
Interactive Lungs Auscultation with Reinforcement Learning Agent
Tomasz Grzywalski
1
, Riccardo Belluzzo
1
, Szymon Drgas
1,2
, Agnieszka Cwali
´
nska
3
and
Honorata Hafke-Dys
1,4
1
StethoMe
R
Winogrady 18a, 61-663 Pozna
´
n, Poland
2
Institute of Automation and Robotics, Pozna
´
n University of Technology, Piotrowo 3a 60-965 Pozna
´
n, Poland
3
PhD Department of Infectious Diseases and Child Neurology Karol Marcinkowski, University of Medical Sciences, Poland
4
Institute of Acoustics, Faculty of Physics, Adam Mickiewicz University, Umultowska 85, 61-614 Pozna
´
n, Poland
agnieszka.cwalinska@kmu.poznan.pl, h.hafke@amu.edu.pl
Keywords:
AI in Healthcare, Reinforcement Learning, Lung Sounds Auscultation, Electronic Stethoscope, Telemedicine.
Abstract:
To perform a precise auscultation for the purposes of examination of respiratory system normally requires the
presence of an experienced doctor. With most recent advances in machine learning and artificial intelligence,
automatic detection of pathological breath phenomena in sounds recorded with stethoscope becomes a reality.
But to perform a full auscultation in home environment by layman is another matter, especially if the patient
is a child. In this paper we propose a unique application of Reinforcement Learning for training an agent that
interactively guides the end user throughout the auscultation procedure. We show that intelligent selection
of auscultation points by the agent reduces time of the examination fourfold without significant decrease in
diagnosis accuracy compared to exhaustive auscultation.
1 INTRODUCTION
Lung sounds auscultation is the first and most com-
mon examination carried out by every general prac-
titioner or family doctor. It is fast, easy and
well known procedure, popularized by La
¨
ennec (Hy-
acinthe, 1819), who invented the stethoscope. Nowa-
days, different variants of such tool can be found on
the market, both analog and electronic, but regardless
of the type of stethoscope, this process still is highly
subjective. Indeed, an auscultation normally involves
the usage of a stethoscope by a physician, thus relying
on the examiner’s own hearing, experience and ability
to interpret psychoacoustical features. Another strong
limitation of standard auscultation can be found in the
stethoscope itself, since its frequency response tends
to attenuate frequency components of the lung sound
signal above nearly 120 Hz, leaving lower frequency
bands to be analyzed and to which the human ear
is not really sensitive (Sovij
¨
arvi et al., 2000) (Sarkar
et al., 2015). A way to overcome this limitation and
inherent subjectivity of the diagnosis of diseases and
lung disorders is by digital recording and subsequent
computerized analysis (Palaniappan et al., 2013).
Historically many efforts have been reported in li-
terature to automatically detect lung sound patholo-
gies by means of digital signal processing and simple
time-frequency analysis (Palaniappan et al., 2013). In
recent years, however, machine learning techniques
have gained popularity in this field because of their
potential to find significant diagnostic information
relying on statistical distribution of data itself (Kan-
daswamy et al., 2004). Palaniappan et al. (2013)
report state of the art results are obtained by using
supervised learning algorithms such as support vector
machine (SVM), decision trees and artificial neural
networks (ANNs) trained with expert-engineered
features extracted from audio signals. However, more
recent studies (Kilic et al., 2017) have proved that
such benchmark results can be obtained through end-
to-end learning, by means of deep neural networks
(DNNs), a type of machine learning algorithm that
attempts to model high-level abstractions in complex
data, composing its processing from multiple non-
linear transformations, thus incorporating the feature
extraction itself in the training process. Among the
most successful deep neural network architectures,
convolutional neural networks (CNNs) together with
recurrent neural networks (RNNs) have been shown
to be able to find useful features in the lung sound
824
Grzywalski, T., Belluzzo, R., Drgas, S., Cwali
´
nska, A. and Hafke-Dys, H.
Interactive Lungs Auscultation with Reinforcement Learning Agent.
DOI: 10.5220/0007573608240832
In Proceedings of the 11th International Conference on Agents and Artificial Intelligence (ICAART 2019), pages 824-832
ISBN: 978-989-758-350-6
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

signals as well as to track temporal correlations be-
tween repeating patterns (Kilic et al., 2017).
However, information fusion between different
auscultation points (APs) and integration of decision
making processes to guide the examiner throughout
the auscultation seems to be absent in the literature.
Reinforcement Learning (RL), a branch of machine
learning inspired by behavioral psychology (Shtein-
gart and Loewenstein, 2014), can possibly provide a
way to integrate auscultation path information, inter-
actively, at data acquisition stage. In the common RL
problem setting, the algorithm, also referred as agent,
learns to solve complex problems by interacting with
an environment, which in turn provides positive or
negative rewards depending on the results of the ac-
tions taken. The objective of the agent is thus to find
the best policy, which is the best action to take, given a
state, in order to maximize received reward and mini-
mize received penalty. We believe that RL framework
is the best choice for the solution of our problem, i.e
finding the lowest number of APs while maintaining a
minimum acceptable diagnosis accuracy. As a result,
the agent will learn what are the optimal locations to
auscultate the patient and in which order to examine
them. As far as we know, this is the first attempt to
use RL to perform interactive lung auscultation.
