
MEV: Visual Analytics for Medication Error Detection
Tabassum Kakar
1,2
, Xiao Qin
1,2
, Cory M. Tapply
1
, Oliver Spring
1
, Derek Murphy
1
, Daniel Yun
1
,
Elke A. Rundensteiner
1
, Lane Harrison
1
, Thang La
2
, Sanjay K. Sahoo
2
and Suranjan De
2
1
Department of Computer Science, Worcester Polytechnic Institute, Worcester, Massachusetts, U.S.A.
2
Center for Drug Evaluation and Research, U.S. Food and Drug Administration, Silverspring, Maryland, U.S.A.
{thang.la, sanjay.sahoo, suranjan.de}@fda.hhs.gov
Keywords:
Visual Analytics, Treemaps, Drug Surveillance Reports, Pharmacovigilance.
Abstract:
To detect harmful medication errors and inform regulatory actions, the U.S. Food & Drug Administration uses
the FAERS spontaneous reporting system to collect medication error reports. Drug safety analysts, however,
review the submitted report narratives one by one to pinpoint critical medication errors. Based on a formative
study of the review process requirements, we design an interactive visual analytics prototype called Medication
Error Visual analytics (MEV), to facilitate the medication error review process. MEV visualizes distributions
of the reports over multiple data attributes such as products, types of error, etc., to guide analysts towards
most concerning medication errors. MEV supports interactive filtering on key data attributes that aim to help
analysts hone in on the set of evidential reports. A multi-layer treemap visualizes the count and severity of the
errors conveyed in the underlying reports, while the interaction between these layers aid in the analysis of the
corresponding data attributes and their relationships. The results of a user study conducted with analysts at the
FDA suggests that participants are able to perform the essential screening and review tasks more quickly with
MEV and perceive tasks as being easier with MEV than with their existing tool set. Post-study qualitative
interviews illustrates analysts’ interest in the use of visual analytics for FAERS reports analysis operations,
opportunities for improving the capabilities of MEV, and new directions for analyzing critical spontaneous
reports at scale.
1 INTRODUCTION
A medication error is a preventable event that may
cause or lead to inappropriate medication use or
patient harm while the medication is in the control
of the health care professional, or patient. Every
year, serious preventable medication errors occur
in 3.8 million inpatient admissions and 3.3 million
outpatient visits with an estimated annual cost burden
of $20 billion (err, 2010). A medication error involves
mistakes that are caused by wrong administration or
handling of drug due to ambiguity of drug label or
carton. Hence, these errors are preventable and should
be detected and corrected earlier to avoid further
damage.
To be able to take immediate regulatory actions
towards the medical products that are prone to
harmful medication errors, the U.S. Food &
Drug Administration (FDA) uses the Adverse
Event Reporting System, FAERS in short, to
collect medication error reports from health care
professionals, consumers, and drug manufacturers.
At the FDA, the Division of Medication Errors
Prevention and Analysis (DMEPA) is responsible for
ensuring the safe use of medications by minimizing
use errors related to the drugname, such as drugnames
that sound or look similar, labeling, packaging, or
design. It is their responsibility to monitor and
analyze reports about medication errors submitted via
FAERS to identify concerns that can be addressed
through regulatory action. These actions may include
revising container labels or instructions for use,
communicating safety issues to the public, and in rare
cases, changing a proprietary drugname.
A safety analyst may determine that a reported
incident corresponds to a more general medication
error concern that may potentially warrant a label
change, drug withdrawal, or other similar action.
Such incident report is then evaluated based on
various factors including the severity, type and the
cause of the error. This evaluation tends to require the
analysis of many other reports over a longer period of
time. In these reports, some useful information such
as demographics of the affected patients are explicitly
72
Kakar, T., Qin, X., Tapply, C., Spring, O., Murphy, D., Yun, D., Rundensteiner, E., Harrison, L., La, T., Sahoo, S. and De, S.
MEV: Visual Analytics for Medication Error Detection.
DOI: 10.5220/0007366200720082
In Proceedings of the 14th International Joint Conference on Computer Vision, Imaging and Computer Graphics Theory and Applications (VISIGRAPP 2019), pages 72-82
ISBN: 978-989-758-354-4
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

captured in the structured fields associated with each
report, while the details of the error are discussed
in-depth only in the text narrative itself. Statistics
about FAERS reports can also be important. For
example, to understand how severe an error is, the
analysts may want to know how its severity compares
to that of a similar type of error within the overall set
of reports.
Currently, drug safety analysts at the FDA
use tools that are supported by Structured Query
Language (SQL) to retrieve reports from FAERS
that refer to their assigned set of products. The
information about a specific error is gathered by
reading through each narrative report. They may
alternatively use SQL to collect basic statistics about a
collection of reports, e.g., they may compute the total
number of reports for a given error type in the last
two weeks or the age distribution of those affected by
this error (e.g., to determine if an older population is
disproportionately affected).
Such mechanisms become problematic as the
volume of reports grows. First, a systematic
method of exploring and categorizing reports based
on their content is missing. Second, information
embedded in the unstructured narrative text can only
be extracted manually. This manual information
extraction from text is inefficient, time consuming
and cognitively demanding. Third, no comprehensive
representation that conveys the overall global
statistics of the suspected errors or products with
respect to different subsets of FAERS reports is
available. Our overarching objective is to design
interactive visualization and analytics techniques to
address these shortcomings.
To design Medication Error Visual analytics
(MEV) tool, we first characterize the current practices
in medication error detection and prevention through
formative interviews with drug safety analysts at the
FDA. This leads us to gain an understanding of the
analysts’ pain points and limitations of current tools.
We then utilize these insights to guide the design of
MEV. The result is MEV– a visual analytics approach
that aims to support the exploration and analysis
of medication error reports. MEV first extracts
key information about the reported incident from
the respective text narrative using recently developed
biomedical natural language processing techniques
(Savova et al., 2010; Xu et al., 2010; Aronson and
Lang, 2010; Wunnava et al., 2017). MEV then
displays this information along with other attributes
associated with a given report such as drugnames
on the treemap visualization (Fig. 2). MEV
provides several visual interactions aim to help safety
analysts sift through these reports to uncover pertinent
information about suspected medication errors.
MEV defines criticality scores for different types
of medication errors based on the severity of the
error and the count of reports reflecting that same
error. This information is encoded in visual features
of the visualization, such as the shape and size of the
treemap components making the severe reports more
quickly discernible as compared to less severe ones.
A timeline view allows analysts to see the overall
distribution of the reports over a period of time.
Demographic displays enable visual analytics based
on the structured information from FAERS reports
such as age, gender and occupation. These interactive
visualizations are intended to allow analysts to see
faceted distributions of the patient characteristics for
selected drugs or errors. Analysts can interactively
choose particular attributes and analyze the resulting
reports.
