
Biomolecular Phage Filter for the Detection of a Small Number of
Pathogens in Large Volumes of Processing Water
Songtao Du
1
, Xu Lu
2
, I-Hsuan Chen
3
, Yuzhe Liu
1
, Shin Horikawa
1
, Tung-shi Huang
4
and Bryan A. Chin
1
1
Material Research and Education Center, Auburn University, Auburn, AL, 36849, U.S.A.
2
Laboratory of Functional Films, Material Science and Engineering, Xi'an University of Technology,
Xi'an, 710048, China
3
Department of Biological Science, Auburn University, Auburn, AL, 36849, U.S.A.
4
Department of Poultry Science, Auburn University, Auburn, AL, 36849, U.S.A.
Keywords: Phage Filter, Me Biosensor, Capture Efficiency.
Abstract: Fresh specialty crop produce such as tomatoes, blueberries, strawberries, sprouts, cantaloupes, lettuce and
leafy greens account for more instances of foodborne illness than any other food category. Recent
announcements to consumers, by the United States (U.S.) Centers for Disease Control (CDCs), to discard all
Romaine lettuce because of bacterial contamination has resulted in hundreds of millions of dollars in losses
to growers and processors. Unfortunately, current microbiological testing of samples of specialty crops
(whole fruits, leaves of spinach, etc.), as specified by FDA’s Bacteriological Analytical Manual (BAM),
requires at least 48 hours to perform the complicated, time-consuming and costly steps of soaking, pre-
enrichment, concentration, enrichment, plate count or PCR to detect pathogens on these samples. Further
complicating the BAM analyses are the realities that: 1) both PCR and ELISA are unable to distinguish
between live and dead cells and 2) only a few samples out of as many as 100,000 fruits, vegetables or leaves
of multi-ton batches of produce can be BAM tested. A Non-clogging Biomolecular Phage Filter has been
developed to simultaneously capture, concentrate and isolate small numbers of pathogens from large
volumes of produce wash water. This phage filter can then be evaluated to screen for live versus dead cells
and ID the specific pathogen in minutes. Capture efficiencies of greater than 94% have been demonstrated.
1 INTRODUCTION
The U.S. Food and Drug Administration (FDA) and
CDCs have, within the last year, taken the
unprecedented step of warning the U.S. public to
discard all Romaine lettuce because of possible
bacterial contamination (CDCs, June 2018; CDCs,
Dec 2018; Staff, 2018). This warning led to all
Romaine lettuce in the food chain being discarded
and a still to be determined loss to producers,
processors and distributors estimated to be in the
hundreds of millions of U.S. dollars. The cost of
foodborne illness in the U.S. is enormous. A report
in 2014 from the U.S. Department of Agriculture
estimated that the direct and indirect costs associated
with illnesses caused by major foodborne pathogens
is more than $15.6 billion per year (Stephen, 2018).
A more recent estimate from Ohio State University
that covered all causes of foodborne illness, not just
illness from the major foodborne pathogens,
concluded the amount was at least $55.5 billion
(Stephen, 2018). Each year in the U.S., about 48
million people get sick from foodborne illness.
These foodborne illnesses result in approximately
128,000 hospitalizations and 3,000 deaths, as
reported by the CDCs (CDCs, Feb 2018). Of the
major foodborne pathogens, Salmonella causes
about 1.2 million illnesses, 23,000 hospitalizations
and 459 deaths in the U.S. each year (CDCs, Nov
2018).
Triple washed, ready-to-eat, salads have found a
ready consumer market in the U.S. The produce that
goes into these ready-to-eat salads typically comes
from numerous farms and are processed in multi-ton
batches. The produce is washed in a series of cleaner
and cleaner water, finally being drip dried on a
vibrating conveyor followed by centrifugal drying.
It is common practice for a few ml samples of the
108
Du, S., Lu, X., Chen, I., Liu, Y., Horikawa, S., Huang, T. and Chin, B.
Biomolecular Phage Filter for the Detection of a Small Number of Pathogens in Large Volumes of Processing Water.
