
FAIRness in Biomedical Data Discovery
Alina Trifan and Jos
´
e Lu
´
ıs Oliveira
IEETA/DETI, University of Aveiro, Portugal
Keywords:
Biomedical Data Discovery, FAIR Guidelines, Data Discovery Platforms, FAIR Metrics.
Abstract:
The FAIR Guiding Principles are a recent, yet powerful set of recommendations for turning data Findable,
Accessible, Interoperable and Reusable. They were designed with the purpose of improving data quality and
reusability. Over the last couple of years they have been adopted more and more by both data owners and
funders as key data management approaches. Despite their increasing popularity and endorsement by multiple
research initiatives from some of the most diverse areas, there are still only a few examples on how these
principles have been translated into practice. In this work we propose an open evaluation of their adoption
by biomedical data discovery platforms. We first overview current biomedical data discovery platforms that
introduce the FAIR guiding principles as requirements of their functioning. We then employ the more recent
FAIR metrics for evaluating the degree to which these biomedical data discovery platforms follow the FAIR
principles. Moreover, we assess their impact on enabling data interoperability and secondary reuse.
1 INTRODUCTION
The FAIR guiding principles - FAIR stands for Find-
able, Accessible, Interoperable and Reusable - were
proposed with the ultimate goal of reusing valuable
research objects (Wilkinson et al., 2016). They rep-
resent a set of guidelines for turning data more mean-
ingful and reusable and they emphasize the need of
making data discoverable and interoperable by ma-
chines. These principles do not provide strict rules
or standards to comply with, but rather focus on con-
ventions that enable data interoperability, stewardship
and compliance against data and metadata standards,
policies and practices.
They are not standards to be rigorously followed,
but rather permissive guidelines. The principles are
aspirational, in that they do not strictly define how to
achieve a state of FAIRness. Depending on the needs
or constraints of different research communities, they
can be open to interpretation. Independently of this
openness, they were designed to assist the interaction
between those who want to use community resources
and those who provide them. When followed, they
are beneficial for both data owners and users that seek
access to the data. These principles have rapidly been
adopted by publishers, funders, and pan-disciplinary
infrastructure programmes as key data management
issues to be taken into consideration. This can be ex-
plained as data management closely relates to inter-
operability and reproducibility (Jansen et al., 2017).
Generic and research-specific initiatives, such as
the European Open Science Cloud
1
, the European
Elixir infrastructure
2
and the USA National Institutes
of Health’s Big Data to Knowledge Initiative
3
are
some of the current initiatives endorsing the FAIR
principles and commiting to provide FAIR ecosys-
tems across multi-disciplinary research areas. More-
over, the European Commission has recently made
available a set of recommendations and demands for
open data research that are explicitly written in the
context of FAIR data
4
.
With regard to biomedical data sources, data inter-
operability and reusability has been a hot topic over
the last decade, strongly correlated with the evolu-
tion of the so called Big Data in Healthcare. De-
spite the incremental increase of the use and storage
of electronic health records, the biomedical commu-
nity still tends to use these data in isolation. Unfortu-
nately more than 80% of the datasets in current prac-
tice are effectively unavailable for reuse (Mons et al.,
2017). This is just one of the factors behind the repro-
ducibility crisis that is manifesting in the biomedical
1
http://eoscpilot.org
2
http://www.elixir-europe.org
3
http://commonfound.nih.gov/bd2k/
4
http://ec.europa.eu/research/participants/data/ref/
h2020/grants manual/hi/oa pilot/h2020-hi-oa-data-
mgt en.pdf
Trifan, A. and Oliveira, J.
FAIRness in Biomedical Data Discovery.
DOI: 10.5220/0007576401590166
In Proceedings of the 12th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2019), pages 159-166
ISBN: 978-989-758-353-7
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
159

arena (Prinz et al., 2011). Apart from data still be-
ing gathered in silos unavailable outside of the owning
institution or country, data privacy concerns and un-
clear data management approaches are critical barri-
ers for sharing and reusing data. The FAIR principles
have been enabling the global debate about better data
stewardship in data-driven and open science, and they
have triggered funding bodies to discuss their applica-
tion to biomedical systems. A wide adoption of these
principles by the data sources and systems that handle
biomedical data has the ability to leverage this repro-
ducibility crisis, by ensuring interoperability among
heterogeneous data sources.
