
Techniques for Usability Risk Assessment during Medical Device
Design
Alice Ravizza
1
, Andres Diaz Lantada
2
, Luis Ignacio Ballesteros Sánchez
2
, Federico Sternini
1
and
Cristina Bignardi
1
1
DIMEAS, Politecnico di Torino, Torino, Italy
2
ETSI Industriales, Universidad Politecnica de Madrid, Madrid, Spain
Keywords: Usability, Human Factors, Risk Management, Medical Device Design.
Abstract: Human errors during the use of medical devices, due to pitfalls in the design of the user interface, may lead
to substantial risk to users and to patients. There are multiple techniques for the identification and for the
assessment of user related risks, that may be chosen according to the step of the design (preliminary
feasibility studies, minimum viable product assessment, verification and validation) and considering
cognitive processes and information processing mechanisms of users, which may lead to errors. Some
techniques are more adequate for a quick-and-dirty approach, during early stages of design: these include
expert reviews, discussions among focus groups, standard reviews and heuristic analyses. Other techniques
are adequate for a more detailed and systematic analysis of risk, in more advanced design stages, with a
failure mode and effect analysis (FMEA) approach, including time-and-motion studies and task analyses.
Lastly, user tests with the help of rapid prototypes, perhaps involving alternative embodiments to be studied,
are very adequate for verification and validation of the interface. Usability analysis techniques should be
part of the toolbox of a biomedical engineer and they should be carefully chosen. Each technique, regardless
the step it is used, should allow the designers to define a precise level of risk in terms of probability,
severity. Moreover, usability risk minimisation measures shall be measurable and able to be quantified, as
well as the impact of risk mitigation strategies. For this reason, usability risk minimisation measures should
be classified according to regulatory requirements as “safe by design”; “alarms and protections” and
“information for safe use”. Each class of risk minimisation measure should be then given a measurable risk
reduction score, so that the risk assessment can be completed in a repeatable and regulatory compliant way.
1 INTRODUCTION
Biomedical engineers routinely include “users’
needs” in the design requirements of medical
devices. But what is a “user need”? Not only the
patient clinical condition, but also the need of a
device that is adequate to his skills, education and
capabilities and can be used safely.
Usability is defined, by the standard IEC 62366,
as “the characteristics or features of the user
interface that facilitate use and thereby effectiveness,
efficiency and user satisfaction in the intended
environment of use” (International Electrotechnical
Commission [IEC], 2015).
It is an essential concept in the design process of
any medical device, for the benefit of healthcare
professionals, patients and all stakeholders. We
believe that usability should be part of the modern
academic education of biomedical engineers,
worldwide. Our group has tested this approach to the
design of innovative open source devices as part of
the UBORA project, including a drop foot frame, a
face splint, a hand rehabilitation tool and more.
Design decisions should be driven not only by
performance, cost or environmental impacts of the
device, but also by its ergonomics and aesthetics,
connected to usability, safety and user experience.
2 POSITION PROPOSAL
In this paper, we present a structured method for the
identification and assessment of use-related risks of
medical devices. These risks need to be considered
during the whole design process, from specification
and conceptualization, towards detailed design,
prototyping, preparation of production and whole
product life cycle.
Ravizza, A., Lantada, A., Sánchez, L., Sternini, F. and Bignardi, C.
Techniques for Usability Risk Assessment during Medical Device Design.
DOI: 10.5220/0007483102070214
In Proceedings of the 12th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2019), pages 207-214
ISBN: 978-989-758-353-7
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
207

We present different techniques as part of this
method, to be chosen according to the kind of device
under assessment, to the level of development of the
device - in terms of ideation, testing and verification
- and to the available resources for the analysis. We
also present how to identify minimisation measures
and how to evaluate their effectiveness.
The method is fully compliant to internationally
recognised standards ISO 14971 (International
Organization for Standardization, 2007) and IEC
62366. The application of this method would be
appropriate during early design stages, during
regulatory approvals and during health technology
assessment, posing important benefits that
overweight the current barriers (Shah, 2007). This
method can be applied by designers, by legal
manufacturers and by authorities involved in health
technology assessment studies.
2.1 Why Is Usability Important for a
BME?
In the upcoming Medical Device Regulation EU
2017/745, the usability of medical devices acquires
extreme importance in the process of certification.
Usability tests are part of an engineering process
that sees the collaboration of a team of experts in the
specific medical sector (physicians, nurses, medical
personnel), clinical and biomedical engineers and
product designers, and include analysis of past
adverse events, related to the use of the device,
design thinking of devices in accordance with the
repetitive and repeatable mental patterns of the
human user, considerations on experience and
technical knowledge of different types of user
(laymen or professional) and the application of
ergonomic principles on the design of the devices.