DOI: 10.5220/0007689901080113
In Proceedings of the 12th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2019), pages 108-113
ISBN: 978-989-758-353-7
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

final wash water to be tested for pathogens using
off-site commercial testing laboratories following
FDA accepted BAM procedures using PCR. Despite
this testing, contaminated batches of Romaine
lettuce have reached consumers and resulted in
illness. The U.S.’s recent failures to identify
contaminated ready-to-eat salads, demonstrates the
need for new technologies that are capable of
capturing and concentrating small numbers of
pathogens from large volumes of produce wash
water. To meet these needs, the authors of this paper
are developing a non-clogging, biomolecular phage
filter. This paper presents the concept and design of
the phage filter, and capture efficiencies of two
different designs of the filter system.
2 PHAGE FILTER DESIGN
2.1 Concept
Magnetoelastic (ME) biosensors immobilized with
filamentous E2 phage have been widely studied and
reported in our previous publications (Nambi and
Nyalamadugu, 2003; Guntupalli and Lakshmanan,
2007; Huang and Yang, 2008a; Huang and Yang,
2008b; Shen and Mathison, 2010; Park and Wikle,
2012; Chai and Li , 2012; Li and Horikawa, 2012;
Guntupalli and Sorokulova, 2012; Chai and
Horikawa, 2013a; Park and Park, 2013a; Park and
Li, 2013b; Chai and Horikawa, 2013b; Chai and
Wikle, 2013c; Horikawa and Chai, 2015). These ME
biosensors are typically rectangular strips made of a
magnetoelastic material, 1000 x 200 x 30 microns in
size. As the specific pathogens are captured by the
phage coated on the ME biosensor, the added mass
of the captured pathogens, causes the resonance
frequency of the ME resonator to decrease as a
direct function of number of captured bacteria. E2
phage has been specifically engineered to bind
Salmonella typhimurium. Because of the selective
and specific binding affinity of E2 phage,
Salmonella pathogens can be captured on surfaces of
ME biosensors and are removed from the test liquid,
when the sensors are collected. The biomolecular
phage filter is composed to many individual ME
biosensors, each biosensor serving as an individual
filter element. Each filter element is held in the filter
by a controlled magnetic field.
The phage filter consists of phage immobilized
ME biosensors and supporting frames. Many layers
of frames (arranged at different orientations) can be
combined together to form a single filter. The ME
filter elements are held by a controlled magnetic
field that is applied to align individual ME elements.
Either electromagnets or permanent magnets can be
used to generate the magnetic field. Openings of
supporting frames are close packed with ME filter
elements each immobilized with E2 phage. The
surfaces of ME filter elements are held
perpendicular to the direction of the flowing liquid.
Salmonella typhimurium that comes into contact
with a filter element immobilized with E2 phage will
bind to that element. Figure 1 shows how the phage
filter works. Each filter element is held at one end to
the supporting frame by the magnetic field. The field
also holds the ME filter elements in the magnetic
plane. Salmonella typhimurium shown in red, is
captured and bound to the filter element by the E2
phage, once collision occurs. At the same time,
debris, such as fruit pulp and sand, will not be
captured by the phage and pass through the ME filter
elements. Large debris (shown in white) will cause
the ME filter elements to open like a gate, passing
through the phage filter. Once the debris has passed,
the ME filter elements will realign due to the planar
magnetic field. Therefore, this phage filter is
pathogen specific and is non-clogging. Auburn
University has engineering different phages that are
designed to capture different pathogens such as
Salmonella enterica, Salmonella typhimurium,
Campylobacter spp., Listeria spp., and E. coli
O157H7, etc.
Figure 2 compares the differences between the
Biomolecular Phage Filter and conventional packed
magnetic bead filters. As shown in the figure, the
antibody immobilized beads capture the specific
pathogen, but become clogged with larger debris
that cannot pass through the interstitials of the
packed spheres. Debris builds up ahead of the
packed bead column and prevents further flow of
liquid and capture of pathogens by the antibody
immobilized beads.
