
Glottal Flow Analysis in Parkinsonian Speech
Patrick Corcoran, Arnold Hensman and Barry Kirkpatrick
TU Dublin, Blanchardstown Campus, Dublin, Ireland
Keywords: Glottal Flow, Parkinson’s Disease, Speech.
Abstract: Speech and vocal impairments are one of the earliest symptoms of Parkinson’s disease (PD). Laryngoscope
examinations have identified that patients with the disease show pathological behaviour of the vocal folds.
The behaviour of the vocal folds is investigated by analysing the glottal flow waveform in Parkinsonian
speech in this study. This study aims to determine the appropriate method for estimating the glottal source in
PD speech and to identify glottal parameters that could be indicative of PD. An experiment was conducted to
analyse a selection of glottal parameters (2 time-domain and 3 frequency-domain) measured from the glottal
flow waveform estimated from speech recordings. A database of 52 healthy speakers and 44 speakers with
Parkinson’s disease was considered for this experiment. Two glottal estimation techniques are considered in
the experiment: iterative and adaptive inverse filtering (IAIF) and quasi-closed phase (QCP) inverse filtering.
The results showed that 2 of the 5 glottal parameters (1 time domain and 1 frequency domain) produced values
indicating a difference between healthy and PD speech files in the database. The results also indicate that
glottal estimates from the IAIF method resulted in parameters discriminating between healthy and PD higher
than glottal estimates from the QCP method.
1 INTRODUCTION
Parkinson’s disease (PD) is a chronic
neurodegenerative disorder of the central nervous
system generally observed in elderly people. It is the
second most common neurodegenerative disease,
after Alzheimer’s, affecting an estimated 10 million
people around the world, with these numbers
expected to double in the next 10 years (Dorsey et al.,
2007). Currently there is no cure for PD but early
diagnosis and drug therapies can decrease the
difficulties of the disorder and improve quality of life.
This study aims to investigate the appropriate method
for estimating the glottal flow in PD speech and
identify glottal parameters that could be indicative of
PD by analysing the behaviour of the glottal flow.
The cause of PD is attributed to the progressive
loss of dopamine in the brain which is the chemical
released by nerve cells to interact with other nerve
cells. This interaction between nerve cells is
responsible for controlling the motor and mental
functions of a person, and the reduction in dopamine
levels leads to PD symptoms. Typical motor
symptoms observed in PD are muscular rigidity,
resting tremor and slowness of movement. Along
with these, many patients develop non-motor
symptoms like sustained depression and memory
loss. Individuals with PD experience different
combinations of these symptoms at different severity
levels. The muscles in the face, mouth and throat can
be affected which results in problems with speech and
swallowing. It is estimated that 89% of PD patients
will suffer some form of vocal impairment (Logeman
et al., 1978) and a vocal disorder may be one of the
earliest symptoms of the disease (Harel et al, 2004).
Speech related symptoms that have been reported to
affect PD patients include harsh or breathy voice,
reduced volume and vocal tremor. The most
commonly used scale for the progression of PD is the
Unified Parkinson’s Disease Rating Scale (UPDRS).
Employing the UPDRS is a complex and lengthy
procedure which requires the subjective evaluation by
a clinical expert. Analysing the speech signal in PD
patients may provide a tool to help clinicians evaluate
and diagnose the disease. This could provide a non-
invasive method of indirectly examining the larynx
which may help with further monitoring of the
disease and could be performed remotely.
Previous studies of the larynx in PD patients have
shown incidences of abnormalities of the vocal folds.
These laryngeal dysfunctions have been observed
through laryngoscope examinations where video
116
Corcoran, P., Hensman, A. and Kirkpatrick, B.
Glottal Flow Analysis in Parkinsonian Speech.
DOI: 10.5220/0007259701160123
In Proceedings of the 12th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2019), pages 116-123
ISBN: 978-989-758-353-7
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
frames of the vocal folds are obtained and analysed
by a clinical expert. Hanson et al (1984) examined 32
PD patients and reported that 94% showed vocal fold
bowing and 81% demonstrated varying degrees of
asymmetry of the vocal folds. Smith et al (1995)
reported that from videostroboscopic examinations of
22 patients, there was a 38% incidence of vocal-fold
bowing and 67% incidence of incomplete glottal
closure. Perez et al (1996) reported irregularities in
the closure and vibration of the vocal folds with 50%
of the patients demonstrating abnormal glottal closure
and 47% demonstrating irregular vibration of the
vocal folds with asymmetric behaviour. Yücetürk et
al. (2002) examined 30 PD patients and reported that
70% had at least one of eight laryngeal dysfunctions
with some of the patients featuring more than one.