The classical techniques of human factors
engineering allow to systematize the approach to
medical device design with a view on usability,
because they allow to describe the different types of
users and to build around them a personalized
interface. In fact, the entire process of usability
assessment allows putting the patient and his/her
needs at the center of the medical device design.
3 ERROR DEFINITION AND
IDENTIFICATION
3.1 What is a User Error
User error is any error made by the user in
interfacing with a device, i.e. any situation caused by
the user that leads to device uses unintended by the
manufacturer. It includes two distinct types of error:
use error and abnormal use. Abnormal use is a
“conscious, intentional act or intentional omission of
an act that is counter to or violates normal use and is
also beyond any further reasonable means of user
interface-related risk control by the manufacturer”
(IEC 62366, 2015).
Use error is “user action or lack of user action
while using the medical device that leads to a
different result than that intended by the
manufacturer or expected by the user” (IEC, 2015).
3.2 The Two Steps of the Usability
Assessment
Assessments regarding usability start early during
the design and are iteratively performed to increase
knowledge about user needs and expectations,
interface solutions that better match those needs,
risks and their mitigation measures.
The standard defines two main steps of usability
assessment: a formative (typically iterative) phase
that is integrated in the development of further
iterations and then a summative phase that is
intended to validate and provide objective evidence
regarding the latest (approved) iteration of the
interface design.
3.2.1 Formative
Formative evaluation is a “user interface evaluation
conducted with the intent to explore user interface
design strengths, weaknesses, and unanticipated use
errors” (IEC 62366, 2015).
It is generally iterative and should be performed
until the manufacturer has reached a finalised
version. Formative evaluation improves user
interface, solving issues in preliminary analysis.
During formative iterations, it may be useful to
identify early phase versus late phase studies.
Early phase studies are characterised by a higher
uncertainty in the possible device variants, with
many specifications not yet completely defined. At
this stage, many prototypes are still available and
they can be radically different one to another, so the
employment of rapid and low-cost prototyping
techniques (i.e. 3D printing, cardboard modelling)
proves quite beneficial for first conceptual
assessments.
Late phase studies are characterised by a better
defined list of requirements and of specifications,
which leads to a shorter list of device variants, with
potentially small but very significant differences.
BIODEVICES 2019 - 12th International Conference on Biomedical Electronics and Devices
208
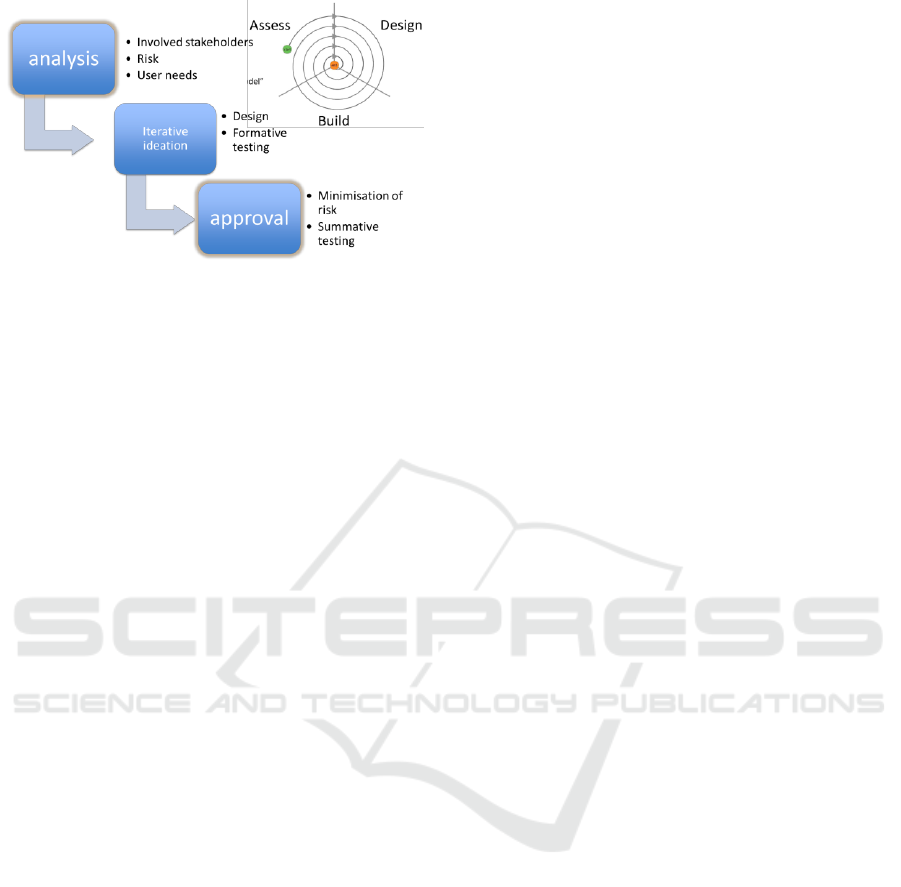
Figure 1: Usability engineering workflow.
