combustion, so the carbon
reacts to become CO
2
and H
2
be a lot, otherwise will
produced the less amount of ash (Siahaan, 2013). The
results of this study in accordance with previous
research that has done on burning coconut shell, in
which increasing temperature and combustion time,
the results yield burning decrease (Tirono, 2011).
3.5 Influence of Extraction Time and
Temperature of Combustion to the
Concentration of K
2
O
The resulting level of K2O from the ashes was
analyzed using Atomic Absorption
Spectrophotometer (AAS) can be seen in Figure 4.
Figure 4 shows the graph that the increase of
temperature of combustion significant influence to
the yield of K
2
O on the ash. Increase temperature will
decrease the yield of K
2
O, but the time of combustion
are not significant different such as at low
temperature or higher temperature. The higher the
time used at low temperature 500C the rendemen of
7,5
7,9
8,3
8,7
9,1
9,5
9,9
10,3
500 550 600 650
3 hours 6 hours
Temperature of Combustion (˚C)
Rendemen of Ash (%)
7,5
7,9
8,3
8,7
9,1
9,5
9,9
10,3
500 550 600 650
3 hours 6 hours
Temperature of Combustion (˚C)
Rendemen of Ash (%)
ICONART 2019 - International Conference on Natural Resources and Technology
232
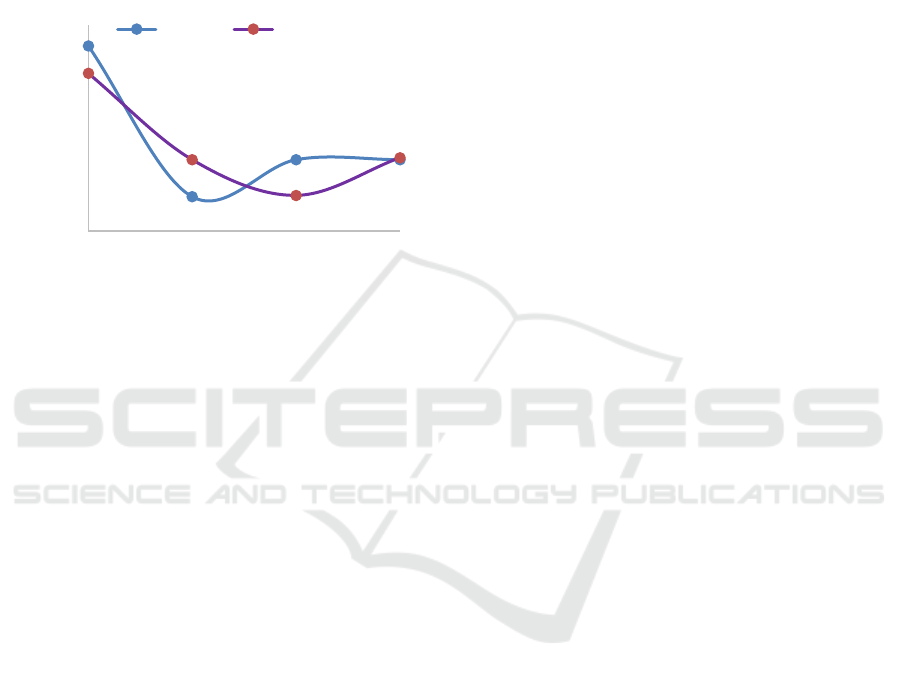
K
2
O was lower. But at temperature combustion at
650 the rendemen both of sample for 3 hours and 6
hours time of combustion are same at 15.7% of
rendemen. It is caused not due to potassium is lost or
evaporates, however, the decrease in K
2
O levels may
occur with increase temperature, because the amount
other alkaline components increases with increasing
temperature at the longer time (Perry, 1999).
Figure 4: Influence of Time and Temperature Combustion
versus levels of K2O.
4 CONCLUSIONS
The conclusions from this research are the highest
yield of K
2
O from kapok pods on the pyrolysis
combustion for 3 hours at 500˚C with levels of K
2
O
of 35.91%. Pyrolysis process time 6 hours is better,
because the ash was obtained in the form of oxide.
While the process time is 3 hours the resulting ash
still contain abundance elements of carbonate.
Increase the temperature of pyrolysis will also
improve the normality and pH, as well as increasing
the time of pyrolisis also will raise the pH and
normality. Potassium content in ash kapok pods can
be used as a source of alkaline solution.
REFERENCES
Abba, H. A. 2013. Review of Agro Waste Plastic
Composites Production. Journal of Minerals and
Materials Characterization and Engineering, 1, 271-
279.
Babayemi, J. O. 2010. Evaluation of the Composition and
Chemistry of Ash and Potash from Various Plant
Materials-A Review. Journal of Applied Sciences, 10:
1820-1824.
Bates, D. M. 2004. Malvales. CD Version: Encyclopaedia
Britannica.
Brown, S. H. 2017. Ceiba pentandra. Horticulture Agent.
Florida, South West Florida: University of Florida.