In this paper we propose an overview of the adop-
tion of these principles by biomedical data discov-
ery platforms, which are considered important en-
ablers of secondary research. In the process of reusing
biomedical data for secondary research, discovery
platforms play an important part as they provide sup-
port for identifying data sources that can answer a
given translational research question. We review cur-
rent biomedical data platforms in order to under-
stand their level of FAIRness. While some of these
platforms already performed self-assesments of their
methodologies for following the FAIR principles, our
exhaustive literature search revealed only a handful
of such assessments. Otherwise the FAIR principles
are identified as system requirements of several dis-
covery platforms, without a deep evaluation of their
adoption. We therefore chose three such platforms
and identify the means through which they answer to
the FAIR guidelines.
This paper is structured in 5 more sections. A de-
tailed presentation of the FAIR principles is covered
in Section 2, followed by an overview of biomedical
data discovery platforms in Section 3. The adoption
of the FAIR principles by these platforms is analyzed
in Section 4. We discuss the importance of this adop-
tion for the biomedical research community in Sec-
tion 5 and we draw the final remarks in Section 6.
2 FAIR GUIDING PRINCIPLES
The FAIR principles were intended as a set of guide-
lines to be followed in order to enhance the reusabil-
ity of any type of data. They put specific emphasis
on enhancing the ability of machines to automatically
find and (re)use the data, in addition to supporting
its (re)use by individuals. The goal is that, through
the pursuit of these principles, the quality of a data
source becomes a function of its ability to be accu-
rately found and reused. Although they are currently
not a strict requirement, nor a standard in biomedi-
cal data handling systems, these principles maximize
their added-value, by acting as a guidebook for safe-
guarding transparency, reproducibility, and reusabil-
ity.
The FAIR principles as initially proposed
by (Wilkinson et al., 2016) are detailed in Table 1.
In a nutshell, if a data source is intended to be FAIR,
sufficient metadata must be provided to automat-
ically identify its structure, provenance, licensing
and potential uses, without having the need to use
specialized tools. Moreover, any access protocols
should be declared where they do or do not exist. The
use of vocabularies and standard ontologies further
benefit to the degree of FAIRness of a data set.
The way these principles should manifest in re-
ality was largely open to interpretation and more
recently some of the original authors revisited the
principles, in an attempt to clarify what FAIRness
is (Mons et al., 2017). They addressed the principles
as a community-acceptable set of rules of engagement
and a common denominator between those who want
to use a community’s resources and those who pro-
vide them. An important clarification was that FAIR
is not a standard and it is not equal to open. The ini-
tial release of the FAIR principles were somehow mis-
leading in the sense that accessibility was associated
with open access. Instead, in the recent extended ex-
planation of what these principles really mean, the A
in FAIR was redefined as “Accessible under well de-
fined conditions”. This means that data do not have to
be open, but the data access protocol should be open
and clearly defined. In fact, data should be “as open
as possible, as closed as needed”.
The recognition that computers must be capable
of accessing a data object autonomously was the core
to the FAIR principles since the beginning. The re-
cent re-interpretation of these principles maintains
their focus on the importance of data being accesi-
ble to autonomous machines and further clarifies on
the possible degrees of FAIRness. While there is no
such notion as unFAIR, the authors discuss the dif-
ferent levels of FAIRness that can be achieved. As
such, the addition of rich, FAIR metadata is the most
important step towards becoming maximally FAIR.
When data objects themselves can be made FAIR and
open for reuse, the highest degree of FAIRness can
be achieved. When all of these are linked with other
FAIR data, the Internet of FAIR data is reached. Ulti-
mately, when a large number of applications and ser-
vices can link and process FAIR data, the Internet of
FAIR Data and Services is attained.
HEALTHINF 2019 - 12th International Conference on Health Informatics
160

Table 1: The FAIR Guiding Principles as originally proposed in (Wilkinson et al., 2016).
Findable F1. (meta)data are assigned a globally unique and persistent identifier.
F2. data are described with rich metadata (defined by R1 below).
F3. metadata clearly and explicitly include the identifier of the data it describes.
F4. (meta)data are registered or indexed in a searchable resource.
Accessible
A1. (meta)data are retrievable by their identifier using a standardized
communications protocol.
A1.1 the protocol is open, free, and universally implementable.
A1.2 the protocol allows for an authentication and authorization procedure,
where necessary.
A2. metadata are accessible, even when the data are no longer available.
Interopera-
ble
I1. (meta)data use a formal, accessible, shared, and broadly applicable language
for knowledge representation.
I2.(meta)data use vocabularies that follow FAIR principles.