3.2.2 Summative
The summative evaluation is conducted at the end of
the development, on the finalized user interface,
with the intent to obtain objective evidence that the
user interface can be used safely. Summative
verifies an acceptable risk-benefit profile under a
usability point of view and also determines and
confirms the expected effectiveness and clinical
benefit.
3.3 Techniques for Usability
Assessment in IEC 62366
System Description. It is an analysis of the main
users and scenarios where the device will be used.
Main functions and sub functions of the device
should be well defined and understood.
Task Analysis. Task analysis broadens the system
description, identifying all the relevant human
interventions in the use of the device and where
errors can occur. It has the objective to understand
and represent in an organized manner the set of tasks
that the human element carries out in the use of the
device. Task analysis may include analysis of
cognitive processes and performance shaping factors
(individual, social and ergonomic) influencing on
the device use. Some methods (Kirwan &
Ainsworth, 1992) for this purpose are divided in
“Task data collection” techniques and “Task
description” techniques.
Task data collection techniques are techniques
which are primarily used for collecting data on
human-system interactions, and which then feed into
other techniques. Some of these techniques are:
Walkthrough, Talkthrough, Critical Incident
Technique, Observation, Questionnaires, Structured
interviews.
Task description techniques represent and
structure the information collected into a systematic
format, serving as a reference material. Some of
these techniques are: Hierarchical Task Analysis,
Tabular Task Analysis, Timeline Analysis,
Decision-Action Diagram. Task description of
various medical devices is present in literature, with
a different level of detail, for example for volumetric
infusion pumps (Chung, 2003)
Human Error Analysis. Including the identification
of possible human errors based on previous Task
analysis. Human errors modes can be analyzed at
two layers: External Errors (Actions) or Internal
Errors (Cognitive). Some techniques to allow human
error identification are Human Hazard and
Operability Study [HAZOP]. Incident analysis, or
use of a Taxonomies and Checklists of possible
generic human errors that might occur during the use
of any device. Human error analysis includes
assessment of probability and severity of each error.
Human Error Reduction and Mitigation. Based
on previous stages a set of recommendations and
requirements for device design are proposed to
reduce and mitigate human errors. Combining the
previous aspects, we can set priorities and propose
strategies for human error reduction.
3.3.1 Which Technique in Each Phase?
The international standard IEC 62366 (IEC, 2016)
presents a series of techniques, that we assessed in:
- early formative
- late formative
- summative
In early formative, a quick-and-dirty approach
identifies the best interface. Then, in late feasibility,
a more structured approach may help to refine the
interface. Lastly, during summative, a frozen version
of the interface is validated to confirm its risk-
benefit profile. Criteria to choose the most
appropriate technique(s) for each phase are:
- Need to involve experts in the technology.
- Need to involve real user(s).
- Time required to assess.
- Time required to report.
- Qualitative results (opinions) vs. quantitative
results (usability scores).
- Depth of analysis.
We have identified some techniques that we
consider particularly appropriate for each step. A
detailed list is shown in Table 1.
The use of rapid prototyping and rapid tooling
techniques, proves interesting for the straightforward
creation of physical models, which can be used to
support most of the aforementioned techniques for
usability assessment. These physical prototypes or
models can support decision making processes for
selecting among different product ideas, on the basis
Techniques for Usability Risk Assessment during Medical Device Design
209

of ergonomics, aesthetics, basic performance,
overall usability and safety, to reach the device
concept in the first stages of the development
process. They can also support in the creation of a
first minimally viable product for interacting with
healthcare professionals, patients, layperson.
Prototypes according to different design iterations,
consequence of the different decisions taken to
mitigate risks and to improve usability, can,
consequently, support the whole methodology and
approach we propose here.
This evaluation has shown that a risk-based
approach is easily adapted to a resource wise
approach. During early formative, low resource
review techniques such as expert reviews, standard
analysis, cognitive walkthrough are easily performed
on documentation and by design experts. They do
not require the participation of a large number of
real users nor the availability of a finalised
prototype, while low-cost replication tools may
provide effective samples to boost discussion.