RL has been successful in solving a variety of
complex tasks, such as computer vision (Bernstein
et al., 2018), video games (Mnih et al., 2013), speech
recognition (Kato and Shinozaki, 2017) and many
others. RL can also be effective in feature selection,
defined as the problem of identifying the smallest sub-
set of highly predictive features out of a possibly large
set of candidate features (Fard et al., 2013). A simi-
lar problem was further investigated (Bi et al., 2014)
where the authors develop a Markov decision process
(MDP) (Puterman, 1994) that through dynamic pro-
gramming (DP) finds the optimal feature selecting se-
quence for a general classification task. Their work
motivated us to take advantage of this framework with
the aim of applying it to find the lowest number of
APs, i.e the smallest set of features, while maximiz-
ing the accuracy in classifying seriousness of breath
phenomena detected during auscultation, which is in
turn directly proportional to diagnosis accuracy.
This work is organized as follows. Section 2 re-
calls the mathematical background useful to follow
the work. Section 3 formally defines our proposed so-
lution and gives a systematic overview of the interac-
tive auscultation application. Section 4 describes the
experimental framework used to design the interactive
agent and evaluate its performance. Section 5 shows
the results of our experiments, where we compare the
interactive agent against its static counterpart, i.e an
agent that always takes advantage of all auscultation
points. Finally, Section 6 presents our conclusions.
2 MATHEMATICAL
BACKGROUND
2.1 Reinforcement Learning
The RL problem, originally formulated in (Sutton,
1988), relies on the theoretical framework of MDP,
which consists on a tuple of (S, A, P
SA
, γ, R) that satis-
fies the Markov property (Puterman, 1994). S is a set
of environment states, A a set of actions, P
SA
the state
(given an action) transitions probability matrix, γ the
discount factor, R(s) the reward (or reinforcement) of
being in state s. We define the policy π(s) as the func-
tion, either deterministic or stochastic, which dictates
what action to take given a particular state. We also
define a value function that determines the value of
being in a state s and following the policy π till the
end of one iteration, i.e an episode. This can be ex-
pressed by the expected sum of discounted rewards,
as follows:
V
π
(s) = E[R(s
0
) + γR(s
1
) + γ
2
R(s
2
) + ...|s
0
= s, π]
(1)
where s
0
, s
1
, s
2
, . . . is a sequence of states within the
episode. The discount factor γ is necessary to moder-
ate the effect of observing the next state. When γ is
close to 0, there is a shortsighted condition; when it
tends to 1 it exhibits farsighted behaviour (Sutton and
Barto, 1998). For finite MDPs, policies can be par-
tially ordered, i.e π ≥ π
0
if and only if V
π
(s) ≥ V
π
0
(s)
for all s ∈ S. There is always at least one policy that
is better than or equal to all the others. This is called
optimal policy and it is denoted by π
∗
. The optimal
policy leads to the optimal value function:
V
∗
(s) = max
π
V
π
(s) = V
π
∗
(s) (2)
In the literature the algorithm solving the RL prob-
lem (i.e finding π
∗
) is normally referred as the agent,
while the set of actions and states are abstracted as be-
longing to the environment, which interacts with the
agent signaling positive or negative rewards (Figure
1). Popular algorithms for its resolution in the case of
finite state-space are Value iteration and Policy itera-
tion (Sutton, 1988).
2.2 Q-learning
Q-learning (Watkins and Dayan, 1992) is a popular al-
gorithm used to solve the RL problem. In Q-learning
Interactive Lungs Auscultation with Reinforcement Learning Agent
825

actionrewardstate
Agent
Environment
R(s
1
)
s
1
0
R(s
0
)
a
Figure 1: RL general workflow: the agent is at state s
0
, with
reward R[s
0
]. It performs an action a and goes from state s
0
to s
1
getting the new reward R[s
1
].
actions a ∈ A are obtained from every state s ∈ S
based on an action-value function called Q function,
Q : S × A → R, which evaluates the quality of the pair
(s, a).
The Q-learning algorithm starts arbitrarily initial-
izing Q(s, a); then, for each episode, the initial state
s is randomly chosen in S, and a is taken using the
policy derived from Q. After observing r, the agent
goes from state s to s
0
and the Q function is updated
following the Bellman equation (Sammut and Webb,
2010):
Q(s, a) ← Q(s, a) + α[r + γ · max
a
0
Q(s
0
, a
0
) − Q(s, a)]
(3)
where α is the learning rate that controls algorithm
convergence and γ is the discount factor. The algo-
rithm proceeds until the episode ends, i.e a terminal
state is reached. Convergence is reached by recur-
sively updating values of Q via temporal difference
incremental learning (Sutton, 1988).
2.3 Deep Q Network
If the states are discrete, Q function is represented
as a table. However, when the number of states is
too large, or the state space is continuous, this for-
mulation becomes unfeasible. In such cases, the Q-
function is computed as a parameterized non-linear
function of both states and actions Q(s, a; θ) and the
solution relies on finding the best parameters θ. This
can be learned by representing the Q-function using
a DNN as shown in (Mnih et al., 2013) (Mnih et al.,
2015), introducing deep Q-networks (DQN).
The objective of a DQN is to minimize the mean
square error (MSE) of the Q-values:
L(θ) =
1
2
[r + max
a
0
Q(s
0
, a
0
;θ
0
) − Q(s, a; θ)]
2
(4)
J(θ) = max
θ
[L(θ)] (5)
Since this objective function is differentiable w.r.t θ,
the optimization problem can be solved using gradi-
ent based methods, e.g Stochastic Gradient Descent
(SGD) (Bottou et al., 2018).
3 RL-BASED INTERACTIVE
AUSCULTATION
3.1 Problem Statement
In our problem definition, the set of states S is com-
posed by the list of points already auscultated, each
one described with a set of fixed number of features
that characterize breath phenomena detected in that
point. In other terms, S ∈ R
n×m
, where n is the num-
ber of auscultation points and m equals the number of
extracted features per point, plus one for number of
times this point has been auscultated.