I3.(meta)data include qualified references to other (meta)data.
Reusable
R1. (meta)data are richly described with a plurality of accurate and relevant
attributes.
R1.1 (meta)data are released with a clear and accessible data usage license.
R1.2 (meta)data are associated with detailed provenance.
R1.3 (meta)data meet domain-relevant community standards.
3 BIOMEDICAL DISCOVERY
PLATFORMS
The integration and reuse of huge amounts of biomed-
ical data currently available in digital format has the
ability to impact clinical decisions, pharmaceutical
discoveries, disease monitoring and the way popula-
tion healthcare is provided globally. Storing data for
future reuse and reference has been a critical factor
in the success of modern biomedical sciences (Raz-
ick et al., 2014). In order for data to be reused, first
it has to be discovered. Finding a dataset for a study
can be burdensome due to the need to search individ-
ual repositories, read numerous publications and ulti-
mately contact data owners or publication authors on
an individual basis. Recent research shows that the
time spent by researchers in searching for and identi-
fying multiple useful data sources can take up to 80%
of their time dedicated to the project or research ques-
tion itself (Press, 2016).
Biomedical data exists in multiple scales, from
molecular to patient data. Health systems, genetics
and genomics, population and public health are all
areas that may benefit from big data integration and
its associated technologies (Martin-Sanchez and Ver-
spoor, 2014). The secondary reuse of citizens’ health
data and investigation of the real evidence of ther-
apeutics may lead to the achievement of personal-
ized, predictive and preventive medicine (Phan et al.,
2012). However, in order for researchers to be able to
reuse data and conduct integrative studies, they first
have to find the right data for their research. Data dis-
covery platforms are one-stop shops that enable clini-
cal researchers to identify datasets of interest without
having to perform individual, extensive searches over
distributed, heterogeneous health centers.
There are currently many data discovery plat-
forms, developed either as warehouses or simply ag-
gregators of metadata that link to the original data
sources. A warehouse platform, the Vanderbilt ap-
proach (Danciu et al., 2014) contains both fully de-
identified research data and fully identified research
that is made available taking into consideration access
protocols and governance rules. A cataloguing toolkit
is proposed by Maelstrom Research, built upon two
main components: a metadata model and a suite of
open-source software applications (Bergeron et al.,
2018). When combined, the model and software
support implementation of study and variable cata-
logues and provide a powerful search engine to facil-
itate data discovery. Disease oriented platforms, such
as The Ontario Brain Institute’s (Brain-CODE) (Vac-
carino et al., 2018) are designed with a very explicit,
yet not limited, purpose of supporting researchers
in better understanding a specific disease. Brain-
CODE addresses the high dimensionality of clinical,
neuroimaging and molecular data related with vari-
ous brain conditions. The platform makes available
integrated datasets that can be queried and linked
to provincial, national and international databases.
Similarly, the breast cancer (B-CAN) platform (Wen
et al., 2017) was designed as a private cancer data
center that enables the discovery of cancer-related
FAIRness in Biomedical Data Discovery
161

data and drives research collaborations aimed at bet-
ter understanding this disease. Still in the spectrum of
cancer discovery, the Project Data Sphere was built
to voluntarily share, integrate, and analyze histori-
cal cancer clinical trial data sets with the final goal
of advancing cancer research (Green et al., 2015).
In the rare disease spectrum, RD-Connect (Gainotti
et al., 2018) links genomic data with patient reg-
istries, biobanks, and clinical bioinformatics tools in
an attempt to provide a FAIR rare disease complete
ecosystem.
Among most established initiatives, Cafe Var-
iome (Lancaster et al., 2015) provides a general-
purpose, web-based, data discovery tool that can be
quickly installed by any genotype–phenotype data
owner and turn data discoverable. MONTRA (Silva
et al., 2018), another full-fledged open-source dis-
covery solution, is a rapid-application development
framework designed to facilitate the integration and
discovery of heterogeneous objects. Both solutions
rely on a catalogue for data discovery and include ex-
tensive search functionalities and query capabilities.
Linked Data is also explored in discovery plat-
forms, such as YummyData (Yamamoto et al., 2018)
which was designed to improve the findability and
reusability of life science datasets provided as Linked
Data. It consists of two components, one that period-
ically polls a curated list of SPARQL endpoints and a
second one that monitors them and presents the infor-
mation measured. Similarly, the Open PHACTS Dis-
covery Platform (Groth et al., 2014) leverages Linked
Data to provide integrated access to pharmacology
databases. Still in the spectrum of Linked Data,
BioSharing is a manually curated searchable portal of
three linked registries (McQuilton et al., 2016) that
cover standards, databases and data policies in the life
sciences.