Later stages of formative assessments may
benefit of more structured techniques, such as a
detailed task analysis that is linked to the FMEA
technique. User tests with 5-10 users may be
planned at later formative steps in order to allow
refinement.
3.3.2 Which Technique for Which Device?
Medical devices belong to varied categories in terms
of technology, intended use, intended users
(layperson or professional), invasiveness in the
human body or expected useful life, which affect
design decisions in connection with usability and
safety. For this reason, we have also assessed each
technique presented by the norm IEC 62366 (IEC,
2016) in terms of adequateness to different kinds of
devices. A detailed evaluation is shown in Table 2 .
In Table 2, the same technique is considered as
adequate or inadequate for devices that may be
apparently very similar from the usability point of
view. However, this is explained by the
technological differences in the device. As an
example, the technique “standard review” proves
“adequate” for very different devices such as heart
valves and nasogastric tubes, but is considered
“adequate with reserve” for Software as a Medical
Device (SaMD). This is due to the poor
standardisation that is still present in the SaMD
sector, while traditional devices can be assessed by
very consistent and complete international standards
and guidelines. Also consider, the technique
“participatory design” that is considered “not
appropriate” for traditional electromedical devices
for the layperson, such as pulse oximeters, but on the
other hand is “adequate” for SaMD and apps for the
layperson. This again is justified as participatory
design may allow the designers to align the medical
app to users’ expectations, by allowing users to
design an intuitive and user friendly app, with a user
interface as similar as possible to a consumer app.
3.4 Linking Usability to Risk
Identification
Each usability evaluation technique allows the
designers to identify risks and potentially hazardous
situations. We describe here some of the techniques
identified above, in terms of capability of the
assessment to be easily linked to a formal risk
analysis as per ISO 14971 (ISO, 2007).
The preferred methods for early feasibility help
the designers to identify risks in general terms and
are potentially adequate to determine risk severity
(worst case consequences of the risk scenario). For
example, at very early stages of ideation of a
electromedical device to be used in emergencies
(e.g. a defibrillator) designers may already be aware
of the importance of high visibility and audibility of
the device, since it is expected to be used in loud,
dark, confusing environments. During late
feasibility, we propose a more structured method, by
application of the Failure Modes and Effects
Analysis FMEA technique. The Application FMEA
technique yields the best results if the question
“what happens if…” is posed at each application
step or phase. So, we propose an integrated
technique: firstly describe the use of the device in
very fine detail by task analysis and then perform
Application FMEA on each step.
We propose a very detailed task analysis and,
where applicable, also a function analysis or use
flowchart. Description of the intended use interface
by a flowchart is particularly adequate for medical
device software, both stand alone and integrated in
an electromedical device. Use of this integrated
method allows a very precise assessment of risk
severity, thanks also to the possibility of obtaining a
description of the chain of events that arise from an
hazardous situation, for example thanks to
brainstorming or focused expert reviews.
If the designers do not have enough past data or
experience-based estimations to determine risk-
related probability, a user test can be very useful to
estimate probability of each hazard. If the user test
are planned in this phase, the task list and use
flowchart already available to designers from the
BIODEVICES 2019 - 12th International Conference on Biomedical Electronics and Devices
210

Application FMEA activity will be used to plan and
record the user tests. For each use error or use
uncertainty observed during user tests, designers can
determine severity and estimate probability.
In late feasibility, user tests can also be
integrated to other techniques to reach a new and
refined iteration of the device interface. We
encourage designers to plan, after user testing
session, additional sessions with the users to gather
information through interviews, SUS questionnaires
(Brooke, 1996) and open-ended questions intended
to encourage participatory design. These interactive
sessions with end users are also very useful to gather
information about expected probability of each
encountered error or uncertainty. For example, late
feasibility studies of a surgically invasive device for
professional use, e.g. catheter for angioplasty, may
include the definition of a task list based on standard
reviews, guidelines, state of the art and interviews.
For each task, designers may identify potential
hazardous situations and their consequences. Then,
user tests on a simulator or dummy may confirm or
improve the estimated risk list; the same users may
be involved after the test to discuss their errors,
determine root causes and suggest improvements in
the catheter shape, pliability or accessory list.
During user tests in the formative phase,
assessments and integrations to reports for
Perception-Cognition-Action technique PCA are
also very common; users can also be invited to
express their thoughts and impressions while they
perform the tasks, as part of participatory design.
A detailed description of the use of different
techniques is given in Table 3.