The set of actions A, conversely, lie in a finite
space: either auscultate another specified point (can
be one of the points already auscultated), or predict
diagnosis status of the patient if confident enough.
3.2 Proposed Solution
With the objective of designing an agent that interacts
with the environment described above, we adopted
deep Q-learning as resolution algorithm. The pro-
posed agent is a deep Q-network whose weights θ are
updated following Eq. 3, with the objective of maxi-
mizing the expected future rewards (Eq. 4):
θ ← θ+α[r +γ·max
a
0
Q(s
0
, a
0
;θ)−Q(s, a; θ)]∇Q(s, a; θ)
(6)
where the gradient in Eq. 6 is computed by back-
propagation. Similarly to what shown in (Mnih et al.,
2013), weight updates are performed through experi-
ence replay. Experiences over many plays of the same
game are accumulated in a replay memory and at each
time step multiple Q-learning updates are performed
based on experiences sampled uniformly at random
from the replay memory. Q-network predictions map
states to next action. Agent’s decisions affect rewards
signaling as well as the optimization problem of find-
ing the best weights following Eq. 6.
The result of the auscultation of a given point is a
feature vector of m elements. After the auscultation,
values from the vector are assigned to the appropri-
ate row of the state matrix. Features used to encode
agent’s states are obtained after a feature extraction
module whose core part consists of a convolutional
recurrent neural network (CRNN) trained to predict
breath phenomena events probabilities. The output of
such network is a matrix whose rows show probabil-
ity of breath phenomena changing over time. This
data structure, called probability raster, is then post-
processed in order to obtain m features, representative
of the agent’s state.
ICAART 2019 - 11th International Conference on Agents and Artificial Intelligence
826
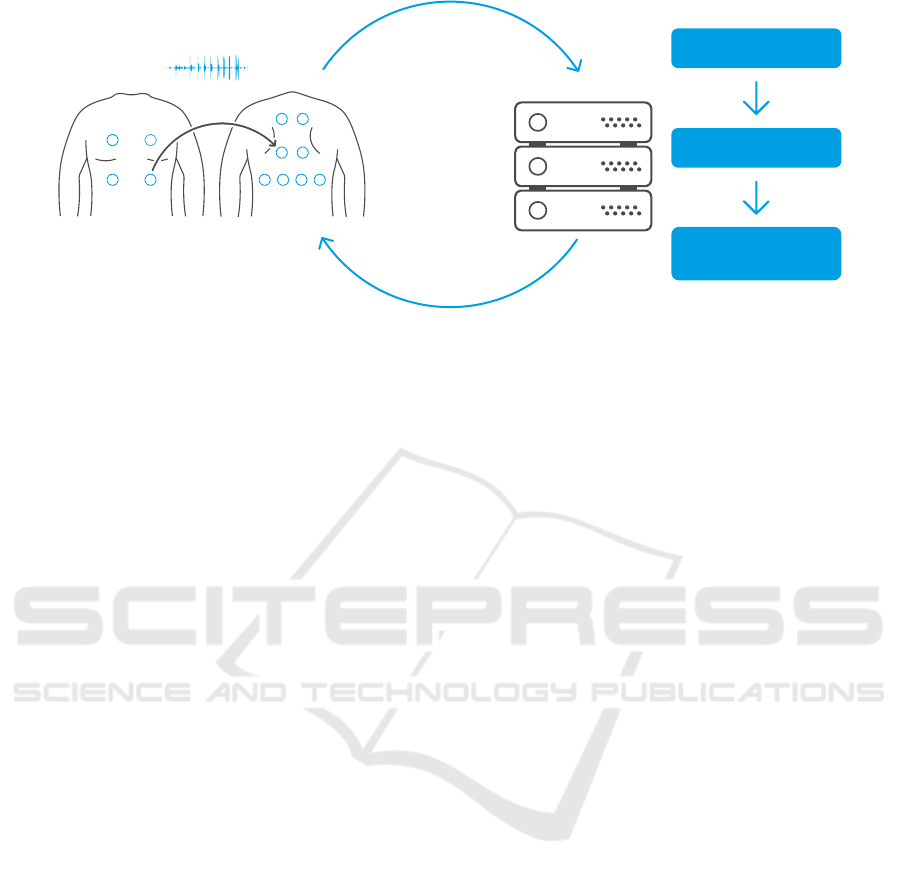
server
recording
next auscultation point
feature extraction
agent
best action
prediction
12
34 1012 119
8 7
6 5
Figure 2: Interactive auscultation: the examiner starts auscultating the patient from the initial point (in this case point number
3), using our proprietary digital and wireless stethoscope, connected via Bluetooth to a smartphone. The recorded signal is
sent to the server where a fixed set of features are extracted. These features represent the input to the agent that predicts the best
action that should be taken. The prediction is then sent back to device and shown to the user, in this case to auscultate point
number 8. The auscultation continues until agent is confident enough and declares predicted alarm value. The application
works effectively even if the device is temporary offline: as soon as the connection is back, the agent can make decisions
based on all the points that have been recorded so far.
Finally, reinforcement signals (R) are designed in
the following way: rewards are given when the pre-
dicted diagnosis status is correct, penalties in the op-
posite case. Moreover, in order to discourage the
agent of using too many points, a small penalty is
provided for each additional auscultated point. The
best policy for our problem is thus embodied in the
best auscultation path, encoded as sequence of most
informative APs to be analyzed, which should be as
shortest as possible.
3.3 Application
The interactive auscultation application consists of
two entities: the pair digital stethoscope and smart-
phone, used as the interface for the user to access the
service; and a remote server, where the majority of
the computation is done and where the agent itself re-
sides. An abstraction of the entire system is depicted
in Figure 2.