Tsuboi et al. (2015) reported that of 22 PD patients
treated with subthalamic nucleus deep brain
stimulation 77% showed an incidence of incomplete
glottal closure and 50% showed signs of
asymmetrical glottal movement.
Research is ongoing in the studies of diagnosing
and monitoring PD with speech and is showing
positive steps towards establishing an objective
measurement of the disease (Little et al., 2009).
Studies implementing advanced signal processing
algorithms have shown that symptoms of PD can be
predicted on the UPDRS scale remotely using non-
invasive speech recordings (Tsanas et al., 2010).
Speech impairments of PD patients were investigated
by features such as jitter, shimmer and harmonic to
noise ratio (Tsanas et al, 2012). Results obtained from
these parameters showed accuracies of up to 98%.
Sharma (2014) also reported jitter and shimmer
showing different values, when tested on 14 PD and
7 healthy subjects. This study also reported the glottal
pulse of the healthy subjects to be symmetric in nature
when compared to the PD patients. The behaviour of
the glottal flow in PD patients has been studied and
parameters have been identified that discriminate
from healthy speakers with accuracies of over 90%
(Hanratty et al., 2016). Additionally, automatic
detection of PD has been researched by analysing the
non-linear behaviour of the vocal folds which affects
the glottal flow signal with accuracies up to 78%
(Belalcázar-Bolaños et al., 2016). Detection of early
stages of Parkinson’s disease using Mel-frequency
cepstral coefficients was investigated by (Jeancolas et
al., 2017), employing a detection framework similar
to that used in speaker recognition and obtained
results between 60% and 91%. It is difficult to
compare results between studies as they have used
different performance metrics on different test
databases and recording protocols.
The aim of this study is to build on previous
studies and contribute to the research of using speech
files to aid in the diagnosis of PD. This will be
achieved by analysing the behaviour of the glottal
flow waveform in PD speech and identifying glottal
parameters which are distinct to healthy speech. The
glottal flow will be estimated from speech signals by
different methods to identify which is the most
applicable to extracting the glottal flow estimate in
Parkinsonian speech.
The rest of this paper is organised as follows
Section 1.1 describes the background on the glottal
flow, glottal estimation techniques and glottal
parameters. Section 2 describes the experimental
procedure and details the data used in the experiment,
Section 3 presents the results of the experiment, and
Section 4 comprises the conclusions of the study.
1.1 Background
The glottal flow is the airflow that is generated from
the lungs and then passed through the vocal folds,
located in the larynx. The vocal folds vibrate which
causes them to open and close periodically. This
airflow is filtered by the vocal tract cavities to
produce human speech (Quatieri, 2006). The glottal
flow waveform is produced from this airflow and is
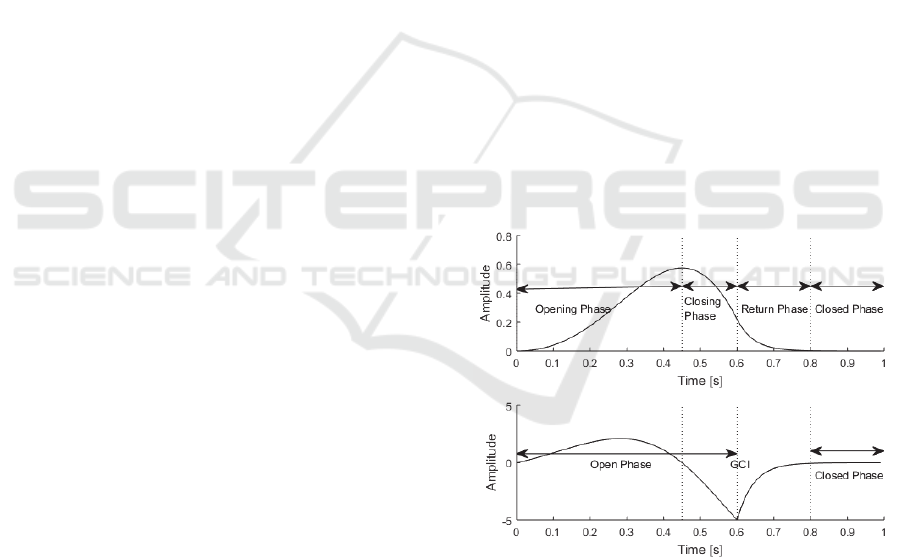
depicted in Figure 1 (Drugman et al., 2012).