4 RISK MITIGATION
TECHNIQUES
4.1 Risk Control Measures in Usability
Regulatory requirements (for example Medical
Device Regulation EU 2017/745, Annex I) on risk
minimisation are clearly indicating a preferred order
in the identification and selection of risk
minimisation measures.
Safe-by- design solutions are preferred and, if
not available or not sufficient, other measures shall
be added in terms of protections and alarms.
Moreover, information for safe use shall be
provided. Designers shall plan in eliminating the
most severe risks by safe-by-design solutions from
very early stages of design. To continue with the
defibrillator example given for early feasibility,
designers may decide to place all the interface
commands on the same (front) side of the device and
review standards for colours and icons at a
preliminary stage of the ideation. Alarms and
protections can be included during all iterations of
the formative stage even adjunct to safe-by- design
measures. For example while designing a software
interface of an electromedical device, the designers
may allow only an “admin” user (e.g. a qualified
medical professional) to set performance parameters
in a predefined interval, as based on state of the art
clinical guidelines. Then designers may place
adequate screens for password input as protection
measures for the “admin” access. Moreover, for all
interface screens designers may provide information
for safe use with reference to the allowed interval for
clinical parameters, tips to proceed to the next
clinically relevant step of the therapy and so forth.
4.2 Summative as Part of Device
Validation
The goal of device validation is to determine if the
device is adequate for its intended purpose and to
confirm its estimated risk benefit profile. No major
modifications are expected at this phase. While not
all parts of the interface may be subject to
summative, designers should plan to validate all the
critical ones. For example, summative assessment of
the interface for the assistance and maintenance
personnel of an electromedical device, when
personnel is directly trained by the device legal
manufacturer, may not be needed.
We propose to plan the summative evaluation by
mirroring activities of the late formative step, on the
final and frozen iteration of the interface. A
complete task analysis should be available and
checked for coherence to the user manual or
instruction leaflet. Moreover, if applicable to the
kind of device, also a complete use flowchart should
be available.
Summative evaluation should be performed with
real users and in a very well simulated or real use
environment, depending on device kind and ethics
considerations. During user tests, additional
techniques may be integrated to determine the length
of time needed for each task (by time-and-motion
studies) and the workload of the user.
It should be noted that, while very adequate for
summative activities, time of use and workload
assessment are not easily evaluated during formative
tests. The interface is still under modification and,
more often than not, the tasks may be interrupted for
Techniques for Usability Risk Assessment during Medical Device Design
211

clarifications and comments from the users, a very
common event if the participating users are aware
that the device is under development and not under
validation: most users are very keen to provide their
feedback and opinions as part of participatory design
activities. Interrupted and commented tasks disrupt
the workload assessment and the time estimation.
The outcome of the summative step is the
confirmation of all parts of the device interface,
including the information for safe use. No additional
risks should be encountered and all the foreseen
risks should be confirmed in terms of severity and
probability. Risk control measures should be
formally reviewed for final implementation and
effectiveness and the risk-benefit profile confirmed.
5 CONCLUSIONS
An integrated approach to usability and risk
management, while complex in general terms, can
be easily adapted to the design step, kind of device
under assessment and available resources. Designers
should be provided with a complete usability
toolbox and be able to choose a adequate tools for
each of their designs.
Integration of usability assessments in the wider
risk management leads to safer and more intuitive
medical devices, for the benefit of patient and
professional users alike.
While our group has tested this method in
multiple instances, we wish that it would be used
widely. With more experience, this method can be
refined, adapted to different cultural settings and
various technical skills, and updated with device-
specific tools. Moreover, this technique may be
integrated with the risk mitigation measures required
for the adequate management and protection of
patient data.
ACKNOWLEDGEMENTS
Authors acknowledge the UBORA “Euro-African
Open Biomedical Engineering e-Platform for
Innovation through Education” project, funded by
the European Union’s “Horizon 2020” research and
innovation programme under grant agreement No
731053.
REFERENCES
Brooke, J. (1996, September). SUS - A quick and dirty
usability scale. Usability evaluation in industry, 189, 4-7.
Council of the European Communities. (1993, July 12).
COUNCIL DIRECTIVE 93/42/EEC of 14 June 1993
concerning medical devices - ANNEX I. Official
Journal of the European Communities, 36, pp. 13-18.
European Parliament and Council of the European Union.
(2017, May 5). Regulation 2017/745 on medical
devices, amending Directive 2001/83/EC, Regulation
(EC) No 178/2002 and Regulation (EC) No 1223/2009
and repealing Council Directives 90/385/EEC and
93/42/EEC. Official Journal of the European Union,
pp. 1-175.