The first element in the pipeline is our propri-
etary stethoscope (StethoMe, 2018). It is a digital
and wireless stethoscope similar to Littmann digital
stethoscope (Littmann 3200, 2009) in functionality,
but equipped with more microphones that sample the
signal at higher sampling rate, which enables it to
gather even more information about the patient and
background noise. The user interacts with the stetho-
scope through a mobile app installed on the smart-
phone, connected to the device via Bluetooth Low
Energy protocol (Gomez et al., 2012). Once the aus-
cultation has started, a high quality recording of the
auscultated point is stored on the phone and sent to
the remote server. Here, the signal is processed and
translated into a fixed number of features that will be
used as input for the agent. The agent predicts which
is the best action to perform next: it can be either to
auscultate another point or, if the confidence level is
high enough, return predicted patient’s status and end
the examination. Agent’s decision is being made dy-
namically after each recording, based on breath phe-
nomena detected so far. This allows the agent to make
best decision given limited information which is cru-
cial when the patient is an infant and auscultation gets
increasingly difficult over time.
4 EVALUATION
This section describes the experimental framework
used to simulate the interactive auscultation applica-
tion described in Subsection 3.3. In particular, in Sub-
section 4.1 the dataset used for the experiments is de-
scribed. In Subsection 4.2 a detailed description of
the feature extraction module already introduced in
Subsection 3.2 is provided. In Subsection 4.3 the in-
teractive agent itself is explained, while in Subsection
4.4 the final experimental setup is presented.
4.1 Dataset
Our dataset consists of a total of 570 real exam-
inations conducted in the Department of Pediatric
Pulmonology of Karol Jonscher Clinical Hospital in
Pozna
´
n (Poland) by collaborating doctors. Data col-
lection involved young individuals of both genders
Interactive Lungs Auscultation with Reinforcement Learning Agent
827
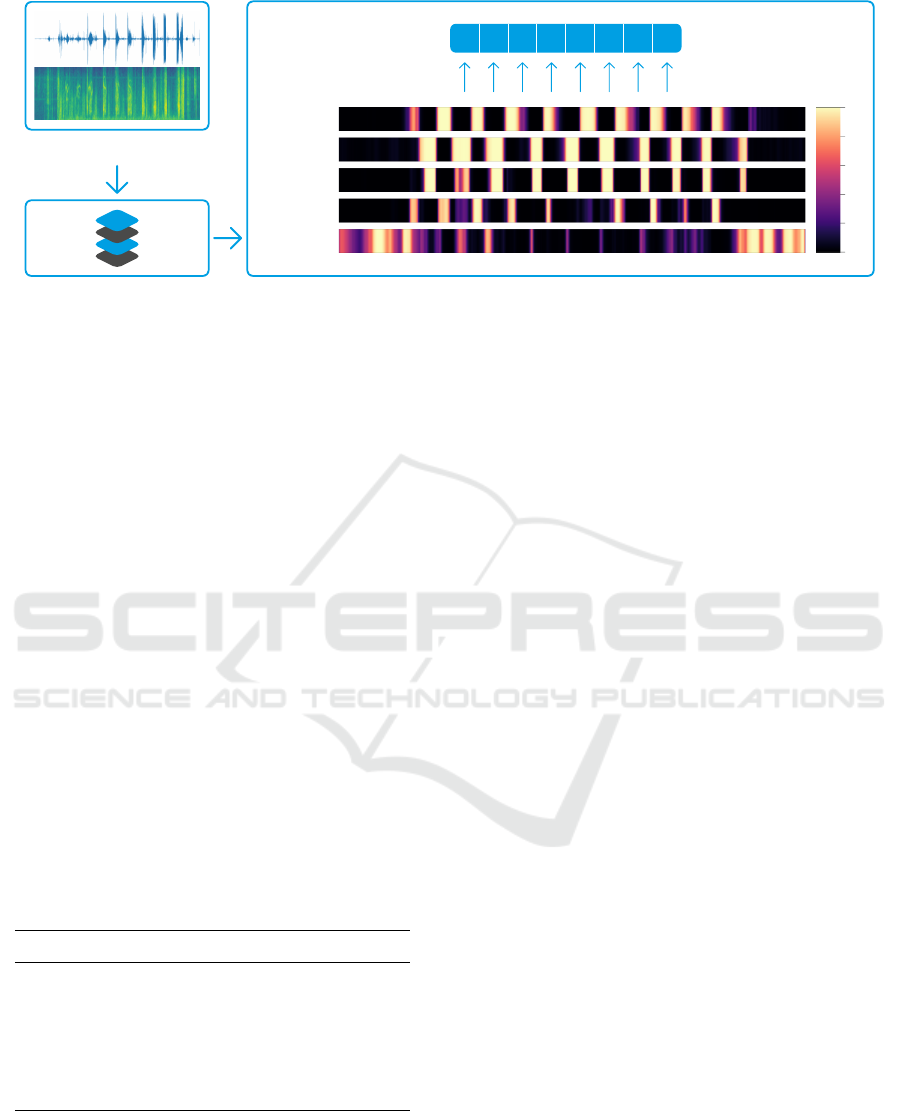
(a)
(b) (c)
1 2 3 4 5 6 7 8
inspiration
expiration
wheezes
crackles
noise
1
0,8
0,6
0,4
0,2
0
Figure 3: Feature extraction module: audio signal is first converted to spectrogram and subsequently fed to a CRNN, which
outputs a prediction raster of 5 classes: inspiration, expiration, wheezes, crackles and noise. This raster is then post-processed
with the objective of extracting values representative of detection and intensity level of the critical phenomena, i.e wheezes
and crackles. More specifically, maximum probability value and relative duration of tags are computed per each inspira-
tion/expiration and the final features are computed as the average of these two statistics along all inspirations/expirations.