A user study with 10 drug safety analysts at
the FDA , who were not involved in the design
process of MEV, suggests that performing several
common review related exploration tasks with MEV
is faster and easier than their existing tool. Further,
qualitative interviews show participants’ enthusiasm
regarding the use of visual analytics for medication
error detection and highlight opportunities for future
improvements.
2 RELATED WORK
We study existing techniques that align with our data
type and goals. The key data elements extracted
using NLP such as type and cause of an error
are categorical, called facets. Facets have been
widely used as interactive filters for searching and
browsing data. FacetMap (Smith et al., 2006)
supports interactive visualizations to explore facets of
a dataset, however, it does not support discovering
relationships among facets. FacetLens (Lee et al.,
2009) extends FacetMap to help users observe trends
and explore relationships within faceted datasets.
Most of these faceted systems (Lee et al., 2009;
Smith et al., 2006) divide their interfaces between a
main viewing area and a secondary facet area which
allows to browse only one data item at a time. For
medication error screening, however, it is crucial to
see the effect of selection of one item on others, so
that data points representing concerning errors can be
identified quickly.
Treemaps (Asahi et al., 2003) have been
widely used in visualization systems (Liu et al.,
2009; Harrison et al., 2012). For example,
SellTrend (Liu et al., 2009), a visualization tool
MEV: Visual Analytics for Medication Error Detection
73

for displaying temporal categorical data, displays
transaction failures using treemaps. NV (Harrison
et al., 2012) utilizes treemaps and histograms to allow
security analysts to discover, analyze, and manage
vulnerabilities on their networks. However, these
tools do not have support for extracting name entities
from textual data, neither do they visualize temporal
patterns and demographics within the data. JigSaw
(Stasko et al., 2008), on the other hand, is a powerful
tool for investigating text data by visualizing name
entities and their relationships to reveal hidden plots
in criminal reports. However, there is a need to
support temporal data analysis for reports screening
and review.
In the medical domain there has been work
on designing systems to avoid medication
errors from arising in the first place, such as
medication-reconciliation tools (Ozturk et al., 2014)
and clinical information systems (Jia et al., 2016).
Varkey et al. (Varkey et al., 2007) study the effect of
interventions on decreasing medication errors related
to the administration of drugs. A patient’s one year
long prescription history is visualized using timeline
charts to be used by clinicians and the emergency
room staff (Ozturk et al., 2014). Other tools are
designed as interfaces to provide a user-friendly
mean of error reporting (Singh et al., 2008). Clinical
decision support systems have been proven to reduce
medication errors during prescription (Jia et al.,
2016). However, these tools are designed with the
goal of reducing medication errors from happening
in the first place during the prescription or the
administration of the drugs.
Our work instead starts after the medication errors
have already occurred and have been reported to the
concerned authorities such as the FDA. For example,
if two drugs have look-alike carton labels for different
dosages and FDA receives error reports about these
dosages being prescribed interchangeably. Then FDA
drug safety analysts after careful examination of such
reports can recommend to change the product carton
label so that different products or dosages can be
differentiated easily. This prevents such errors from
happening in the future. To the best of our knowledge,
no visual analytics tool exists that can be used to help
analysts explore medication error reports.
3 REQUIREMENT ANALYSIS
Before designing a system for medication error
analysis, we conducted formative interviews with the
FDA drug safety analysts to understand their data,
current workflow, exiting tools for reports review and
their limitations and challenges.
3.1 Interviews with Domain Experts
We organized a series of semi-formal interview
sessions with five drug safety analysts at the
Division of Medication Error Prevention and Analysis
(DMEPA) at the FDA. Our primary objective was
to understand the current report review process and
to identify the challenges these analysts face in
analyzing medication error reports. From these
interviews, we learned that certain information was
critical to their workflow. We also observed the
limitations of current tools.
To develop and refine the design of the specific
visualizations MEV uses, we showed these analysts
sketches of design alternatives, such as parallel
coordinates and variations of node-link diagrams.
This activity helped us gather additional design
requirements, such as readability of the visualization.
In subsequent interviews, we presented these analysts
with a working prototype of MEV to evaluate their
perceptions of the degree to which MEV meets their
needs, and to receive further feedback on the visual
and interaction design. In the final session, a larger
group of analysts (ten), who were not involved in the
design process of MEV, participated in the user study
to evaluate MEV and provide additional insights on
the utility of MEV.
3.2 FAERS Data Description
We briefly describe the data reviewed by FDA
analysts based on our initial discussions with the
domain experts. FDA maintains an Adverse
Event Reporting System (FAERS) (FDA, 1995)
as a part of its post-marketing drug surveillance
program for medications and therapeutic biologic
products. Reports submitted to FAERS include
mandatory reports submitted by drug manufacturers
and voluntary reports submitted by health care
professionals and consumers. These reports are
semi-structured in nature, that is, they contain
structured information about patient demographics,
drugs taken, therapies, and adverse reactions
or medication errors. They also contain an
unstructured textual narrative that describes the
incidents associated with medication errors or adverse
reactions in detail and contains richer information
such as the details of the incident to help analysts
decide if the incident is worthy of investigation.
Majority of the key information used in the analysis
is categorical, with drugs having the highest number
of categories (50-100) per analyst.
IVAPP 2019 - 10th International Conference on Information Visualization Theory and Applications
74

Safety analysts review reports based on the
classes of products assigned to them. On average
a safety analyst can have 50-100 medical products
and reviews 200 reports on average on a weekly
basis. However, for detail analysis of a product
thousands of reports are retrieved for several months.
These numbers vary from team to team based on the
assigned products. For example, a new approved
product might be causing more medication errors as
compared to an old product that has been in market
for a long time and people are familiar with its proper
usage.
Natural
Language
Processor
Structured
Information
Unstructured
Information
MEV Visualizer
FAERS
Reports
FAERS
Database
Query
Executer
MEV Data
Store
Figure 1: The MEV framework.
3.3 Domain Experts Workflow
At the FDA, drug safety analysts review FAERS
reports daily for potential severe medication errors.
The analysts receive reports related to the drugs
assigned to them. The first task is to screen the reports
for potential signals based on severe outcomes such
as hospitalization or death, age groups, products and
error types. Then the report narrative of the prioritized
errors are analyzed. Once any alarming error is
found, then other sources are analyzed to investigate
a particular error.
3.4 Limitations of Current Tools – MEV
Design Rationale
Currently, existing tools at the FDA are mostly
SQL based. That is, they allow finding reports
related to an age group or a product. However,
our interviews revealed that the tool lacks the ability
to interactively guide the user towards most critical
errors and ultimately help them in forming hypothesis
about a potential dangerous medication error. That
is, by using the current tools it is not trivial to
analyze data distributions and relationships among
core attributes. Particularly, it is cumbersome to ask
questions such as which errors are associated with
a particular product, or which age group is more
prevalent in the reports associated with a particular
error? Moreover, questions such as what stage a
particular error is happening at? or what are most
reported root causes of error? are not supported by
the tool at all.