Figure 1: Glottal flow waveform (upper) and glottal flow
derivative waveform (lower) with open phase and closed
phase displayed.
Each period of the glottal flow waveform can be
separated into three main parts, the open phase, the
return phase and the closed phase. During the open
phase, the air pressure gradually increases until it
comes to an abrupt stop when the glottis closes, which
is called the glottal closure instant (GCI; Drugman
and Dutoit, 2009). In healthy speech, during the
Glottal Flow Analysis in Parkinsonian Speech
117
closed phase, there is no air flow through the vocal
folds and the amplitude of the signal has returned to
zero. The open phase of the glottal flow waveform is
divided into two phases, the opening phase and the
closing phase. The opening phase refers to the
timespan up to the maximum positive amplitude of
the glottal pulse, while the closing phase is the period
after this until the GCI. After the open phase, there is
a period where the waveform returns to the initial
state, this is called the return phase (Drugman et al.,
2012). The glottal flow can be represented as a glottal
flow derivative waveform, as shown in Figure 1, as it
reflects some characteristics that are not represented
in the glottal flow waveform (Plumpe et al., 1999).
1.1.1 Estimating the Glottal Source
A speech signal can be represented as being made up
of two main components, the glottal flow (source) and
the vocal tract (filter) (Fant, 1971). Glottal inverse
filtering (GIF) is a technique used to estimate the
glottal flow waveform from a speech signal. The idea
of GIF is to estimate a model for the vocal tract filter
from a recorded speech signal and then filter the
recorded signal through the inverse of this model to
cancel the effects, resulting in an estimate of the
glottal flow signal (Alku, 2011). Modern GIF
methods can be categorised as (1) closed-phase
methods (2) iterative methods and (3) spectral
decomposition methods. Closed-phase methods use
the closed-phase of the glottal flow signal as there is
said to be less interaction from the vocal tract and it
provides a more accurate model of the vocal tract,
resulting in more accurate glottal estimates (Wong et
al., 1979). Iterative methods utilise the whole pitch
period to remove the influence of the glottal
waveform and estimate the vocal tract. This vocal
tract estimate is then used by inverse filtering to
provide an estimate of the glottal flow (Alku, 1992).
Spectral decomposition methods involve estimating
the glottal flow by separating the speech by maximum
and minimum phase components (Alku, 2011). All
methods except iterative methods require accurate
identification of glottal closure instants (GCI) and
glottal opening instants (GOI; Drugman and Dutoit,
2009). Most studies when evaluating GIF methods
will use synthetic speech because the glottal flow
signal cannot be measured directly from the human