International Electrotechnical Commission. (2015). IEC
62366-1:2015 Medical devices -- Part 1: Application
of usability engineering to medical devices (1st ed.).
International Electrotechnical Commission. (2016). IEC
TR 62366-2:2016 Medical devices – Part 2: Guidance
on the application of usability engineering to medical
devices (1st ed.).
International Organization for Standardization. (2007).
ISO 14971:2007 Medical devices -- Application of risk
management to medical devices (2nd ed.).
International Organization for Standardization. (2016).
ISO 13485:2016 Medical devices -- Quality
management systems -- Requirements for regulatory
purposes (3rd ed.).
Kirwan, B., & Ainsworth, L. (1992). A Guide To Task
Analysis: The Task Analysis Working Group. Taylor &
Francis Ltd.
U.S. Food & Drug Administration. (2016, February 3).
Applying Human Factors and Usability Engineering to
Medical Devices. Guidance for Industry and Food and
Drug Administration Staff. Retrieved from (last access
Nov. 2018)
https://www.fda.gov/downloads/medicaldevices/.../uc
m259760.pdf
Shah, S., & Robinson, I. (2007).Benefits of and barriers to
involving users in medical device technology
development and evaluation.International Journal of
Technology Assessment in Health
Chung, P. H., Zhang, J., Johnson, T. R., & Patel, V. L.
(2003). An extended hierarchical task analysis for
error prediction in medical devices. AMIA ... Annual
Symposium proceedings. AMIA Symposium, 2003,
165-9.
APPENDIX
Tables.
BIODEVICES 2019 - 12th International Conference on Biomedical Electronics and Devices
212
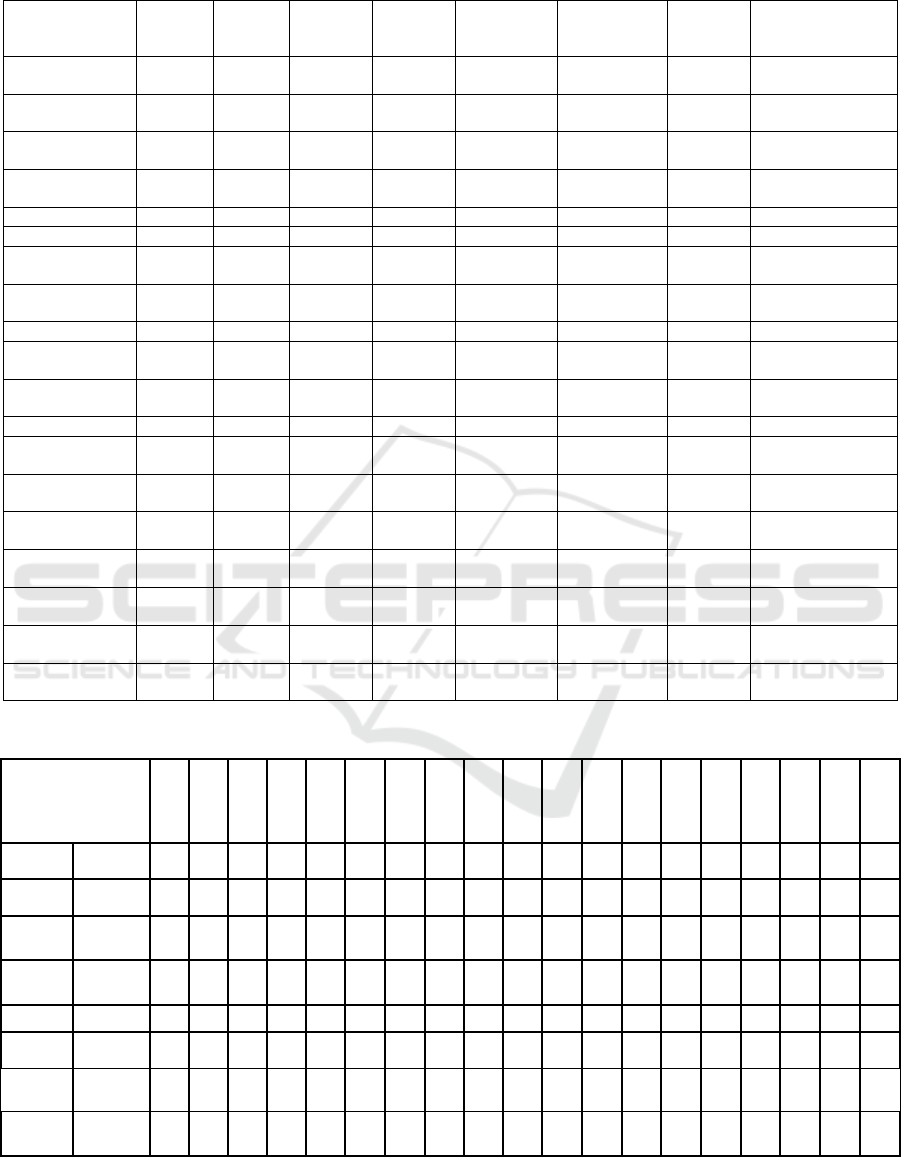
Table 1: Assessment of each evaluation technique according to predefined criteria.