The reason is that first, the key information such
as stages and causes of error must be learned from
the text narrative, as this information is not stored in
the structured fields associated with a given report.
Second, these existing tools are only designed to help
analysts filter a set of reports using the structured
information, which can be analyzed further using
Microsoft Excel spreadsheets.
Therefore, an automatic way is needed to first
extract this information from text narratives and
then to allow analysts to interactively query this
information along with other structured information
to make the report review process efficient. Our MEV
system is designed to assist analysts in this signal
screening phase by supporting the analysis of data
distributions to help in hypothesis formation about
critical errors.
3.5 The MEV Framework
Following the workflow of domain analysts, MEV
depicted in Fig. 1 is designed to explore the reports
efficiently. As described earlier, FAERS reports
contain both structured as well unstructured text
narrative explaining the event in detail. In case
of medication errors, the core information related
to the type or cause of the errors is not captured
in the structured parts of the report. Instead,
it tends to be mostly mentioned within the text
narrative. To support analysts in finding important
information concerning medication errors quickly,
we use rule-based name-entity recognition techniques
(Wunnava et al., 2017) to extract key information
from the text narrative.
We use domain specific lexicons (NCC-MERP,
1995; Brown et al., 1999) to extract key data
attributes. These attributes include types of
medication errors (e.g., taking a wrong drug or
dosage), the root causes of the errors (e.g., name
confusion and container label confusion), and the
stage in which error has occurred (e.g., dispensing and
administration). The Natural Language Processor
(Fig. 1) after preprocessing the text, such as stemming
and tokenizing, extracts these core data elements.
This extracted information is then standardized
by mapping it to NCC-MERP terms using edit
distance based string matching (Du, 2005) for
smooth exploration and analysis. Currently, analysts
manually summarize each narrative by adding these
terminologies into the Excel spreadsheet. After
standardization, on average each of the extracted
entity contain approximately 15-20 categories.
The extracted information along with structured
information about demographics is stored in the
MEV: Visual Analytics for Medication Error Detection
75
a
b
c
d
20,234
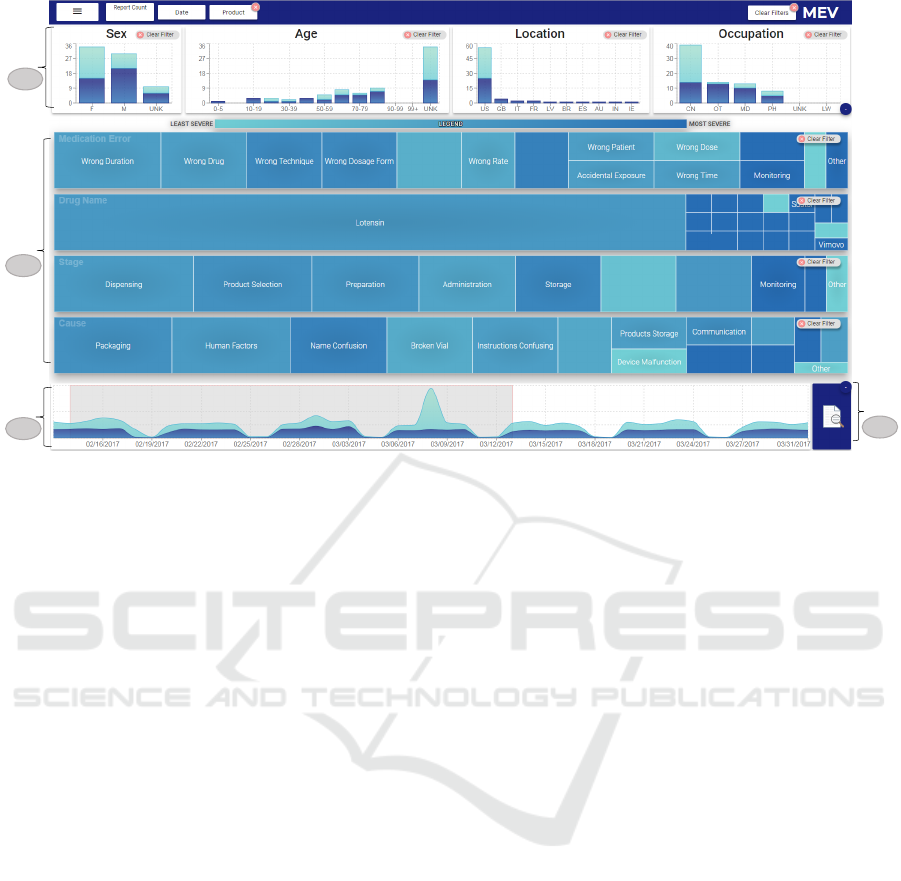
Figure 2: The user interface of MEV (a) The demographics panel. (b) The treemap panel. (c) The timeline panel. (d) Reports
icon to access the reports view to analyze the associated report narratives.
MEV Data Store (Fig. 1). The MEV Query
Executor handles processes requests on the data
store specified through online MEV visual interface.
Results from frequent interactions are cached to
improve user experience. The MEV assists analysts
in exploring the data interactively using linked
interactive visualizations described below.
4 MEV INTERFACE OVERVIEW
Our MEV tool consists of four main interactive
displays (Fig. 2), the treemap view, the demographics
panel, the timeline panel and the reports view.
4.1 The Treemap Panel
A treemap visualization (Fig. 2b) displays the
distribution of each of the multi-value categorical
attributes extracted from structured data as well as
unstructured text. These attributes include drugname,
the root cause of the error, the stage where the error
has occurred, and the error type. Each of these
attributes have multiple values. In each treemap,
each rectangle represents a data value within an
attribute, e.g., for the product treemap, each rectangle
represents a drugname. The size of each rectangle is
mapped to the count of reports related to that specific
data value, while the color depicts the count of severe
outcomes which is a structured data field.
This treemap design allows analysts to
interactively filter even large number of items,
such as a large number of drugnames can be
visualized in a compact way (Liu et al., 2009). The
analyst can select one or multiple data values on each
treemap and the system will immediately show what
other data attributes correspond to a selected value.
This direct manipulation of data allows the analysts
to narrow down their search based on the items
distributions that need the most attention, which may
be achieved through multiple tidy steps using their
current tools.
Although, treemaps are often used to visualize
hierarchical data, here we leverage the capability of
displaying categorical data as well as showing many
values though space filling techniques. Another
advantage of treemaps is their ability to effectively
make use of both size and color for encoding
additional properties about each categorical choice.
While alternate multi-dimensional visualization
techniques, such as parallel coordinates or scatter
plot matrices are possible, for scalability and avoiding
visual clutter, treemaps are used to guide analysts
in the screening of their assigned reports. Treemaps
are one possible design, but other design choices
including bar-charts or lists (Stasko et al., 2008)
having similar functionality may have desirable
properties.