larynx (Airaksinen, 2014). A recent study (Chien et
al., 2017) has shown that closed-phase and iterative
methods perform well and show stability on different
voice qualities of sustained synthetic vowels, while
spectral decomposition methods provided a less
stable performance on the tested database. Breathy
voice quality is one of the reported speech disorders
of Parkinson’s disease and this paper reported that
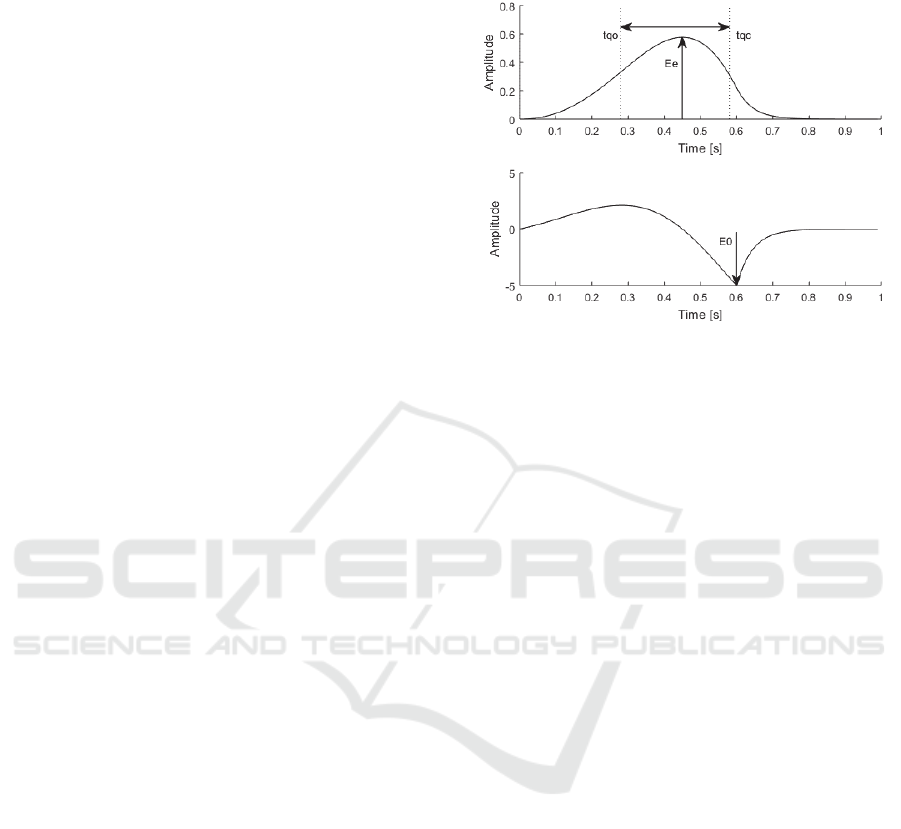
Figure 2: Glottal flow pulse (top) and glottal flow derivative
pulse (bottom) with measurements for quasi-open quotient
(QOQ) and normalised amplitude quotient (NAQ).
closed-phase and iterative methods display
robustness on this voice quality across a number of
synthetic vowels.
1.1.2 Glottal Flow Parameters
Many parametrisations of the glottal flow exist but
not all are suitable for Parkinsonian speech.
Parkinsonian speech is known to show harsh and
breathy characteristics among other pathologies. This
indicates that the glottal parameters must be robust to
noise for effective measurement. This section gives
an overview of the glottal parameters selected to be
considered in this study.
The time-domain parameters, Quasi-open
quotient (QOQ) and normalised amplitude quotient
(NAQ), were selected as they are known to be robust
measurements of the glottal waveform in adverse
conditions. Previous studies have reported that they
show potential for separating PD and healthy speech
(Hanratty et al., 2016).
Quasi-open Quotient (QOQ): This parameter
measures the duration of the open phase from when
the amplitude of the glottal pulse crosses the 50%
marker line at the point, t
qc
until it falls below it again
at t
qo
as shown in Figure 2. The marker t
qc
is defined
as the point at which the amplitude of the glottal pulse
reaches 50% of its maximum value and t
qo
marks the
point at which the glottal pulse goes below 50% of the
maximum value. The timing distance between these
two points is referred to as the quasi-open phase. This
duration is subsequently normalised with respect to
the pitch period, T
0
. It was designed as a more robust
version of the open quotient (OQ) (Airas, 2008; Kane,
BIOSIGNALS 2019 - 12th International Conference on Bio-inspired Systems and Signal Processing
118

2012) and mitigates the issue of noise corrupting the
measurement of the instant of glottal opening. The
formula for QOQ is displayed in Equation 1 (Hacki,
1989).
QOQ
t
t
T
(1)
Normalised Amplitude Quotient (NAQ): This
parameter is measured from the peak amplitude of the
glottal flow, E
e,
and maximum negative amplitude of
the glottal flow derivative, E
0
, as shown in Figure 2.
This is then normalised with respect to the pitch
period, T
0
, as shown in Equation 2 (Alku et al., 2002).
This is selected as a glottal parameter as it
representative of the glottal pulse and glottal
derivative pulse. It is robust to variations in recording
conditions as it is normalised with respect to
amplitude.