(46% females and 54% males.) and different ages:
13% of them were infants ([0, 1) years old), 40%
belonging to pre-school age ([1, 6)) and 43% in the
school age ([6, 18)).
Each examination is composed of 12 APs,
recorded in pre-determined locations (Figure 2).
Three possible labels are present for each exami-
nation: 0, when no pathological sounds at all are
detected and there’s no need to consult a doctor;
1, when minor (innocent) auscultatory changes are
found in the recordings and few pathological sounds
in single AP are detected, but there’s no need to
consult a doctor; 2, significant auscultatory changes,
i.e major auscultatory changes are found in the
recordings and patient should consult a doctor. This
ground truth labels were provided by 1 to 3 doctors
for each examination, in case there was more than
one label the highest label value was taken. A resume
of dataset statistics is shown in Table 1.
Table 1: Number of examinations for each of the classes.
Label Description N
examinations
0 no auscultatory
changes - no alarm 200
1 innocent auscultatory
changes - no alarm 85
2 significant auscultatory
changes - alarm 285
4.2 Feature Extractor
The features for the agent are extracted by a fea-
ture extractor module that is composed of three main
stages, schematically depicted in Figure 3: at the be-
ginning of the pipeline, the audio wave is converted
to its magnitude spectrogram, a representation of the
signal that can be generated by applying short time
fourier transform (STFT) to the signal (a). The time-
frequency representation of the data is fed to a con-
volutional recurrent neural network (b) that predicts
breath phenomena events probabilities in form of pre-
dictions raster. Raster is finally post-processed in or-
der to extract 8 interesting features (c).
4.2.1 CRNN
This neural network is a modified implementation of
the one proposed by C¸ akir et al. (2017), i.e a CRNN
designed for polyphonic sound event detection
(SED). In this structure originally proposed by the
convolutional layers act as pattern extractors, the
recurrent layers integrate the extracted patterns over
time thus providing the context information, and
finally the feedforward layer produce the activity
probabilities for each class (C¸ akir et al., 2017). We
decided to extend this implementation including
dynamic routing (Sabour et al., 2017) and applying
some key ideas of Capsule Networks (CapsNet), as
suggested in recent advanced studies (Vesperini et al.,
2018) (Liu et al., 2018). The CRNN is trained to
detect 5 types of sound events, namely: inspirations,
expirations, wheezes, crackles (Sarkar et al., 2015)
and noise.
4.2.2 Raster Post-processing
Wheezes and crackles are the two main classes of
pathological lung sounds. The purpose of raster
ICAART 2019 - 11th International Conference on Agents and Artificial Intelligence
828
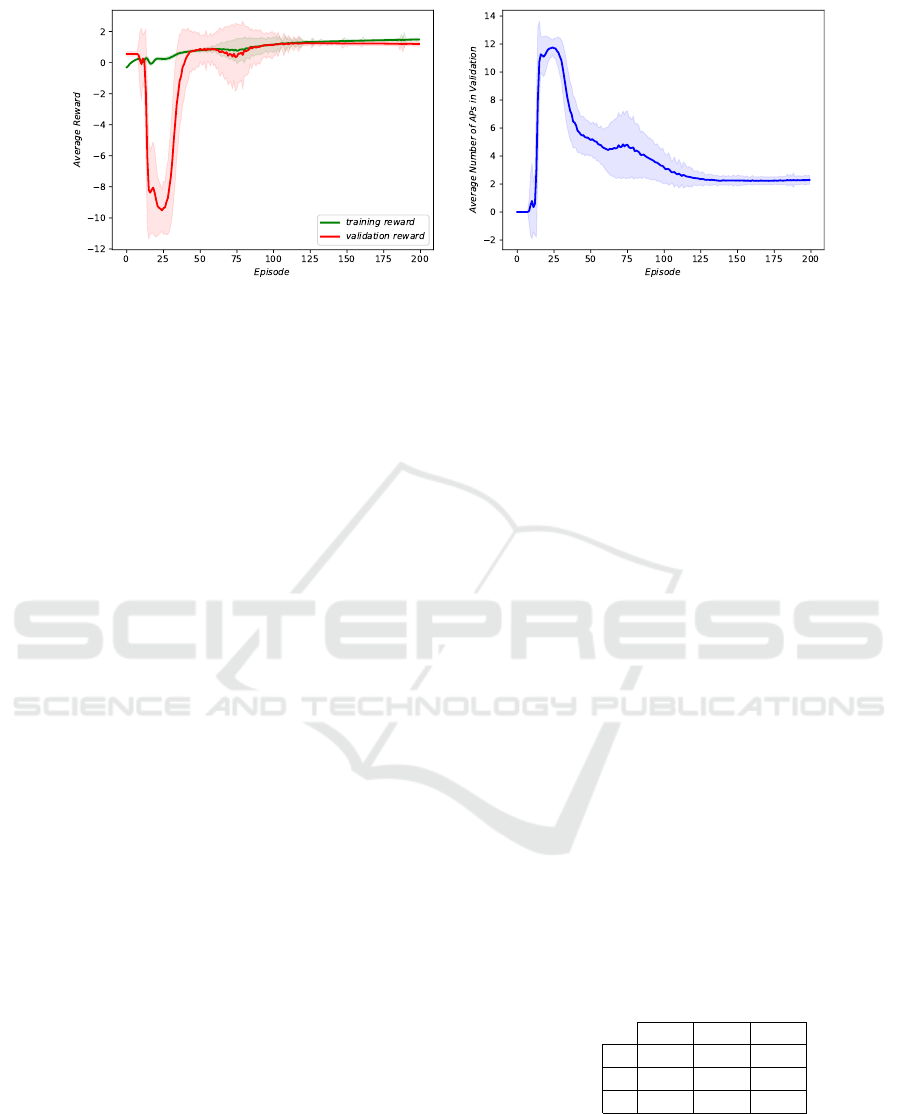
(a) (b)
Figure 4: Interactive agent learning curves: in the very first episodes the agent randomly guesses the state of the patient,
without auscultating any point. Next comes the exploration phase when the agent auscultates many points, often reaching
the 12-point limit which results in high penalties. Finally, as the agent plays more and more episodes, it starts learning the
optimal policy using fewer and fewer points until he finds the optimal solution.