4.2 The Timeline Panel
The timeline panel (Fig. 2c) displays the overall
report distribution as well as their severity over a
IVAPP 2019 - 10th International Conference on Information Visualization Theory and Applications
76
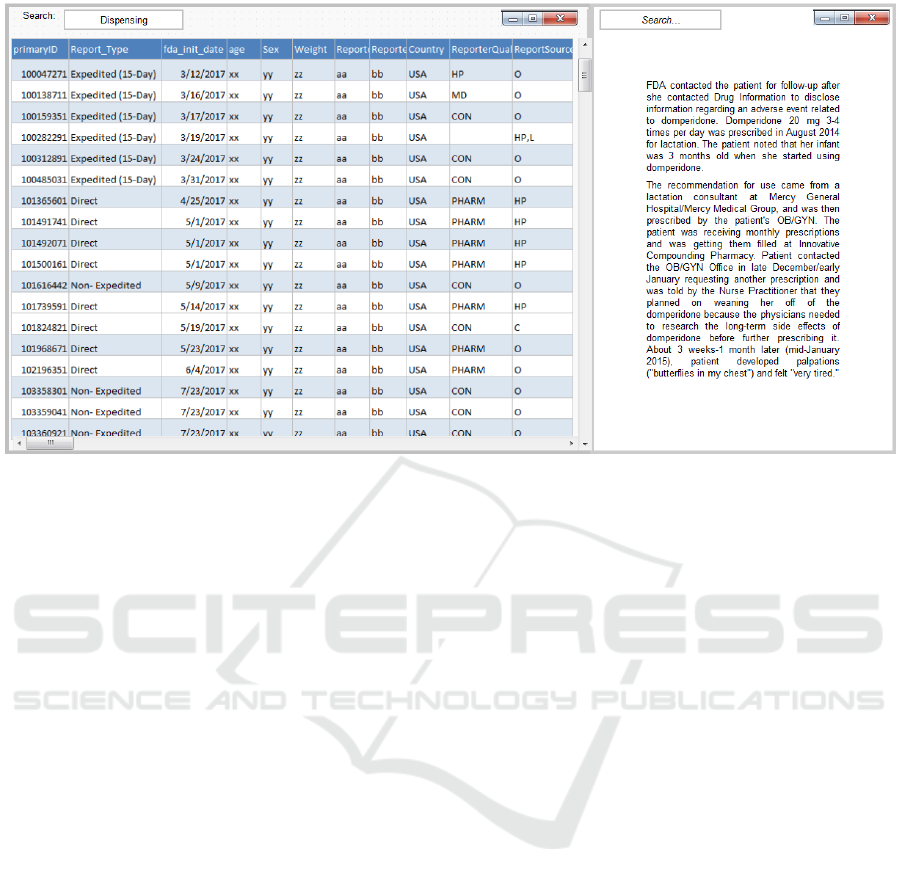
Figure 3: Reports view with no personal information (Left). Example of a de-identified text narrative (Right).
period of time using a temporal area chart. This
allows us to detect a spike in the severity associated in
the incidence of certain products. Interactive brushing
and selection through zooming is provided to allow
the safety analysts to drill into a particular date range
and explore the associated reports. Once a date range
is selected, other displays are updated to reflect only
data from the selected date range.
4.3 The Demographic Panel
The demographics of patients also play an important
role in the analysis of the reports. For instance,
for a particular drug there might be many more
severe outcomes in a particular age group than in
the other groups. The graphs in the demographics
panel (Fig. 2a) assist the analysts in selecting reports
related to a particular demographic attribute, such
as, location, gender or age group. Drug safety
analysts can not only prioritize reports based on these
attributes to hone in on respective reported medication
errors, but they can also upon selecting any data value
immediately view the distribution of reports for each
demographic view through linked displays.
4.4 The Reports View
After safety analysts select a particular product or
medication error of interest, they can view the
respective reports and investigate them further to find
if the reports indeed are indicative of errors with
serious consequences for patient health warranting
regulatory action. For this, by clicking on the reports
icon (Fig. 2d) the selected reports are accessible. The
reports view displays the line listing of the screened
data elements (Fig. 3-left). Analysts can drill into the
narrative of each report to further examine the report
in great detail (Fig. 3-right).
4.5 System Implementation
MEV is a web based tool developed using React
and JavaScript for front-end and PostgreSQL for the
back-end database. The tool also leverages a cache
(Redis) for efficient data retrieval and to improve user
experience. The extracted data elements are stored in
the database along-with other structured information.
5 EVALUATION
5.1 Pharmacovigilance Usage Scenario
To understand how MEV can help with reports
screening, we now discuss a use case of Alex, a
drug safety analyst, reviewing reports related to her
assigned products using MEV. From the timeline
panel, she sees an overall weekly distribution of the
number of new reports received over this last month.
At a glance, she can see in Fig. 2 that no reports
have been submitted over the weekend, while new
reports have been received during weekdays. She
explains that the FDA does not populate any reports
into the database during the weekend. She notices a
MEV: Visual Analytics for Medication Error Detection
77
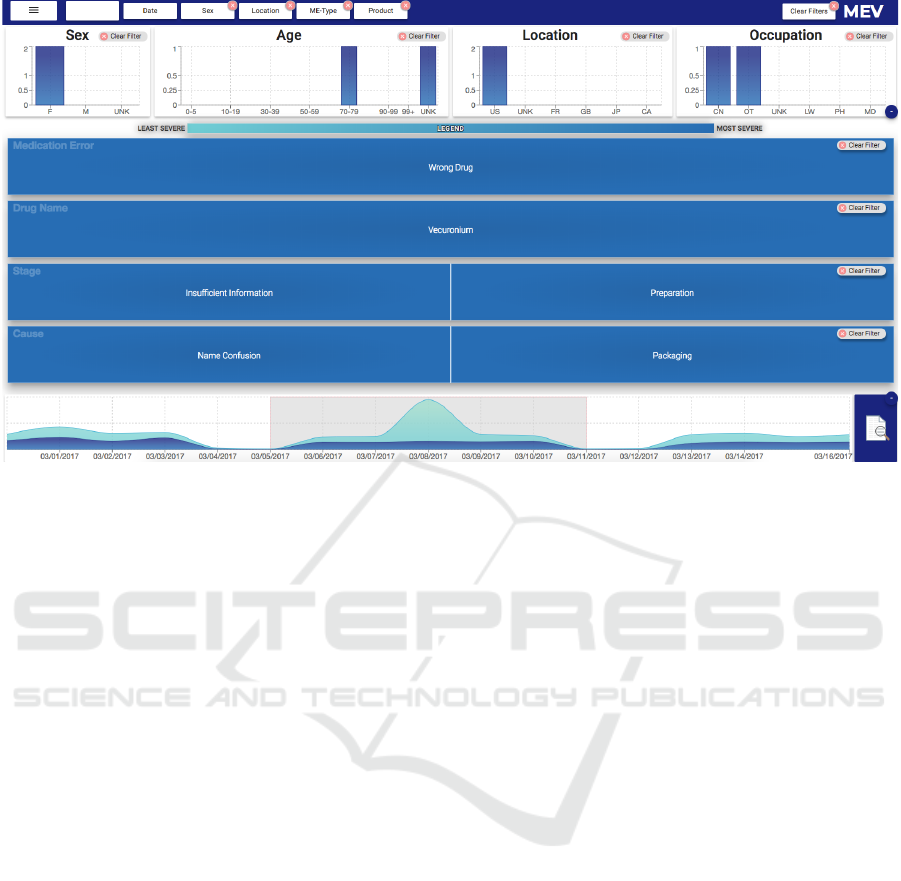
Report Count
49
Figure 4: MEV with selection of date range, demographics, drugname and medication error type.