2.2 Design and Simulation
Magnetic fields were used to attach, align and
release the ME filter elements from the filter and
supporting frames. In the research, two different
methods of filtering were investigated: 1) stationary
filter with gravity-driven, vertical liquid flow and 2)
rotational moving filter driven by electric motor.
Figure 1: White debris passing through phage filter.
Biomolecular Phage Filter for the Detection of a Small Number of Pathogens in Large Volumes of Processing Water
109

Figure 2: Difference between phage filter and
conventional bead filter.
2.2.1 Vertical Flow Model
For the vertical flow model, the magnetic field was
created by electromagnetic coils that were wound
around supporting frames. The solenoid coils were
made of plastic coated electrical wire (28GA). The
ME filter elements were aligned and held to the
frame when current was applied to the coils. The
ME filter elements could therefore be easily released
by turning off the current. The supporting frames
were fabricated from one kind of soft magnetic
material, Permalloy 80, by electric discharge
machining (EDM). A grooved structure of
supporting frame (Figure 3) was selected to
concentrate the magnetic field between the
supporting frames. The distance between frame
members was determined by the length of ME filter
elements and the size of the pipe. Figure 3 shows a
3D model and photo of the phage filter, including
supporting frames and electromagnetic coils. The
ME filter elements were attached by the magnetic
field to the supporting frames perpendicularly to
cover the opening of supporting frame.
Figure 3: 3D model & photo of vertical model phage filter.
The magnetic field was modeled using ANSYS
Maxwell. The 3D model was designed using NX
Unigraphics software. The predicted magnetic field
is shown in Figure 4. ME filter elements align
parallel to the magnetic field vectors. Iron powder
was used to experimentally verify predicted
magnetic flux lines. In addition, the magnetic force
was measured by Gauss meter. A value of 69.72
gauss generated by an electrical current of 1.5A was
enough to hold ME filter elements to supporting
frames.
Figure 4: Simulation and iron powder test of magnetic flux
vectors of vertical model phage filter.
2.2.2 Rotating Filter Model
For the rotating model, permanent magnets (Grade
N52) with diameter of 3/16" were used to generate
the magnetic field instead of electromagnetic coils.
Figure 5 (left) shows the 3D model of the rotating
phage filter with ME filter elements attached.
Supporting frames were made of Permalloy 80 wire
with a diameter of 1mm. Two Permalloy 80 sheets
were fixed on the end of magnets, in order to
improve uniformity of the magnetic field. The
modified 3D model (improved) of the phage filter is
shown in Figure 5 (right). Simulation results are
shown in Figure 6. The magnetic flux vectors were
also perpendicular to the supporting frames of phage
filter, especially for the corners of the supporting
frame. Therefore, the ME filter elements can be
coupled to the supporting frames with the same
direction of magnetic flux vector, passing parallel to
the long axis of ME biosensors.
2.3 Testing of Vertical and Rotating
Filter Systems
Tests of both the Vertical Flow and Rotating Filter
Systems were conducted. The systems were both
constructed of transparent food-grade plastic.
Figure 5: 3D model of phage filter of rotating model
before & after modification.
4mm
4mm
BIODEVICES 2019 - 12th International Conference on Biomedical Electronics and Devices
110

Figure 6: Simulation of magnetic flux vectors of rotating
phage filter.
2.3.1 Vertical Flow System
The vertical gravity-fed system consisted of a square
pipe, phage filters and a valve. The valve was used
to control the flow of pathogen solution. For the
phage filter, solenoid coils were placed outside the
pipe to guarantee all the pathogen solution could
pass through the phage filter plane containing the
ME filter elements. ME filter elements with size of
1mm x 0.2mm x 0.03mm were used. Figure 7 shows
the 3D model of vertical system. For multiple layers
of phage filters, each layer was horizontally rotated
90-degree relative to the previous layer. In this way,
a higher packing density of ME filter elements was
obtained, in addition to increasing the probability of
impact and hence capture by phage of the target
pathogen.