All these platforms address data discovery from
different perspectives, integrating or linking to differ-
ent types of biomedical data. Another aspect that they
share is that they identify the FAIR principles as re-
quirements of their architectures, as well as enablers
of data discovery. Although the high majority of these
platforms emphasize the importance of providing a
way for machines to discover and access the data sets,
they are heterogeneous in the way they address the
FAIR guidelines. For this evaluation, we have chosen
three of the previously overviewed data discovey plat-
forms for understanding their approaches in following
the FAIR guiding principles. We first overview the
scope and methods of these platforms and we present
in a narrative form their partial or total compliance
with the FAIR principles.
4 ADOPTION OF THE FAIR
PRINCIPLES
In lifesciences, initiatives such as GOFAIR
5
make
use of infrastructures that already exist in European
countries to create a federated approach for turning
the FAIR principles a working standard in science.
Dataverse (Magazine, 2011), for instance, is an open-
source data repository software designed to support
public community or institutional research reposito-
ries. Another example is FAIRDOM
6
, a web plat-
form built for collecting, managing, storing, and pub-
lishing data, models, and operating procedures. Both
solutions follow the FAIR guiding principles in an
attempt to improve research management practices.
Open PHACTS
7
, a data integration platform for drug
discovery, UniProt (Pundir et al., 2017), an online re-
source for protein sequence and annotation data and
the EMIF Catalogue (Trifan and Oliveira, 2018), are
some of the few FAIR self-assessed data discovery
and integration platforms.
Among the three platforms we chose for this as-
sessment, the Maelstrom Research cataloguing toolkit
presented by (Bergeron et al., 2018) is built upon two
main components: a metadata model and a suite of
open-source software applications. The model sets
out specific fields to describe study profiles, charac-
teristics of the subpopulations of participants, timing
and design of data collection events and variables col-
lected at each data collection event. The model and
software support implementation of study and vari-
able catalogues and provide a powerful search en-
gine to facilitate data discovery. Developed as an
open source and generic tool to be used by a broad
range of initiatives, the Maelstrom Research cata-
loguing toolkit serves several national and interna-
tional initiatives. The FAIR principles have been
identified from early on as a requirement of its ar-
chitecture. With respect to Findability, each dataset
is complemented by rich metadata. To ensure qual-
ity and standardization of the metadata documented
across networks, standard operating procedures were
implemented. In what concerns Accessibility, when
completed, study and variable-specific metadata are
made publicly available on the Maelstrom Research
website. Using information found in peer-reviewed
journals or on institutional websites, the study out-
line is documented and validated by study investiga-
tors. Thus, the linkage with other FAIR metadata is
achieved. Where possible, data dictionaries or code-
5
http://go-fair.org
6
https://fair-dom.org/about-fairdom/
7
http://www.openphactsfoundation.org/
HEALTHINF 2019 - 12th International Conference on Health Informatics
162

books are obtained, which contributes to the data in-
teroperability.
Many life science datasets are nowadays repre-
sented via Linked Data technologies in a common
format (the Resource Description Framework). This
makes them accessible via standard APIs (SPARQL
endpoints), which can be understood as one of the
FAIR requirements. While this is an important step
toward developing an interoperable bioinformatics
data landscape it also creates a new set of obsta-
cles as it is often difficult for researchers to find the
datasets they need. YummyData provides researchers
the ability to discover and assess datasets from differ-
ent providers (Yamamoto et al., 2018). This assess-
ment can be done in terms of metrics such as service
stability or metadata richness. YummyData consists
of two components: one that periodically polls a cu-
rated list of SPARQL endpoints monitoring the states
of their Linked Data implementations and content and
another one that presents the information measured
for the endpoints and provides a forum for discus-
sion and feedback. It was designed with the purpose
to improve the findability and reusability of life sci-
ence datasets provided as Linked Data and to foster its
adoption. Apart from making data available to soft-
ware agents via an API, the adoption of Linked Data
principles has the potential to make data FAIR.
BioSharing is a manually curated searchable por-
tal of three linked registries (McQuilton et al., 2016).
These resources cover standards, databases and data
policies in the life sciences broadly encompassing the
biological environmental and biomedical sciences.