spike in the number of reports between ”3/5/2017 -
3/11/2017” of which 39% of reports are severe and
61% are non-severe reports (Fig. 4-bottom). She
decides to investigate reports by selecting this week
using the brush tool on the timeline panel. She
observes that the number of reports for this one week
are 28,123. The demographics and treemap charts
both are updated for the selected date. She notices
there are more female patients than male and the age
group is mostly between “30-80” years old. That
is expected, as her assigned products are mostly for
elderly women. From the demographics, she selects
females with a location in the U.S. to see the reported
drugs and errors. This reduced the target set to 11,174
reports.
On the treemap, she now notices that the
medication error “wrong-technique” has most of
the count with severe outcomes. She questions
which products are administered with this
“wrong-technique” error. Alex thus selects
wrong-technique in the first treemap by clicking
on the rectangle labeled ‘wrong-technique’. This
reduces the reports count to 2,786 reports. She
observes the reported drug Lotensin has the highest
number of severe reports. She selects Lotensin from
drugnames on the second treemap. Now she wants
to know what causes this “wrong-technique” error
in Lotensin products and at which stage these errors
arise. Looking at 3rd and 4th treemaps corresponding
to the cause and stage of errors respectively, she
notices that most have causes such as “name
confusion” and “packaging”. She adds “It seems an
error in preparation of the drug”. She also observes
that a total of 49 reports remain that she needs to
analyze in detail (Fig. 4). She speculates whether
these reports indeed have compelling evidence about
these errors. She clicks on the reports icon (Fig. 2d)
to read the details of each narrative in the reports
view (Fig. 3). Hence, MEV interactively guides
the analyst towards concerning errors by supporting
exploration and screening of reports.
5.2 User Study
5.2.1 Study Design
We invited eleven drug safety evaluators (10 females,
1 male) at the Division of Medication Error and
Prevention Analysis (DMEPA) at the FDA for a one
hour in-person study session. One of the participants
withdrew participation. These participants were
within the age range of 30-50 years with the majority
having experience with basic visualizations. These
participants were pharmacists, conducting regular
report reviews to identify any medication error that
would need regulatory action.
Assessment Measures. We specified a set of nine
tasks (Table. 1) commonly performed during the
report review process to evaluate the usefulness of
MEV. These tasks were derived from the initial
interviews conducted with the users to understand
the review workflow. These tasks varied from a
one-step task of finding a particular attribute value
IVAPP 2019 - 10th International Conference on Information Visualization Theory and Applications
78
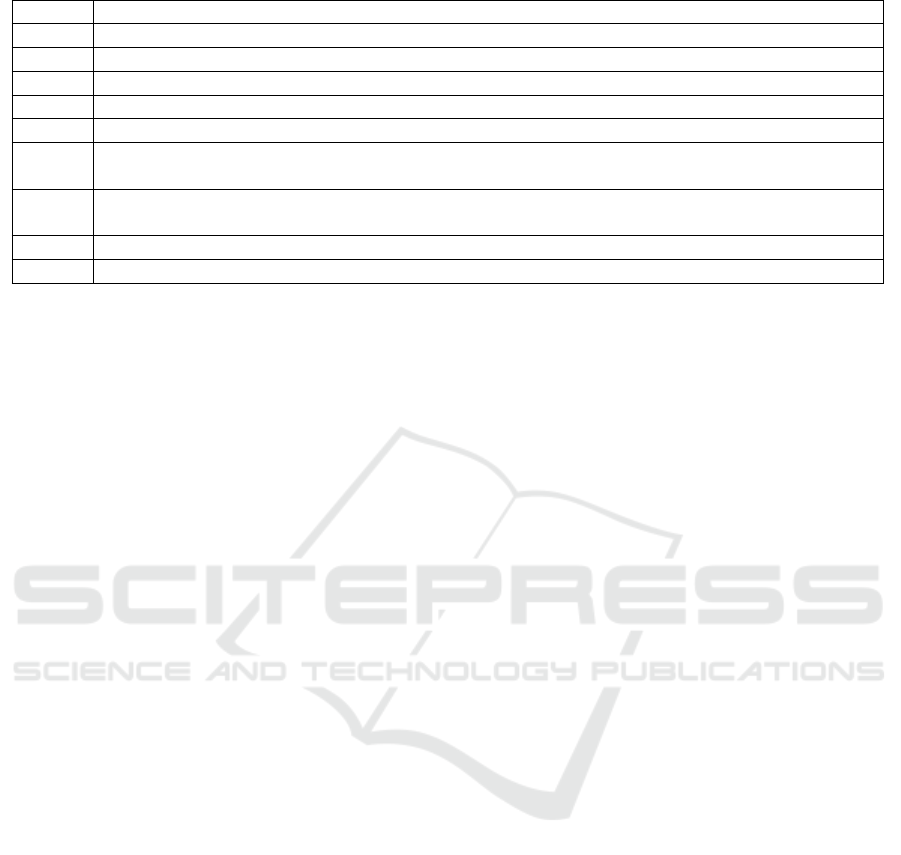
Table 1: List of 9 Tasks designed to evaluate the effectiveness of MEV.
Task # Description
T1 How many total reports have been reported during a time period?
T2 Which medication error is reported the most for a time period?
T3 Which drug has most severe outcomes for a selected medication error?
T4 Which gender and age have most severe outcomes?
T5 Which age group is most prevalent in reports related to a selected product?
T6 What are the two most frequent medication errors reported with a select product, age group, and
gender?
T7 Given the report distribution of a drug for female patients with a specified age group, what are
the critical medication errors that need to be analyzed?
T8 What are the two most frequent root causes of error for a selected drug and medication error?
T9 What are the two most common reported stages of errors for a drug and a medication error?