post-processing is to extract a compact representa-
tion that will be a good descriptions of their pres-
ence/absence and level of intensity. Thus, for each
inspiration and expiration event we calculate two val-
ues for each pathological phenomena (wheezes and
crackles): maximum probability within said inspira-
tion/expiration and relative duration after threshold-
ing (the level in which the inspiration/expiration is
filled, or covered with the pathological phenomenon).
All extracted values are then averaged across all in-
spirations and expirations separately. We therefore
obtain 8 features: average maximum wheeze proba-
bility on inspirations (1) and expirations (2), average
relative wheeze length in inspirations (3) and expira-
tions (4) and the same four features (5, 6, 7 and 8) for
crackles.
4.3 Reinforcement Learning Agent
Our agent consists of a deep fully connected neural
network. The network takes as input a state matrix of
size 12 rows × 9 columns; then processes the input
through 3 hidden layers with 256 units each followed
by ReLU nonlinearity (Nair and Hinton, 2010); the
output layer is composed by 15 neurons which repre-
sent expected rewards for each of the possible future
actions. This can be either to request one of the 12
points to be auscultated, or declare one of the three
alarm status, i.e predict one of the three labels and
finish the examination.
The state matrix is initially set to all zeros, and
the i
th
row is updated each time i
th
AP is auscultated.
First 8 columns of state matrix correspond to eight
features described in previous section, while the last
value is a counter for the number of times this aus-
cultation point was auscultated. At every interaction
with the environment, the next action a to be taken is
defined as argmax of the output vector. At the be-
ginning of the agent’s training we ignore agent’s pref-
erences and perform random actions, as the training
proceeds we start to use agent’s recommended actions
more and more often. For a fully trained model, we al-
ways follow agent’s instructions. The agent is trained
to predict three classes, but classes 0 and 1, treated as
agglomerated not alarm class, are eventually merged
at evaluation phase.
The agent is trained with objective of minimizing
Eq. 4, and Q-values are recursively updated by tem-
poral difference incremental learning. There are two
ways to terminate the episode: either make a classi-
fication decision, getting the reward/penalty that fol-
lows table 2; or reaching a limit of 12 actions which
results in a huge penalty of r = −10.0. Moreover,
when playing the game a small penalty of r = −0.01
is given for each requested auscultation, this is to en-
courage the agent to end the examination if it doesn’t
expect any more information coming from continued
auscultation.
Table 2: Reward matrix for Reinforcement Learning Agent
final decisions.
predicted
0 1 2
actual
0 2.0 0.0 -1.0
1 0.0 2.0 -0.5
2 -1.0 -0.5 2.0
4.4 Experimental Setup
We compared the performance of reinforcement
learning agent, from now on referred to as interactive
agent, to its static counterpart, i.e an agent that always
Interactive Lungs Auscultation with Reinforcement Learning Agent
829
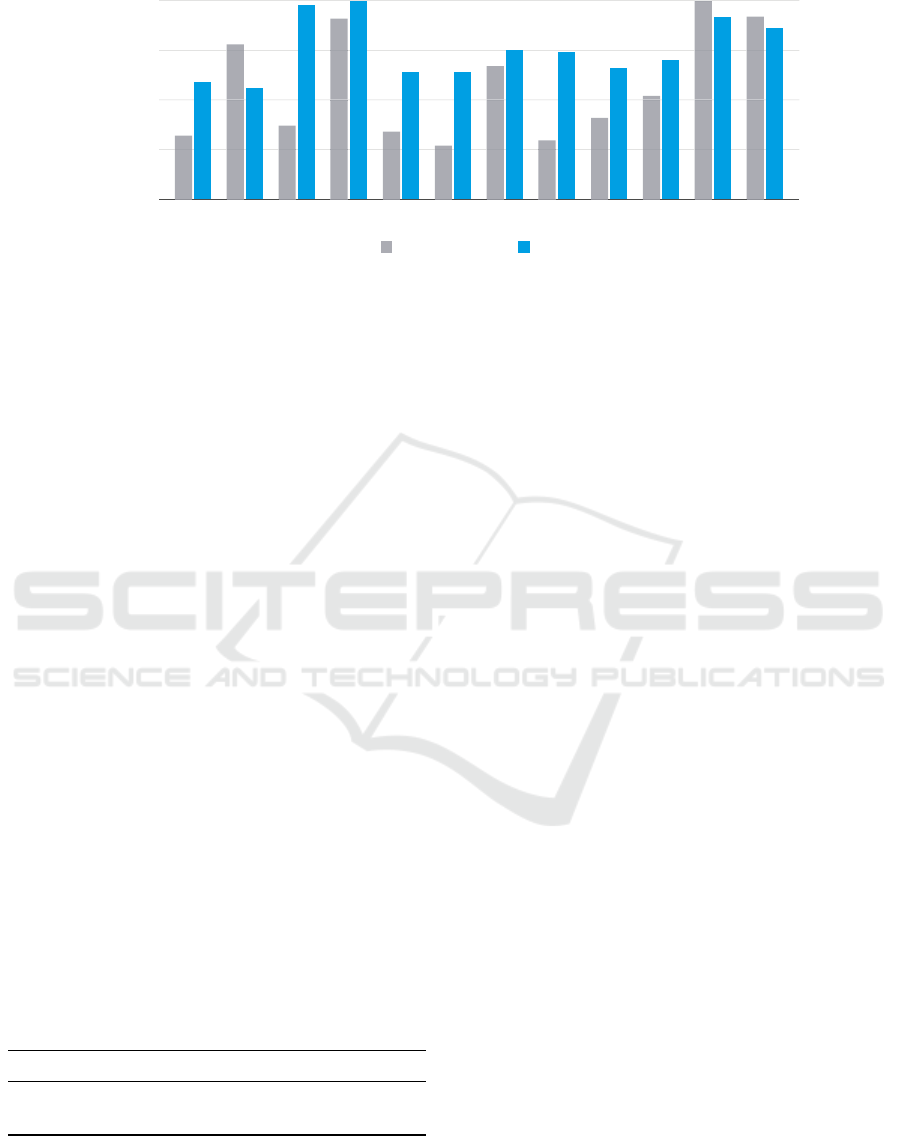
frequency
1
0,75
0,50
0,25
0
1 2 3 4 5 6 7 8 9 10 11 12
Interactive agent Doctors
Figure 5: Histograms: we compared the distribution of most used points by the agent against the ones that doctors would
most often choose at examination time. Results show that the agent learned importance of points without any prior knowledge
about human physiology.