The manifest of the initiative is that BioSharing
makes these resources findable and accessible - the
core of the FAIR principle. Every record is designed
to be interlinked providing a detailed description not
only on the resource itself but also on its relations
with other life science infrastructures. BioSharing is
working with an increasing number of journals and
other registries and its focus is to ensures that data
standards, biological databases and data policies are
registered, informative and discoverable. Thus, it is
considered a pivotal resource for the implementation
of the ELIXIR-supported FAIR principles.
4.1 FAIR Metrics
Along with the narrative analysis of their FAIR ap-
proaches, we propose an assessment following the
FAIR metrics recently proposed by some of the orig-
inal authors of the FAIR guiding principles (Ta-
ble 2). The increasing ambiguity behind the ini-
tially published principles, along with the need of
data providers and regulatory bodies to evaluate their
translation into practice led to the establishment of
the FAIR metrics group
8
, with the purpose of defin-
ing universal measures of data FAIRness. Neverthe-
less, these universal metrics can be complemented by
resource-specific ones that can reflect the expectations
of one or multiple communities.
The second part of our assessment follows the pre-
viously identified FAIRness metrics, applied to each
of the 13 items of the FAIR guiding principles. For
each of the principles, we outline next the questions
that we tried to answer in the evaluation and the name
of the metric, within brackets. The following infor-
mation is a summary of the FAIR metrics description
proposed by some of the original authors of the guid-
ing principles
9
:
• F1 (Identifier uniqueness) Whether there is a
scheme to uniquely identify the digital resource.
• F1 (Identifier persistence) Whether there is a pol-
icy that describes what the provider will do in the
event an identifier scheme becomes deprecated.
• F2 (Machine-readability of metadata) The avail-
ability of machine-readable metadata that de-
scribes a digital resource.
• F3 (Resource identifier in metadata) Whether the
metadata document contains the globally unique
and persistent identifier for the digital resource.
• F4 (Indexed in a searchable resource) The degree
to which the digital resource can be found using
web-based search engines.
• A1.1 (Access Protocol) The nature and use limi-
tations of the access protocol.
• A1.2 (Access authorization) Specification of a
protocol to access restricted content.
• A2 (Metadata longevity) The existence of meta-
data even in the absence/removal of data.
• I1 (Use a knowledge representation language) The
use of a formal, accessible, shared, and broadly
applicable language for knowledge representa-
tion.
• I2 (Use FAIR Vocabularies) The metadata val-
ues and qualified relations should themselves be
FAIR, for example, terms from open, community-
accepted vocabularies published in an appropriate
knowledge-exchange format.
• I3 (Use qualified references) Relationships within
(meta)data, and between local and third-party
data, have explicit and ’useful’ semantic meaning.
8
http://fairmetrics.org
9
https://github.com/FAIRMetrics/Metrics/blob/master/
ALL.pdf
FAIRness in Biomedical Data Discovery
163
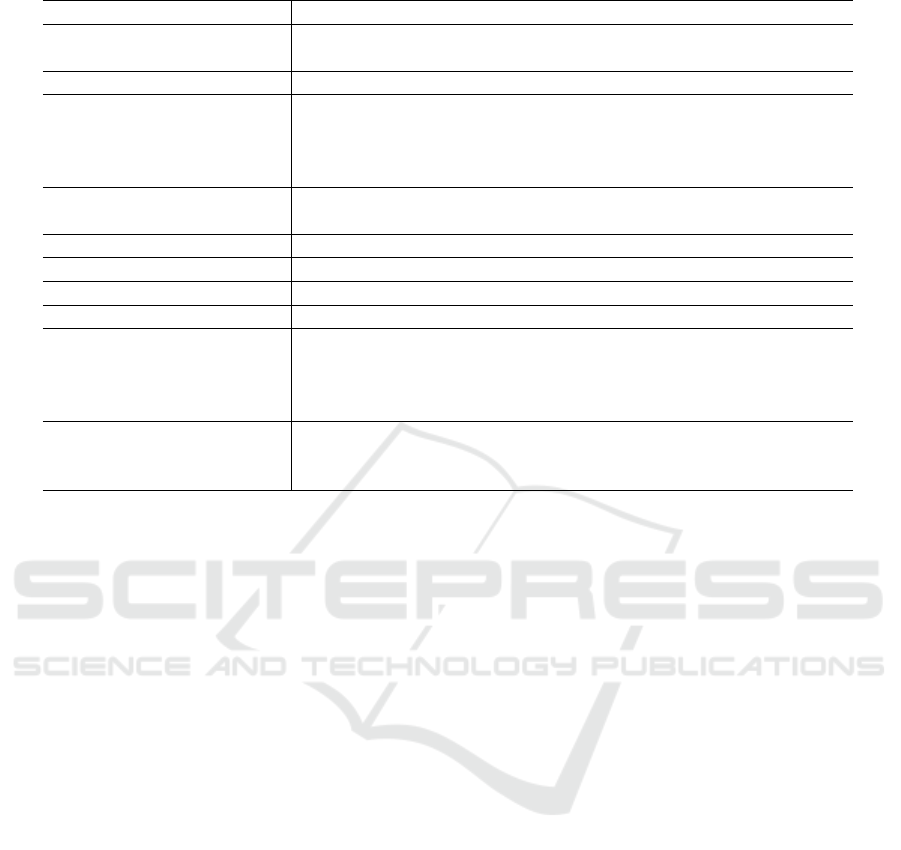
Table 2: The template for creating FAIR Metrics retrieved from https://github.com/FAIRMetrics.