(T1-T2) to two-step tasks of finding reports associated
with analysis of two attributes (T3-T5). We included
multi-step tasks of finding interesting reports to be
prioritized based on the distribution of multiple data
attributes (T6-T9). These composite tasks involved
filtering based on examination of relationships among
data attributes. We considered two metrics, one, time
to successfully complete each task and two, how easy
the participants rated each task. Data loading in their
existing tool takes longer time, so the task completion
time was recorded after FAERS data for one week
(from 2017) was loaded in both tools. The perceived
ease from each task was recorded on a 5-point Likert
scale (5 extremely easy and 1 extremely difficult).
We reported the time taken by each participant to
successfully accomplish each task. Participants were
asked to perform the same set of tasks with their
existing tool (Control) as well as MEV to compare
both tools.
Study Procedure. To get detailed feedback from
the participants and observe them closely interacting
with the system, the study was conducted via a one
hour in-person interview session. Upon successful
completion of the demonstration and training session
(20 minutes), the participants were asked to perform
the set of prescribed tasks (Table. 1) using the FDA
adverse event reporting (FAERS) data from 2017
using both the MEV tool as well as their existing
tool. At the end of each session, participants were
provided with a post-study questionnaire, which was
not timed. The first section of the questionnaire
contained questions related to the demographics
of the participant such as age and gender. The
second part had questions about the usability (Brooke
et al., 1996) of MEV on a 5-point Likert scale
(5 strongly agree & 1 strongly disagree). Finally,
an open-ended questionnaire was offered to solicit
qualitative feedback about MEV.
Analysis. For some tasks, the time and perceived
ease score collected from the study were not normally
distributed. Hence, to find out whether performing the
prescribed tasks is quicker and easier with MEV than
the existing tools, we performed the non-parametric
Mann-Whitney U Test (Wilcoxon Rank Sum Test)
to compare conditions. We also report the 95%
confidence intervals for both time as well as perceived
ease score for all tasks.
5.2.2 Study Results
We now analyze the participants’ performance on
the tasks and their response about the overall system
usability.
Quantitative Analysis: From Table. 2, we see
that for majority of the tasks, there are significant
differences between the recorded time and perceived
ease score for completing them using our proposed
system and their existing tool control with the
exception of T1. T1 was a one-step task involving
finding the total number of reports for a given duration
of time. One possible explanation for this difference
is that participants were used to their current tool and
knew exactly where they will find this information.
On the other hand, being new to MEV tool they
took little longer (M=5.11 seconds [3.47, 8.76])
as compared to their current tool (M=3.62 [1.80,
5.44]). This task was also scored easier under
control condition than using MEV. Neither time nor
perceived ease were significantly different for T1. T2
involved finding the most reported medication errors
for a selected time period. There was significant
difference between the performance under control
condition (M = 7.54 seconds [3.57, 11.52]) and using
MEV (M =31.84 [15.78, 47.91]). In addition to time,
participants also found it easy to perform the task
using MEV (M = 4.9 [4.70, 5.10]) than under control
MEV: Visual Analytics for Medication Error Detection
79
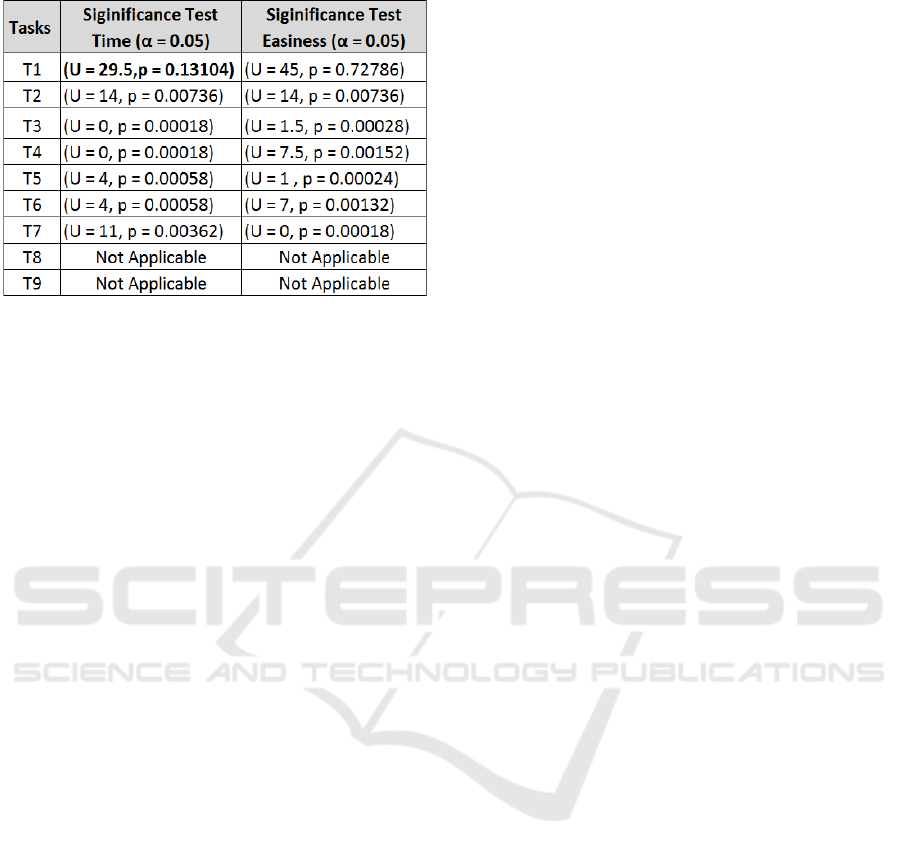
Table 2: U-Test for both time and perceived ease.
condition (M=4.0 [3.59, 4.41]).
For the multi-step tasks (T3-T7), that involved
retrieving data based on analyzing distribution and
severity across multiple attributes, both time and
perceived ease have significant differences (Table. 2).