Chaiarrekij, S. A. 2011. Kapok 1: Characteristics of Kapok
Fibre as A Potential Pulp Source for Paper Making.
Journal of Bioresources , 7 (1), 475-488.
Chekuboyina, R. K. 2012. Physico-Chemical
Characterization and Antimicrobial Activity of Ceiba
Pentandra (Kapok) Seed Oil. Journal Alt med studs, 2-
9.
Green, R. H. 1999. Perry's Chemical Engineer's Handbook.
New York: McGraw-Hill Companies, Inc.
Haygreen, J., G. 1996. Hasil Hutan dan Ilmu Kayu, Suatu
Pengantar.Hadikusumo SA, Penerjemah. In
Terjemahan dari: Forest Products and Wood Science:
an Introduction. Yogyakarta: Gadjah Mada University
Pr.
Hendra D., Winarni, I. 2003. Sifat Fisis dan Kimia Briket
Arang Campuran Limbah Kayu Gergajian dan Sebetan
Kayu. Jurnal Penelitian Hasil Hutan, 21(3), 211-226.
Anigo, K., M., Dauda, B., M., D., Sallau, A., B., and
Chindo, I., E., 2013. Chemical Composition of Kapok
(Ceibapentandra) Seed and Physicochemical Properties
of its Oil. Nigerian Journal of Basic and Applied
Science, 21(2): 105-108.
Kapok Tree (Ceiba Pentandra). (2012, September 13).
Retrieved June 29, 2017, from http://www.rainforest-
alliance.org/species/kapok-tree.
Laird, D., A. 2009. Review of the pyrolysis platform for
coproducing bio-oil and biochar†. Biofpr (Biofuels,
bioproducts and biorefining), 1-16.
Misra, M., K. 1993. Wood Ash Composition As a Function
of Furnace Temperature. Biomass and Bioenergy, 4(2)
113.
Mojica, E., R. 2002. Fiber of Kapok (Ceiba pentandra) as
Component of A Metal Sensor for Lead in Water
Samples. Philippines Journal of Crop Science, 27 (2),
37-42.
Ningrum, N., P. 2013. Pemanfaatan Minyak Goreng Bekas
dan Abu Kulit Buah Kapuk Randu (Soda Qie) Sebagai
Bahan Baku Pembuatan Sabun Organik Berbasis
Teknologi Ramah Lingkungan. Journal Teknologi
Kimia dan Industri, 2 (2), 275-285.
Orwa, C., M. 2009. Agroforestree Database: a Tree
Reference and Selection Guide Version 4.0. Kenya:
World Agroforestry Centre.
Perry, R., H. 1999. Chemical Engineers Handbook Physical
and Chemical Data (Vol. 7). Mc Graw Hill Company
Purbasari, A., S. 2008. Pembuatan Pupuk Kalium-Fosfat
dari Abu Kulit Kapok dan Tepung Fosfat secara
Granulasi . Jurnal Ilmiah Bidang Ilmu Kerekayasaan ,
29-2.
Putra, I., T. 2014. Karakteristik Kimia Kulit Buah Kapok
Randu sebagai Bahan Energi Massa .
Putri, E., M. 2012. Biodiesel Production from Kapok Seed
Oil (Ceiba Pentandra) through The Transesterification
process by using cao as catalyst. Global Journal of
Researches in Engineering Chemical Engineering, 12
(2), 6-11.
Rainforest-alliance.org. (2018, September). Retrieved from
https://www.rainforest-alliance.org/species/kapok-tree.
5
10
15
20
25
30
35
500 550 600 650
3 hours 6 hours
Temperature of Combustion (˚C)
Amounts of K
2
O (%)
Pyrolisis of Kapok (Ceiba pentandra) Pods Wastes as Sources of Potassium Oxide (K2O)
233

Siahaan, S., H., 2013. Penentuan Kondisi Optimum Suhu
dan Waktu Karbonisasi pada Pembuatan Arang dari
Sekan Padi. Jurnal Teknik Kimia, 2 (1).
Tirono, M., S. 2011. Efek Suhu pada Proses Pengarangan
Terhadap Nilai Kalor Arang Tempurung Kelapa. Jurnal
Neutrino, 3 (2).
Wibowo, W., A. 2012. Pengaruh Laju Alir Pelarut dan
Tinggi Tumpukan Bahan Terhadap Nilai Koefisien
Transfer Massa Volumetris Pada Proses Ekstraksi Soda
Ki dari Abu Klotok randu. Ekuilibrium , 11 (2), 57-61.
Wikipedia. 2017. Ceiba Pentandra. Retrieved from
https://en.wikipedia.org/wiki/Ceiba_pentandra.
Wikipedia. 2018. https://en.wikipedia.org/wiki/Ceiba_
pentandra.
Winanti, M., S. 2017. Pabrik Bio-Oil dari Jerami Padi
dengan Proses Pirolisis Cepat Teknologi Dynamotive.
Surabaya.
ICONART 2019 - International Conference on Natural Resources and Technology
234