FIELD DESCRIPTION
Metric Identifier
FAIR Metrics should, themselves, be FAIR objects, and thus
should have globally unique identifiers.
Metric Name A human-readable name for the metric.
To which principle does it
apply
Metrics should address only one sub-principle, since each FAIR
principle is particular to one feature of a digital resource; metrics
that address multiple principles are likely to be measuring multiple
features, and those should be separated whenever possible.
What is being measured
A precise description of the aspect of that digital resource that is
going to be evaluated.
Why should we measure it Describe why it is relevant to measure this aspect.
What must be provided What information is required to make this measurement?
How do we measure it In what way will that information be evaluated?
What is a valid result What outcome represents “success” versus “failure”?
For which digital
resource(s) is this relevant
If possible, a metric should apply to all digital resources; however,
some metrics may be applicable only to a subset. In this case, it is
necessary to specify the range of resources to which the metric is
reasonably applicable.
Example of their
application across types of
digital resource
Whenever possible, provide an existing example of success, and an
example of failure.
• R1.1 (Accessible Usage License) The existence of
a license document, for both (independently) the
data and its associated metadata, and the ability to
retrieve those documents.
• R1.2 (Detailed Provenance) That there is prove-
nance information associated with the data, cov-
ering at least two primary types of provenance in-
formation: who/what/when produced the data (i.e.
for citation) and why/how was the data produced
(i.e. to understand context and relevance of the
data).
• R1.3 (Meets Community Standards) Certification,
from a recognized body, of the resource meeting
community standards.
This evaluation allowed us to identify the FAIR re-
quirements already satisfied and the ones that are not
undressed, or unclear. Our findings show a high level
of FAIRness achieved by the three platforms, mainly
favored by the rich metadata with which each of these
platform complement the actual data sources. In all
cases the metadata can be accessed both by humans
and machines through a unique and persistent iden-
tifier, mostly in the form of an URI. Moreover, the
use of FAIR standards and vocabularies contributes
to their degree of FAIRness. This is complemented
in two of the platforms by the ability to link to other
FAIR metadata, which speaks for the data interoper-
ability and reusability. Still related to reusability, the
use of Linked Data by two of the platforms is one of
its strong enablers. Last but not least, all of the plat-
forms support machine discoverability and access, by
providing dedicated APIs. The main unclear aspect
was the access protocol, which was not trivial to iden-
tify. Another weak point was the lack of quantifiable
certification that the resources meet community stan-
dards. We present our summarized assessment in Ta-
ble 3.
5 DISCUSSION
Researchers need tools and support to manage, search
and reuse data as part of their research work. In the
biomedical area, data discovery platforms, either in
the shape of data warehouses or metadata integrators
that link to original data silos support the researcher
in the process of finding the right data for a given re-
search topic. However, finding the right data is not
sufficient for conducting a study. Data should be not
only qualitative and accessible under clear and well-
defined protocols, but it should also be interoperable
and reusable in order to maximize the research out-
comes. The FAIR guiding principles are recommen-
dations on the steps to follow in order to increase the
meaningfulness and impact of data and are strongly
related to data management. FAIR compliant biomed-
ical data discovery platforms have the ability to sup-
port biomedical researchers throughout all the steps
from finding the right data source to reusing it for
HEALTHINF 2019 - 12th International Conference on Health Informatics
164

Table 3: Assessment of the FAIRness of each of the three discovery platforms based on the FAIRness metrics. X represents a
satisfied requirement and - means that no proof to support the requirement was found.