2.3.2 Rotating Filter System
The rotating system was constructed using a
transparent food grade plastic chamber, phage filter
on a spindle and a motor with speed control unit.
Figure 8 shows the 2D model and photo of rotating
filter system. ME filter elements of size 4mm x
0.8mm x 0.03mm were used. All the phage filter
layers can be rotated along the spindle, which was
driven by a speed-controlled electric motor. The
pathogen containing solution was filtered by the
phage filter when rotated.
3 MATERIAL AND METHODS
3.1 ME Filter Elements
ME filter elements were fabricated from Metglas
2826MB as cast ribbon. The raw material was diced
to form individual ME filter elements that were
rectangular in shape. An Ultraviolet (UV) sensitive
Figure 7: 3D model of vertical flow system.
Figure 8: 2D model and photo of rotating flow system.
film was used to hold the Metglas material during
dicing with an automated saw (Disco 3220).
Acetone was used to wash the UV sensitive film
with the ME filter elements prior to exposure to UV
radiation to release the ME filter elements from the
film. After release, ME filter elements were
ultrasonically cleaned in acetone followed by
methanol. The ME filter elements were then vacuum
annealed at 220℃ for 3 hours to remove residual
stresses caused by the dicing process. Chromium
(30µm) and then gold (150µm) were sputtered onto
all surfaces of the ME filter elements. The Cr layer
was used to improve the adhesion of Au on surfaces
of ME filter elements. The Au layer provided a
bioactive surface for phage immobilization and
corrosion resistance.
3.2 E2 Phage and Surface Blocking
The filamentous E2 phage was prepared and
provided by the Department of Biological Sciences.
E2 phage is a genetically engineered landscape
phage with bio-receptors designed to capture
Salmonella typhimurium bacteria. The E2 phage
solution was diluted to 5x10
11
vir/ml with Tris-
Buffered-Saline (TBS) solution. ME filter elements
were incubated in a 1.5ml centrifuge tube for 1 hour
using a 3D rotating incubator at a speed of 8 rpm.
After attaching the phage to the ME filter elements,
the ME filter elements were washed in TBS solution.
The ME filter elements were then incubated in super
Biomolecular Phage Filter for the Detection of a Small Number of Pathogens in Large Volumes of Processing Water
111
blocking buffer (1x, Thermo Fisher Scientific) for
40mins, in order to reduce non-specific binding.
After blocking, the ME filter elements were
washed with TBS solution and filtered deionized
water one time. After final washing, the ME filter
elements were ready for placement into the filters to
capture Salmonella pathogen.
3.3 Phage Filter Performance
The Salmonella pathogen with an original
concentration of 5 x 10
8
cfu/ml was prepared and
provided by the Department of Biological Sciences.
The concentration of Salmonella solution was
diluted to 5 x 10
4
cfu/ml and 5 x 10
3
cfu/ml with
filtered deionized water. The total theoretical
number of Salmonella pathogen input were 1650cfu
in the vertical gravity-fed system and 5 x 10
4
cfu in
the rotating flow system, respectively. The phage-
immobilized ME filter elements were aligned
compactly on phage filters and placed into the test
system. For the vertical gravity-fed system, a liquid
velocity of 3mm/s was controlled by the valve. The
tested Salmonella solution was passed through
phage filter one-time in. For the rotating flow
system, single and double layers were tested,
separately. A rotational speed of 12 rpm was
maintained for 30mins.
4 RESULTS AND DISCUSSION
4.1 Vertical Flow System
The stability of the vertical flow system was tested
using filtered deionized water. Experiments showed
that the ME filter elements could be suspended
perpendicular up to the maximum obtained
volumetric flow rate of 20 l/min (bottom exit valve
wide open). In all tests, no ME filter elements
detached from the filter frame elements, except
when the current was removed.
Figure 9: Capture efficiency of the vertical flow system.
Plate counts were conducted on input and exit
solutions to determine the capture efficiencies. For
fluid velocities of 3mm/s, capture efficiencies of
94%+ were obtained, which was shown in figure 9.