performs an exhaustive auscultation (uses all 12 APs).
In order to compare the two agents we performed 5-
fold cross validation for 30 different random splits of
the dataset into training (365 auscultations), valida-
tion (91) and test (114) set. We trained the agent for
200 episodes, setting γ = 0.93 and using Adam opti-
mization algorithm (Kingma and Ba, 2014) to solve
Eq. 4, with learning rate initially set to 0.0001.
Both in validation and test phase, 0 and 1 labels
were merged as single not alarm classes. There-
fore results shown in the following refer to the binary
problem of alarm detection: we chose as compara-
tive metrics balanced accuracy (BAC) defined as un-
weighted mean of sensitivity and specificity; and F1-
score, harmonic mean of precision and recall, com-
puted for each of the two classes.
5 RESULTS
In Table 3 we show the results of the experiments we
conducted. The interactive agent performs the auscul-
tation using on average only 3 APs, effectively reduc-
ing the time of the examination 4 times. This is a very
significant improvement and it comes at a relatively
small cost of 2.5 percent point drop in classification
accuracy.
Table 3: Results of experiments.
Agent BAC F1
alarm
F1
not alarm
APs
Static 84.8 % 82.6 % 85.1 % 12
Interactive 82.3 % 81.8 % 82.6 % 3.2
Figure 4 shows learning curves of rewards and
number of points auscultated by the agent. In the very
first episodes the agent directly guesses the state of
the patient, declaring the alarm value without auscul-
tating any point. As soon as it starts exploring other
possible action-state scenarios, it often reached the
predefined limit of 12 auscultation points which sig-
nificantly reduces its average received reward. How-
ever, as it plays more episodes, it starts converging to
the optimal policy, using less points on average.
In order to assess the knowledge learned by the
agent, we conducted a survey involving a total of
391 international experts. The survey was distributed
among the academic medical community and in hos-
pitals. In the survey we asked each participant to
respond a number of questions regarding education,
specialization started or held, assessment of their own
skills in adult and child auscultation, etc. In particular,
we asked them which points among the 12 proposed
would be auscultated more often during an exami-
nation. Results of the survey are visible in Figure 5
where we compare collected answers with most used
APs by the interactive agent. It’s clear that the agent
was able to identify which APs carry the most infor-
mation and are the most representative to the overall
patient’s health status. This is the knowledge that all
human experts gain from many years of clinical prac-
tice. In particular the agent identified points 11 and
12 as very important. This finding is confirmed by
the doctors who strongly agree that these are the two
most important APs on patient’s back. On the chest
both doctors and the agent often auscultate point num-
ber 4, but the agent prefers point number 2 instead of
3, probably due to the distance from the heart which
is a major source of interference in audio signal.
The agent seems to follow two general rules dur-
ing the auscultation: firstly, it auscultates points be-
longing both to the chest and to the back; secondly,
it tries to cover as much area as possible, visiting not-
subsequent points. For instance, the top 5 auscultation
paths among the most repeating sequences that we
observed are: [4, 9, 11], [8, 2, 9], [2, 11, 12], [7, 2, 8],
ICAART 2019 - 11th International Conference on Agents and Artificial Intelligence
830

[4, 11, 12]. These paths cover only 3% of the possi-
ble paths followed by the agent: this means the agent
does not follow a single optimal path or even cou-
ple of paths, but instead uses a wide variety of paths
depending on breath phenomena detected during the
examination.
6 CONCLUSIONS
We have presented a unique application of reinforce-
ment learning for lung sounds auscultation, with the
objective of designing an agent being able to perform
the procedure interactively in the shortest time possi-
ble.
Our interactive agent is able to perform an intelli-
gent selection of auscultation points. It performs the
auscultation using only 3 points out of a total of 12,
reducing fourfold the examination time. In addition
to this, no significant decrease in diagnosis accuracy
is observed, since the interactive agent gets only 2.5
percent points lower accuracy than its static counter-
part that performs an exhaustive auscultation using all
available points.
Considering the research we have conducted, we
believe that further improvements can be done in the
solution proposed. In the near future, we would like
to extend this work to show that the interactive solu-
tion can completely outperform any static approach to
the problem. We believe that this can be achieved by
increasing the size of the dataset or by more advanced
algorithmic solutions, whose investigation and imple-
mentation was out of the scope of this publication.