Platform
F1 F2 F3 F4 A1.1 A1.2 A2 I1 I2 I3 R1.1 R1.2 R1.3
Maelstrom catalogue X X X X X – – X X X – X –
YummyData X X X X X X – X X – X X –
Biosharing X X X X X X – X X X X X –
secondary research. This can ultimately lead to bet-
ter health and healthcare outcomes. Ultimately, these
principles give an important contribution to the repro-
ducibility of research.
The FAIR guiding principles have been widely
endorsed by publishers, funders, data owners and
innovation networks across multiple research areas.
Up until recently, they did not strictly define how
to achieve a state of FAIRness and this ambiguity
led to some qualitatively different self-assessments of
FAIRness. A new template for evaluating the FAIR-
ness of a data set or a data handling system, recently
proposed by some of the original authors of the prin-
ciples, offers a benchmark for a standardized evalua-
tion of such self-assessments. In this paper we have
applied them to three different biomedical data dis-
covery platforms in order to estimate their FAIRness.
Moreover, we sought to understand the impact that
the adoption of these guidelines has in the quality of
the output produced by these platforms and to what
degree ensuring data reusability and interoperability
turns data more prone to be reused for secondary re-
search.
This analysis revealed that the adoption of
the FAIR principles is an ongoing process within
the biomedical community. However, the FAIR-
compliance of a resource or system can be distinct
from its impact. The platforms discussed exposed a
high level of FAIRness and an increased concern for
enabling data discovery by machines. While FAIR is
not equal to Linked Data, Semantic Web technologies
along with formal ontologies fulfill the FAIR require-
ments and can contribute to the FAIRness of a discov-
ery platform.
With digital patient data increasing at an expo-
nential rate and having understood the importance of
reusing these data for secondary research purposes, it
is highly important to ensure its interoperability and
reusability. The assessment of data FAIRness is a
key element for providing a common ground for data
quality to be understood by both data owners and data
users. If up until recently the open interpretation of
the FAIR guidinig principles could lead to assessment
biases, the recently published FAIR metrics support
more than ever the implementation of the common
ground. For this, the biomedical research community
should continue to challenge and refine their imple-
mentation choices in order to achieve a desirable In-
ternet of FAIR Data and Services.
6 CONCLUSIONS
The FAIR principles demand well-defined qualities
and properties from data resources but at the same
time they allow a great deal of freedom with respect
to how they should be implemented. In this work
we evaluated the approaches followed by three differ-
ent biomedical data discovery platforms in providing
FAIR data and services. This evaluation was strictly
done based on the analysis of the scientific publica-
tions describing these platforms. As future work, we
intend to extend this assessment by exploring these
platforms hands-on, in an attempt to address spe-
cific driving medical questions. Nevertheless, these
fresh examples highlighted the increasing impact of
the FAIR principles among the biomedical research
community. Moreover, by acting in accordance with
the FAIR metrics we, as a community, can reach an
agreed basis for the assessment of data quality.
REFERENCES
Bergeron, J., Doiron, D., Marcon, Y., Ferretti, V., and
Fortier, I. (2018). Fostering population-based cohort
data discovery: The Maelstrom Research cataloguing
toolkit. PloS one, 13(7):e0200926.
Danciu, I., Cowan, J. D., Basford, M., Wang, X., Saip, A.,
Osgood, S., Shirey-Rice, J., Kirby, J., and Harris, P. A.
(2014). Secondary use of clinical data: the Vanderbilt
approach. Journal of biomedical informatics, 52:28–
35.
Gainotti, S., Torreri, P., Wang, C. M., Reihs, R., Mueller,
H., Heslop, E., Roos, M., Badowska, D. M., Paulis, F.,
Kodra, Y., et al. (2018). The RD-Connect Registry &
Biobank Finder: a tool for sharing aggregated data and
metadata among rare disease researchers. European
Journal of Human Genetics, 26(5):631.
Green, A. K., Reeder-Hayes, K. E., Corty, R. W., Basch, E.,
Milowsky, M. I., Dusetzina, S. B., Bennett, A. V., and
Wood, W. A. (2015). The project data sphere initia-
FAIRness in Biomedical Data Discovery
165

tive: accelerating cancer research by sharing data. The
oncologist, 20(5):464–e20.
Groth, P., Loizou, A., Gray, A. J., Goble, C., Harland, L.,
and Pettifer, S. (2014). Api-centric linked data inte-
gration: the open PHACTS discovery platform case
study. Web Semantics: Science, Services and Agents
on the World Wide Web, 29:12–18.