Tasks T8 and T9 involved composite filtering to
retrieve the root causes and stages of errors related to
severe outcomes. As these data entities were extracted
using NLP and their current tools do not provide
them, the comparison was not possible. Additionally,
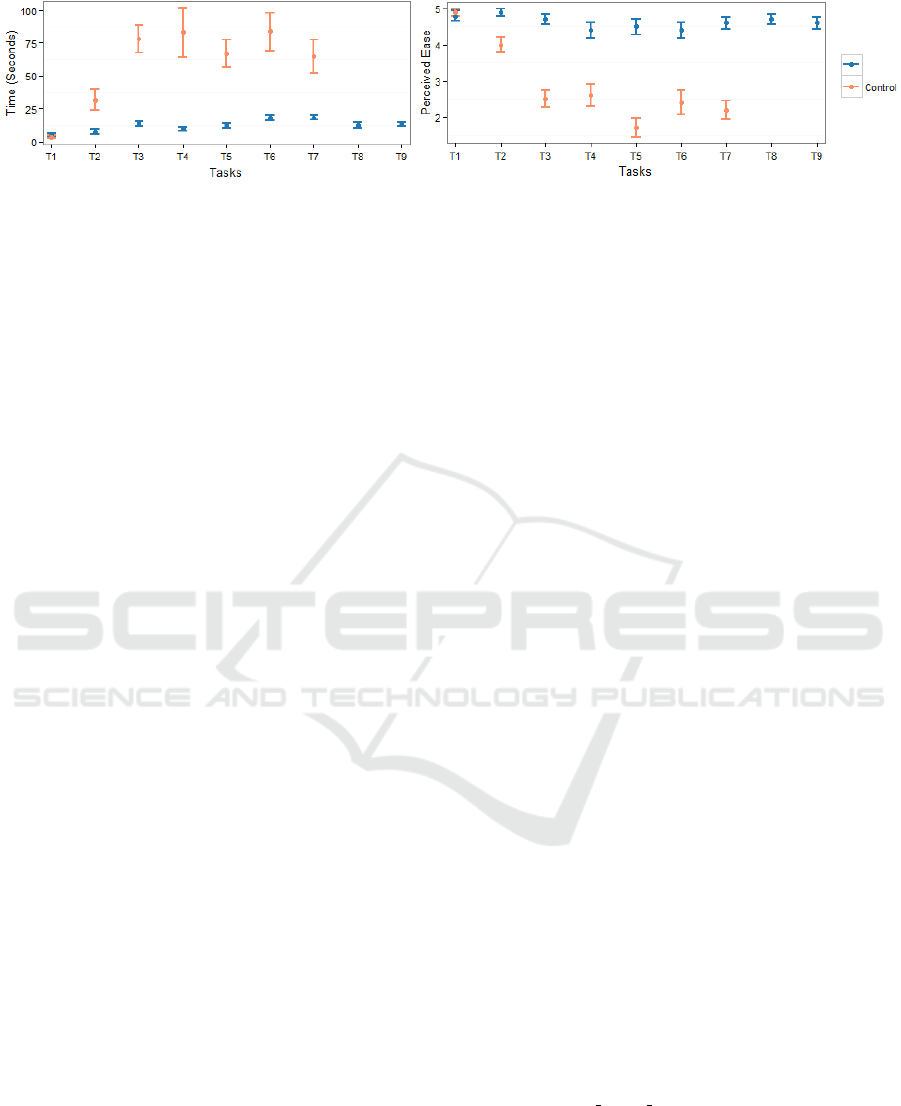
from Fig. 5 (Left), we see that participant’s
performance is relatively consistent/stable for all
tasks, that is, all participants were able to quickly
perform the tasks using MEV. On the other hand,
participants had highly varied performance for tasks
(T2-T7) using the existing tool.
Similarly, for perceived ease, Fig. 5 (Right)
depicts that participants perceived it easier to perform
tasks (T2-T9) using MEV than the existing tool. T5
was rated the most difficult to perform under control
condition, as it involved analysis of distribution of age
for a selected product. Exploring the distribution of
data attributes with their existing tool is tedious as it
requires filtering for each attribute value individually
and then analyzing the outcome.
Lastly, we aggregated the responses from
all participants on the system usability (SUS)
questionnaire (Brooke et al., 1996). MEV received
an SUS score of 85 out of 100.
Qualitative Analysis & Overall Impression of
MEV: The focus of qualitative questionnaires was
on the participants’ subjective impression of the
tool and their experience using it. Our analysis
of comments on the questionnaire suggests that
the participants’ experiences with the tool differed
depending on their prior experience with similar
interactive visualizations. For instance, some
participants found the timeline visualization difficult
to interact with, while others liked it.
Overall, the majority of participants agreed with
the general premise of the tool, and found its goal
of analyzing drug-related medication errors with
severe outcomes and promoting individuals’ ability to
explore data to be promising and potentially useful.
According to the study participant P10: “Well, I think
this tool makes it very easy to see what the reports
are describing without going into much detail”. 6 out
of 10 participants explicitly mentioned the usefulness
of integrating name-entities into the visualization and
the intuitiveness of the tool itself. Participant P2
mentioned: “Though the text-extraction is not perfect
but it gives us a big sense of what kind of errors
are being reported”. Participant P5 said: “It takes
sometime to get used to the tool, then it is very easy
and intuitive to use”.
Constructive feedback for potential improvements
of the design of the tool were also solicited using an
open response option. For instance, four participants
suggested that an individual search option on each
treemap for looking up a particular drug or error
would be useful to achieve the presented tasks.
6 DISCUSSION
The aim of this work centers on developing
visualization-enabled systems that support domain
experts in pharmacovigilance. Our results indicate
that users can in fact perform review tasks in
pharmacovigilance data by analyzing the distribution
of various data attributes using the provided views,
and conduct investigative tasks from within MEV.
More broadly, additional challenges and opportunities
in the space of human-in-the-loop systems for
medical professionals have been uncovered through
interaction with drug analysts.
One key issue in modern systems is scale. As
the goal of MEV is to be used by each drug safety
analyst for reports screening of their assigned set of
products on a weekly basis that constitutes a count
of thousands of reports. We tested MEV with data
from one year (2017) which constitutes over 1.82
million reports, where it takes several seconds to
load data and transform it for the initial overview.
Other challenges of scale relate to the visualizations
themselves. If the analyst were to steer to a view with
hundreds or more drugs, the treemap may display
only tiny rectangles, a source of visual clutter (Peng
et al., 2004). One solution to this clutter problem
is to display a subset of drugs on the treemap along
with a search option to access a desired drugname.
Adding a layer of drug classes on the treemap can
IVAPP 2019 - 10th International Conference on Information Visualization Theory and Applications
80
(a)$ (b)$ (c)$
MEV$
Figure 5: 95% Confidence Interval for performing tasks using both MEV and existing tool (Control). (Right): Perceived Ease
Score. (Left): Time (in sec). Task 8 & 9 are not supported by Control.
be another alternative to address the scaling issue.
Analyst can select a drug class and the drugs under
that class can be visualized on the treemap. We could
also incorporate domain practices into the system. For
example, the maximum number of distinct products
in the reports for each user does not exceed 100, so
clutter is not a problem for typical use cases of MEV.
During our qualitative interviews while majority
of the analysts acknowledged MEV’s usefulness in
reports screening, few analysts mentioned that they
would prefer to read each and every report narrative
rather than using MEV for screening, if the number
of reports is few, i.e., ten or twenty. For such users, a
feature of highlighting the key information within the
report narratives can be added. During our user study,
we also noticed that the extracted information were
incorrect, when users fetched the reports to analyze
the narratives. We leveraged the MEFA (Wunnava
et al., 2017) name-entity extractor in this work for
extracting information such as the stage and cause of
the error. More advanced extraction techniques using
deep learning (Jagannatha and Yu, 2016) could be
plugged into MEV to improve the entity extraction
accuracy. However, name-entity extraction in the
medical domain itself is known to be a challenging
problem and research efforts towards more accurate
techniques continue.