REFERENCES
Bernstein, A., Burnaev, E., and N. Kachan, O. (2018). Re-
inforcement Learning for Computer Vision and Robot
Navigation.
Bi, S., Liu, L., Han, C., and Sun, D. (2014). Finding the
optimal sequence of features selection based on rein-
forcement learning. In 2014 IEEE 3rd International
Conference on Cloud Computing and Intelligence Sys-
tems, pages 347–350.
Bottou, L., Curtis, F. E., and Nocedal, J. (2018). Optimiza-
tion methods for large-scale machine learning. SIAM
Review, 60:223–311.
C¸ akir, E., Parascandolo, G., Heittola, T., Huttunen, H., and
Virtanen, T. (2017). Convolutional recurrent neu-
ral networks for polyphonic sound event detection.
CoRR, abs/1702.06286.
Fard, S. M. H., Hamzeh, A., and Hashemi, S. (2013). Using
reinforcement learning to find an optimal set of fea-
tures. Computers & Mathematics with Applications,
66(10):1892 – 1904. ICNC-FSKD 2012.
Gomez, C., Oller, J., and Paradells, J. (2012). Overview
and evaluation of bluetooth low energy: An emerging
low-power wireless technology.
Hyacinthe, L. R. T. (1819). De l’auscultation m
´
ediate ou
trait
´
e du diagnostic des maladies des poumons et du
coeur (On mediate auscultation or treatise on the di-
agnosis of the diseases of the lungs and heart). Paris:
Brosson & Chaud
´
e.
Kandaswamy, A., Kumar, D. C. S., Pl Ramanathan, R.,
Jayaraman, S., and Malmurugan, N. (2004). Neu-
ral classification of lung sounds using wavelet coef-
ficients. Computers in biology and medicine, 34:523–
37.
Kato, T. and Shinozaki, T. (2017). Reinforcement learning
of speech recognition system based on policy gradient
and hypothesis selection.
Kilic, O., Kılıc¸, z., Kurt, B., and Saryal, S. (2017). Clas-
sification of lung sounds using convolutional neural
networks. EURASIP Journal on Image and Video Pro-
cessing, 2017.
Kingma, D. P. and Ba, J. (2014). Adam: A method for
stochastic optimization. CoRR, abs/1412.6980.
Littmann 3200 (2009). Littmann
R
. https:
//www.littmann.com/3M/en_US/
littmann-stethoscopes/products/, Last ac-
cessed on 2018-10-30.
Liu, Y., Tang, J., Song, Y., and Dai, L. (2018). A capsule
based approach for polyphonic sound event detection.
Mnih, V., Kavukcuoglu, K., Silver, D., Graves, A.,
Antonoglou, I., Wierstra, D., and Riedmiller, M.
(2013). Playing atari with deep reinforcement learn-
ing.
Mnih, V., Kavukcuoglu, K., Silver, D., Rusu, A. A., Ve-
ness, J., Bellemare, M. G., Graves, A., Riedmiller,
M., Fidjeland, A. K., Ostrovski, G., Petersen, S.,
Beattie, C., Sadik, A., Antonoglou, I., King, H., Ku-
maran, D., Wierstra, D., Legg, S., and Hassabis, D.
(2015). Human-level control through deep reinforce-
ment learning. Nature, 518(7540):529–533.
Nair, V. and Hinton, G. E. (2010). Rectified linear units im-
prove restricted boltzmann machines. In Proceedings
of the 27th International Conference on International
Conference on Machine Learning, ICML’10, pages
807–814, USA. Omnipress.
Palaniappan, R., Sundaraj, K., Ahamed, N., Arjunan, A.,
and Sundaraj, S. (2013). Computer-based respiratory
sound analysis: A systematic review. IETE Technical
Review, 33:248–256.
Puterman, M. L. (1994). Markov Decision Processes: Dis-
crete Stochastic Dynamic Programming. John Wiley
& Sons, Inc., New York, NY, USA, 1st edition.
Sabour, S., Frosst, N., and Hinton, G. E. (2017). Dynamic
routing between capsules. In NIPS.
Sammut, C. and Webb, G. I., editors (2010). Bellman Equa-
tion, pages 97–97. Springer US, Boston, MA.
Sarkar, M., Madabhavi, I., Niranjan, N., and Dogra, M.
(2015). Auscultation of the respiratory system. An-
nals of thoracic medicine, 10:158–168.
Interactive Lungs Auscultation with Reinforcement Learning Agent
831

Shteingart, H. and Loewenstein, Y. (2014). Reinforcement
learning and human behavior. Current opinion in neu-
robiology, 25:93–8.
Sovij
¨
arvi, A., Vanderschoot, J., and Earis, J. (2000). Stan-
dardization of computerized respiratory sound analy-
sis. Eur Respir Rev, 10.
StethoMe (2018). Stethome
R
, my home stethoscope.
https://stethome.com/, Last accessed on 2018-
10-30.
Sutton, R. S. (1988). Learning to predict by the methods of
temporal differences. Machine Learning, 3(1):9–44.
Sutton, R. S. and Barto, A. G. (1998). Introduction to Re-
inforcement Learning. MIT Press, Cambridge, MA,
USA, 1st edition.
Vesperini, F., Gabrielli, L., Principi, E., and Squartini, S.
(2018). Polyphonic sound event detection by using
capsule neural network.
Watkins, C. J. C. H. and Dayan, P. (1992). Q-learning. In
Machine Learning, pages 279–292.
ICAART 2019 - 11th International Conference on Agents and Artificial Intelligence
832