Method as per
table E.1 of IEC
62366-2:2016
Involve
experts
Involve
real
user(s)
Time to
assess
Time to
report
Qualitative
results
(opinions)
Quantitative
results
(scores)
Depth of
analysis
Proposed for step
Advisory panel
reviews
Yes
No
Medium
Low
Yes
No
Low
Early formative
Brainstorm use
scenarios
Yes
No
Low
Low
Yes
No
Low
Early formative
Cognitive
walkthrough
Yes
No
Low
Low
Yes
No
Medium
Early formative
Expert reviews
Yes
No
Low
Low
Yes
No
Low
Early formative
and summative
FMEA and FTA
Yes
Yes
High
High
No
Yes
High
Late formative
Focus groups
No
No
Low
Low
Yes
No
Low
Early formative
Function
analysis
Yes
No
Medium
Low
Yes
Yes
High
Early formative
Heuristic
analysis
Yes
No
Medium
Medium
Yes
Yes
High
Late formative
Observation
No
Yes
Medium
Medium
Yes
Yes
Medium
Early formative
One-on-one
interviews
No
Yes
Medium
Medium
Yes
No
Medium
Early formative
and late formative
Participatory
design
Yes
Yes
Medium
Medium
Yes
No
Medium
Late formative
PCE analysis
Yes
Yes
High
High
Yes
Yes
High
Late formative
Simulation
Yes
Yes
High
High
Yes
Yes
High
Late formative and
summative
Standards
reviews
Yes
No
Low
Low
Yes
Yes
Medium
Early formative
Surveys
No
Yes
Low
Low
Yes
Yes
Low
Late formative and
summative
Task analysis
Yes
Yes
High
High
Yes
Yes
High
Late formative and
summative
Time-and-
motion studies
No
Yes
Medium
Medium
Yes
Yes
Medium
Late formative and
summative
Usability tests
Yes
Yes
High
High
Yes
Yes
High
Late formative and
summative
Workload
assessment
No
Yes
High
High
Yes
No
Medium
Late formative and
summative
Table 2: Assessment of each evaluation technique related to device kind.
Advisory panel
reviews
Brainstorm use
scenarios
Cognitive
walkthrough
Expert reviews
FMEA
Focus groups
Function
analysis
Heuristic
analysis
Observation
One-on-one
interviews
Participatory
design
PCA analysis
Simulation
Standards
reviews
Surveys
Task analysis
Time
-and-
motion studies
Usability tests
Workload
assessment
Method as per table
E.1 of IEC 62366-
2:2016
Implantable,
electro-
medical
e.g.:
implantable
defibrillator
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
No
Yes
May
be
Yes
Yes
Yes
No
Yes
No
No
No
Implantable,
not electro-
Medical
e.g.: heart
valve
Yes
Yes
Yes
Yes
Yes
Yes
May
be
Yes
No
Yes
May
be
May
be
Yes
Yes
No
Yes
May
be
May
be
No
Electro-
Medical for
professional
e.g: ecg
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
No
Yes
Yes
Yes
May
be
Electro-
Medical for
layperson
e.g: home
thermometer
Yes
Yes
Yes
Yes
Yes
May
be
Yes
Yes
Yes
No
May
be
Yes
Yes
Yes
May
be
Yes
May
be
Yes
No
Samd for
professional
e.g: surgical
planning
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
May
be
No
Yes
May
be
Yes
May
be
Samd for
layperson
e.g: app for
treatment
adherence
Yes
Yes
Yes
Yes
Yes
May
be
No
Yes
No
Yes
May
be
Yes
Yes
May
be
May
be
Yes
May
be
Yes
No
Not active
device-
professional
e.g:
nasogastric
tube
Yes
Yes
Yes
Yes
Yes
Yes
No
Yes
Yes
Yes
Yes
No
Yes
Yes
No
Yes
May
be
Yes
May
be
Not active
device-
layperson
e.g.: contact
lenses
Yes
Yes
Yes
Yes
Yes
May
be
No
Yes
No
No
May
be
No
Yes
Yes
May
be
Yes
May
be
Yes
No
Techniques for Usability Risk Assessment during Medical Device Design
213

Table 3: Risk identification related to each evaluation technique. Methods as per Table E.1 of IEC 62366-2:2016.