Figure 9 demonstrates that the capture rate of
Salmonella increases with an increase in number of
ME filter elements.
4.2 Rotating Flow System
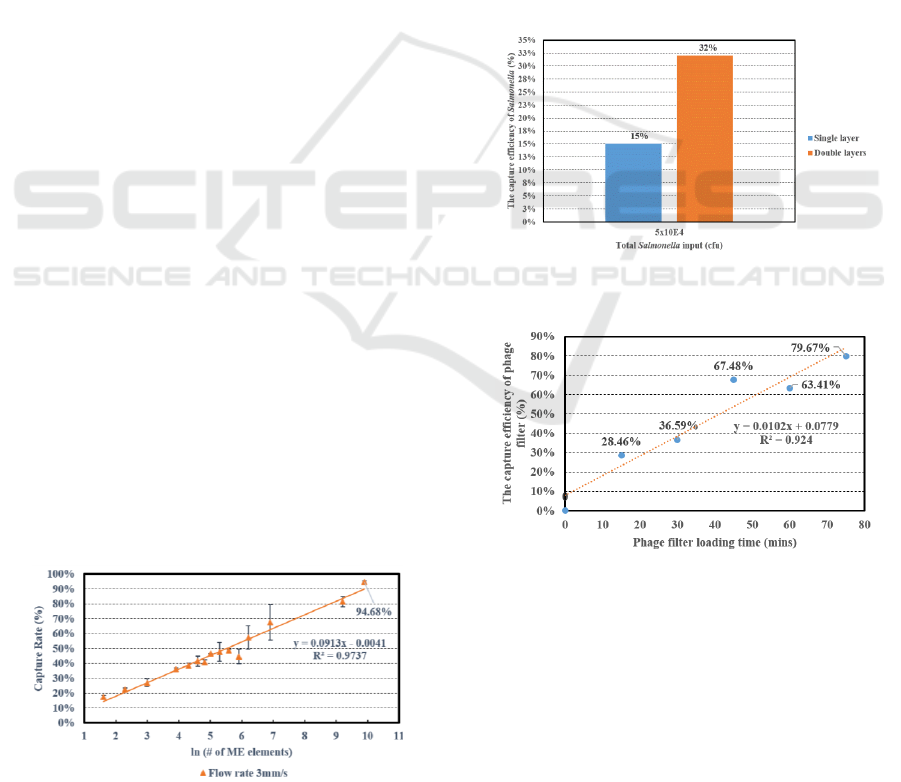
Figure 10 shows the capture efficiency of single
layer and double layers for the rotating flow filter
system in 30mins. The capture efficiency increases
with increasing the number of filter layers.
In addition, the capture efficiency was measured
as a function of time for double filter layers. Figure
11 shows the capture efficiency of the rotating filter
also increased with time. A capture efficiency of
nearly 80% was obtained for a two-layer system
after 75 minutes.
Figure 10: Capture efficiencies of single and double layers
rotating systems.
Figure 11: Capture efficiency increases with time, rotating
filter.
5 CONCLUSIONS
The concept, design and testing of a Non-clogging
Biomolecular Phage Filter has been presented in this
paper. This phage filter when deployed will be able
to simultaneously capture, concentrate and isolate
small numbers of pathogens from large volumes of
BIODEVICES 2019 - 12th International Conference on Biomedical Electronics and Devices
112

produce wash water. Two different filter designs
(vertical, gravity driven and rotating system) are
presented and tested. Adding multiple layers of ME
filter elements increases capture efficiencies.
Capture efficiencies of greater than 94% have been
demonstrated for flow velocity of 3mm/s.
Future research will address the effects of
temperature, flow rate, and water organic content on
capture efficiencies.
ACKNOWLEDGEMENTS
This material is based upon work, which is
supported by the National Institute of Food and
Agriculture (NIFA), U.S. Department of Agriculture
(USDA) and the Auburn University Detection Food
Safety Center (AUDFS).