Jansen, C., Beier, M., Witt, M., Frey, S., and Krefting, D.
(2017). Towards reproducible research in a biomed-
ical collaboration platform following the FAIR guid-
ing principles. In Companion Proceedings of the10th
International Conference on Utility and Cloud Com-
puting, pages 3–8. ACM.
Lancaster, O., Beck, T., Atlan, D., Swertz, M., Thangavelu,
D., Veal, C., Dalgleish, R., and Brookes, A. J. (2015).
Cafe Variome: General-purpose software for making
genotype–phenotype data discoverable in restricted or
open access contexts. Human mutation, 36(10):957–
964.
Magazine, D.-L. (2011). The dataverse network
R
: an
open-source application for sharing, discovering and
preserving data. D-lib Magazine, 17(1/2).
Martin-Sanchez, F. and Verspoor, K. (2014). Big data in
medicine is driving big changes. Yearbook of medical
informatics, 9(1):14.
McQuilton, P., Gonzalez-Beltran, A., Rocca-Serra, P.,
Thurston, M., Lister, A., Maguire, E., and Sansone,
S.-A. (2016). Biosharing: curated and crowd-sourced
metadata standards, databases and data policies in the
life sciences. Database, 2016.
Mons, B., Neylon, C., Velterop, J., Dumontier, M.,
da Silva Santos, L. O. B., and Wilkinson, M. D.
(2017). Cloudy, increasingly FAIR; revisiting the
FAIR data guiding principles for the European
Open Science Cloud. Information Services & Use,
37(1):49–56.
Phan, J. H., Quo, C. F., Cheng, C., and Wang, M. D. (2012).
Multiscale integration of-omic, imaging, and clini-
cal data in biomedical informatics. IEEE reviews in
biomedical engineering, 5:74–87.
Press, G. (2016). Cleaning big data: Most time-consuming,
least enjoyable data science task, survey says. Forbes,
March, 23.
Prinz, F., Schlange, T., and Asadullah, K. (2011). Believe
it or not: how much can we rely on published data on
potential drug targets? Nature reviews Drug discov-
ery, 10(9):712.
Pundir, S., Martin, M. J., and O’Donovan, C. (2017).
Uniprot protein knowledgebase. Protein Bioinformat-
ics: From Protein Modifications and Networks to Pro-
teomics, pages 41–55.
Razick, S., Mo
ˇ
cnik, R., Thomas, L. F., Ryeng, E., Drabløs,
F., and Sætrom, P. (2014). The eGenVar data manage-
ment system-cataloguing and sharing sensitive data
and metadata for the life sciences. Database, 2014.
Silva, L. B., Trifan, A., and Oliveira, J. L. (2018). Montra:
An agile architecture for data publishing and discov-
ery. Computer methods and programs in biomedicine,
160:33–42.
Trifan, A. and Oliveira, J. L. (2018). A FAIR market-
place for biomedical data custodians and clinical re-
searchers. In 2018 IEEE 31st International Sympo-
sium on Computer-Based Medical Systems (CBMS),
pages 188–193. IEEE.
Vaccarino, A. L., Dharsee, M., Strother, S. C., Aldridge,
D., Arnott, S. R., Behan, B., Dafnas, C., Dong, F.,
Edgecombe, K., El-Badrawi, R., et al. (2018). Brain-
code: A secure neuroinformatics platform for man-
agement, federation, sharing and analysis of multi-
dimensional neuroscience data. Frontiers in neuroin-
formatics, 12:28.
Wen, C.-H., Ou, S.-M., Guo, X.-B., Liu, C.-F., Shen, Y.-
B., You, N., Cai, W.-H., Shen, W.-J., Wang, X.-Q.,
and Tan, H.-Z. (2017). B-CAN: a resource sharing
platform to improve the operation, visualization and
integrated analysis of TCGA breast cancer data. On-
cotarget, 8(65):108778.
Wilkinson, M. D., Dumontier, M., Aalbersberg, I. J., Apple-
ton, G., Axton, M., Baak, A., Blomberg, N., Boiten,
J.-W., da Silva Santos, L. B., Bourne, P. E., et al.
(2016). The FAIR Guiding Principles for scientific
data management and stewardship. Scientific data, 3.
Yamamoto, Y., Yamaguchi, A., and Splendiani, A. (2018).
YummyData: providing high-quality open life science
data. Database, 2018.
HEALTHINF 2019 - 12th International Conference on Health Informatics
166