Advisory
panel reviews
By brainstorming and review of past experiences, panels may identify potentially hazardous situations,
assess probability and severity, describe the risk minimization measures present in the state of the art
Brainstorm
use scenarios
Designers involved in the brainstorming may identify user errors and misuse/abnormal use; designers
may also identify risk control measures
Cognitive
walkthrough
Designers involved in the brainstorming may identify usability pitfalls in the design and describe the
hazardous situations that may arise; designers may also identify risk control measures
Expert
reviews
Experts may point out usability strengths and pitfalls during their review. Usability pitfalls may then be
linked to the hazardous situations; designers may also identify risk control measures. We recommend
that experts answers to questions as per ISO 14971 ann. C
FMEA and
FTA
FMEA technique is a very thorough method for the identification of risks. We recommend that this
method is used in conjunction with a very detailed task analysis
Focus groups
During a focus group, designers may guide the discussion with users leading to the identification
usability pitfalls in the design and describe the hazardous situations that may arise; designers may also
identify risk control measures and ask participants to the focus group to comment the proposed measures
Function
analysis
A functional flow diagram, commented with the identification of machine functions and user functions
may be used in conjunction with a FMEA technique for a thorough identification of risks
Heuristic
analysis
During heuristic analysis, usability experts may use heuristic principles to identify and give usability
scores to usability pitfalls. They may describe the hazardous situations that may arise; designers may
also identify risk control measures and ask experts participating to the heuristic review to comment the
proposed measures and score their capability to lower the risk
Observation
During observation, designers may identify user uncertainties or errors; root cause should be discussed
with the users to ensure that the hazardous situation is well understood by the designers; we believe that
observation alone cannot provide sufficient information regarding risk and that it should be backed up
with interviews or surveys as a de-brief activity
One-on-one
interviews
Interviews are useful when used in conjunction with techniques involving users that perform actual
tasks on the device, from observation to cognitive walkthrough to usability tests. Interviews are best
used as de-briefing activities as they allow to identify not only the hazardous situations, but also their
root causes
Participatory
design
Partecipatory design very powerful tool when used in conjunction with techniques involving users that
perform actual tasks on the device, from observation to cognitive walkthrough to usability tests.
Focused on defining risk mitigation measures and their perceived effectiveness
PCA analysis
PCA analysis can be integrated in the task analysis and therefore in the FMEA analysis to provide a
complete evaluation of risk; most applicable to complex tasks and/or interfaces
Simulation
We believe that simulation is one of the core techniques, as it can easily be adapted to all devices thanks
to the use of mockups, dummies, animal models and other simulated settings. This allows the planning
of all usability assessment activities in a cost-effective and ethical fashion
Standards
reviews
We believe that standards review should be applied whenever an internationally recognized document is
available, be it an ISO norm, a guideline from a scientific society, a local procedure. Non- fulfillment of
standard requirements is a potential source of significant risk
Surveys
Surveys are useful tools in some situations, where the use of the medical device is difficult to observe;
typically if it is used by the layperson as part of private life (contact lenses, in vitro testing for
pregnancy, and so on). Surveys are not adequate to investigate root causes of hazardous situations
Task analysis
Task analysis is the most powerful tool for linking usability assessment to risk management. It is best
used as an input to the FMEA technique but can also be used during preliminary steps of the device
design to determine the user needs and consequent testable technical requirements. Non fulfillment of
one of those requirements shall be treated as significant risk
Time-and-
motion
studies
We believe that time and motion studies are most adequate to assess risk of those devices in which the
time of execution is a risk control measure, e.g. If a fast execution improves patient safety (for example,
lowering chances of bacterial contamination or improving chances of patient recovery)
Usability
tests
Usability tests are a very powerful tool to determine those risks that not identified by the designers,
using techniques that do not directly involve users (such as brainstorming, standard reviews…).
Usability tests allow to estimate the probability of an hazardous situation; they also allow designers to
consolidate the task list
Workload
assessment
Workload assessment reviews may allow designers to identify some kind of use errors related to
overload or environmental distractions; we believe that this technique is most appropriate when
professional users are involved, as they are more prone to burn out and also more aware of the impact of
overload on their performance at work
BIODEVICES 2019 - 12th International Conference on Biomedical Electronics and Devices
214