REFERENCES
CDC, June 2018, https://www.cdc.gov/ecoli/
2018/o157h7-04-18/index.html.
CDC, December 2018, https://www.cdc.gov/ecoli/
2018/o157h7-11-18/index.html.
CDC, February 2018, https://www.cdc.gov/
foodsafety/foodborne-germs.html.
CDC, November 2018, https://www.cdc.gov/ salmonella/
index.html.
Chai Y, Li S, (2012) Rapid and sensitive detection of
Salmonella typhimurium on eggshells by using
wireless biosensors. Journal of Food Protection, 75
(4), 631-636.
Chai Y, Horikawa S, (2013a) A surface-scanning coil
detector for real-time, in-situ detection of bacteria on
fresh food surfaces, Biosens. Bioelectron, 50, 311.
Chai Y, Horikawa S, (2013b) Surface-scanning coil
detectors for magnetoelastic biosensors: A comparison
of planar-spiral and solenoid coils, Appl. Phys. Lett,
103, 173510.
Chai Y, Wikle H. C, (2013c) Design of a surface-scanning
coil detector for direct bacteria detection on food
surfaces using a magnetoelastic biosensor, J. Appl.
Phys, 114, 104504.
Guntupalli R, Lakshmanan R, (2007) Magnetoelastic
biosensor for the detection of Salmonella typhimurium
in food products, Sensing and Instrumentation for
Food Quality and Safety, Volume 1, Issue 1, Pages 3-
10. doi:10.1007/s11694-006-9003-8.
Guntupalli R, Sorokulova I, (2012) Detection and
identification of methicillin resistant and sensitive
strains of Staphylococcus aureus using tandem
measurements, J. Microbiol. Methods, 90(3), 182-91.
Huang S, Yang H, (2008a) The Effect of Salt and Phage
Concentrations on the Binding Sensitivity of
Magnetoelastic Biosensors for Bacillus anthracis
Detection, Biotechnol. Bioeng, 101, 1014-1021.
Huang S, Yang H, (2008b) Optimization of Phage-Based
Magnetoelastic Biosensor Performance, NSTI
nanotech 2008 conference, symposium on Phage
Nano-biotechnology, pp. 642-645.
Horikawa S, Chai Y, (2015) Direct detection of
Salmonella on fresh produce. ECS Transactions 69
(38), 25-31.
Li S, Horikawa S, (2012) Amorphous metallic glass
biosensors, Intermetallics 30, 80-85.
M. K. Park, J. W. Park, (2013a) Evaluation of phage-
based magnetoelastic biosensors by comparison with
TaqMan-based quantitative PCR, Sens. Actuators, B:
Chem, 176, 1134-1140.
Nambi S, Nyalamadugu S, (2003) Radio Frequency
Identification Sensors for Food Safety, Wireless
Communications Systems and Circuits Design in the
7
th
World Multi-Conference on Systemics, Cybernetics,
and Informatics, pp. 386-390.
Park M.K, Wikle H. C, (2012) The effect of incubation
time for Salmonella typhimurium binding to phage-
based magnetoelastic biosensors, Food Control,
Vol.26 (2), pp 539-545.
Park M. K, Li S, (2013b) Detection of Salmonella
typhimurium grown directly on tomato surface using
phage-based magnetoelastic biosensors, Food
Bioprocess Tech, 6(3), 682-689.
Staff, November 2018, FDA Update on Romaine Lettuce
Outbreak as Yuma, AZ, Growing Season Begins,
Food Safety Magazine.
Stephen Ostroff, M.D. June/July 2018. The Costs of
Foodborne illness, Product Recalls Make the Case for
Food Safety Investments. Food Safety Magazine.
Shen W, Mathison L.C, (2010) Design and
characterization of a magnetoelastic sensor for the
detection of biological agents, Journal of Physics D:
Applied Physics, 43 (2010) 015004. DOI:
10.1088/0022-3727/43/1/015004.
Biomolecular Phage Filter for the Detection of a Small Number of Pathogens in Large Volumes of Processing Water
113