Our user study has a number of limitations. First,
participants are familiar with their existing tool; this
familiarity allowed participants to complete some
complicated tasks in a short time using their existing
tool. Also, for a few participants some tasks were
deemed as not relevant. For instance, participants
who usually investigate one particular drug found it
irrelevant to look for reports related to multiple drugs
based on severity of reports. Study participants, while
a small number, are real drug safety analysts who
would be ultimately users in every day analysis. Long
term studies with these analysts would help to further
assess MEV in their task flow.
There are a few possible directions to work on in
future. First, we plan to integrate interactive support
for report text analysis into MEV to support the full
workflow of the analysts. Second, direct access to
external sources such as PubMed and DailyMed from
within MEV so that analysts can confirm or reject a
hypothesis about a possible medication error formed
using the treemap by investigating these sources
would simplify the analysis. Third, visual provenance
(Groth and Streefkerk, 2006) would also add value by
allowing analysts to share their thought-processes and
findings with their team members.
7 CONCLUSION
In this paper, we introduce MEV – a prototype
tool for visual analytics of medication errors from
spontaneous reporting databases. MEV assists
analysts in exploring and screening spontaneous
reports via an interactive treemap, interactive
bar charts showing demographics and a timeline
visualization. Analysts can pinpoint severe reports
visually and compare data distributions across many
weeks of data. Results from a task-based user study
with 10 drug safety analysts at the FDA suggest that
performing review tasks using MEV is both efficient
and perceived easier than their current tool. Study
results also suggest that analysts find MEV intuitive
and easy to interact with and that it would likely align
with the existing workflow of medication error reports
analysis. Lastly, qualitative interviews suggested
opportunities for improvements in the current design.
REFERENCES
(2010). A $21 Billion Opportunity National
Priorities Partnership convened by
the National Quality Forum file.
http://www.nehi.net/bendthecurve/sup/documents/
Medication Errors Brief.pdf [Accessed:
2018-01-07].
Aronson, A. R. and Lang, c¸.-M. (2010). An overview
of metamap: historical perspective and recent
advances. Journal of the American Medical
Informatics Association, 17(3):229–236.
Asahi, T., Turo, D., and Shneiderman, B. (2003). Visual
decision-making: using treemaps for the analytic
MEV: Visual Analytics for Medication Error Detection
81

hierarchy process. In The Craft of Information
Visualization, pages 235–236. Elsevier.
Brooke, J. et al. (1996). Sus-a quick and dirty usability
scale. Usability evaluation in industry, 189(194):4–7.
Brown, E. G., Wood, L., and Wood, S. (1999). The medical
dictionary for regulatory activities (meddra). Drug
safety, 20(2):109–117.
Du, M. (2005). Approximate name matching. NADA,
Numerisk Analys och Datalogi, KTH, Kungliga
Tekniska H
¨
ogskolan. Stockholm: un, pages 3–15.
FDA (1995). FDA Adverse Event Reporting. http://
www.fda.gov/Drugs/GuidanceComplianceRegulatory
Information/Surveillance/AdverseDrugEffects/
ucm070434.htm [Accessed: 2018-01-10].
Groth, D. P. and Streefkerk, K. (2006). Provenance
and annotation for visual exploration systems.
IEEE Transactions on visualization and Computer
Graphics, 12(6):1500–1510.
Harrison, L., Spahn, R., Iannacone, M., Downing, E., and
Goodall, J. R. (2012). Nv: Nessus vulnerability
visualization for the web. In Proceedings of the ninth
International Symposium on Visualization for Cyber
Security, pages 25–32. ACM.
Jagannatha, A. N. and Yu, H. (2016). Bidirectional rnn for
medical event detection in electronic health records.
In Proceedings of the conference. Association for
Computational Linguistics. North American Chapter.
Meeting, volume 2016, page 473.
Jia, P., Zhang, L., Chen, J., Zhao, P., and Zhang, M. (2016).
The effects of clinical decision support systems on
medication safety: an overview. Public Library of
Science One, 11(12):e0167683.
Lee, B., Smith, G., Robertson, G. G., et al. (2009).
Facetlens: exposing trends and relationships to
support sensemaking within faceted datasets. In
Proceedings of the SIGCHI Conference on Human
Factors in Computing Systems, pages 1293–1302.
ACM.
Liu, Z., Stasko, J., and Sullivan, T. (2009). Selltrend:
Inter-attribute visual analysis of temporal transaction
data. IEEE Transactions on Visualization and
Computer Graphics, 15(6):1025–1032.
NCC-MERP (1995). National Coordinating Council
for Medication Error Reporting and Prevention.
http://www.nccmerp.org/ [Accessed: 2018-02-28].
Ozturk, S., Kayaalp, M., and McDonald, C. J. (2014).
Visualization of patient prescription history data
in emergency care. In AMIA Annual Symposium
Proceedings, volume 2014, page 963. American
Medical Informatics Association.
Peng, W., Ward, M. O., and Rundensteiner, E. A.
(2004). Clutter reduction in multi-dimensional
data visualization using dimension reordering. In
Information Visualization, 2004. INFOVIS 2004.
IEEE Symposium on, pages 89–96. IEEE.
Savova, G. K., Masanz, J. J., Ogren, P. V., et al. (2010).
Mayo clinical text analysis and knowledge extraction
system (ctakes): architecture, component evaluation
and applications. Journal of the American Medical
Informatics Association, 17(5):507–513.
Singh, R., Pace, W., Singh, A., et al. (2008). A visual
computer interface concept for making error reporting
useful at care point.
Smith, G., Czerwinski, M., Meyers, B. R., et al.
(2006). Facetmap: A scalable search and browse
visualization. IEEE Transactions on Visualization and
Computer Graphics, 12(5).
Stasko, J., Gørg, C., and Liu, Z. (2008). Jigsaw:
supporting investigative analysis through interactive
visualization. Information Visualization,
7(2):118–132.
Varkey, P., Cunningham, J., and Bisping, S. (2007).
Improving medication reconciliation in the outpatient
setting. Joint Commission Journal on Quality and
Patient Safety, 33(5):286–292.
Wunnava, S., Qin, X., Kakar, T., et al. (2017).
Towards transforming fda adverse event narratives
into actionable structured data for improved
pharmacovigilance. In Proceedings of the Symposium
on Applied Computing, pages 777–782. ACM.
Xu, H., Stenner, S. P., Doan, S., et al. (2010). Medex: a
medication information extraction system for clinical
narratives. Journal of the American Medical
Informatics Association, 17(1):19–24.
IVAPP 2019 - 10th International Conference on Information Visualization Theory and Applications
82