NAQ
E
E
T
(2)
Frequency-domain parameters, H1-H2, harmonic
richness factor (HRF) and parabolic spectral
parameter (PSP), were selected for this study.
Measurements in the glottal source spectrum give an
alternative approach to the time-domain parameters
and they are known to distinguish between voice
qualities (Alku, 2011). Two of these glottal
parameters, H1-H2 and HRF, have been tested in
combination with time-domain parameters on
Parkinsonian and healthy speech (Belalcázar-Bolaños
et al., 2016). Discrimination between healthy speech
and Parkinsonian speech was made using a Support
Vector Machine (SVM) and produced accuracies of
up to 78%. These parameters were selected to
determine their individual performance in detecting
PD.
H1-H2: This is a measure of the change in amplitudes
of the first two harmonics, H1 and H2, of the
differentiated glottal source spectrum (Fant, 1995).
This measurement has been used as a glottal
parameter as it is reported that changes in the open-
quotient of the glottal cycle produce a corresponding
change in H1-H2 (Doval et al., 2006). This has been
used to detect different phonation types by analysing
the measurement.
Harmonic Richness Factor (HRF): This spectral
parameter is a measurement computed by the sum of
the amplitudes of the harmonics above the
fundamental harmonic. This is then normalised with
respect to the first harmonic, H
1
, and is shown in
Equation 3 (Childers and Lee, 1991). This parameter
represents the spectral tilt of the glottal flow and has
been used to identify different phonation types.
HRF
∑
H
H
(3)
Parabolic Spectral Parameter (PSP): This is a
measure to model frequency domain characteristics
within the glottal signal. It is computed by fitting a
parabola to the lower frequencies in the glottal source
spectrum (Alku et al., 1997). This parameter was
introduced as a robust measurement of the spectral
decay in the glottal signal to detect phonation type.
2 EXPERIMENTAL PROCEDURE
The objective of this experiment was to identify the
appropriate method for estimating the glottal source
in Parkinsonian speech. This was completed by
analysing the glottal signal from PD and healthy
speech recordings using different estimation
techniques and identifying parameters that behave
different. These parameters would then be tested to
quantify if a separation exists between PD and heathy
speech.
The performance of the parameters was quantified
using receiver operating characteristic (ROC) curves
and the area under the ROC curve (AUC) (Fawcett,
2006). The ROC curve and AUC value quantify the
performance of the glottal parameters in their task to
separate between PD and healthy speech. The AUC
value can range from 0 and 1 and it can be interpreted
as the probability of making the correct decision on
classifying a particular file correctly. An AUC value
of 0.5 indicates no separation.
2.1 Data
The data used in the experiment was taken from three
components to create one database, containing
healthy and Parkinsonian speech files.
2.1.1 Parkinsonian Speech
The Parkinson’s disease speech recordings consisted
of a combination of two databases from different
sources.
The first database was recorded in a quiet
environment with a Zoom H2n recorder at St. Mary’s
Hospital in Dublin, Ireland as reported in (Hanratty et
al., 2016). The signals were sampled at 44.1 kHz per
channel with a 16 bit resolution. The database
contains 22 Parkinson’s disease patients who were
asked to make a sustained sound of the vowel ‘a’ for
as long as possible, therefore the signals are of
varying durations.
Glottal Flow Analysis in Parkinsonian Speech
119

The second Parkinson’s disease speech database
was recorded by a Trust MC-1500 microphone placed
10 cm from the speaker’s lips as reported in (Sakar et
al., 2013). The signals were sampled at 44.1 kHz per
channel with a 16 bit resolution. The database
contains 28 Parkinson’s disease patients with an age
range from 39 – 79 and who are suffering with the
disease for 0 – 13 years. The patients recorded
sustained vowels ‘a’ and ‘o’ three times with varying
durations. For this study, the ‘a’ sounds were taken
from this database to correspond with the recordings
taken from the previous database.
2.1.2 Healthy Speech
The healthy speech database was obtained from
(Childers, 1999). This database was recorded in a
professional single-wall sound room with an Electro-
Voice RE-10 cardioid microphone. The microphone
was placed 15 cm from the speaker’s lips and the
signals were sampled at 10 kHz per channel with a
16-bit resolution. The database contains 52 subjects
(25 male and 27 female) with a normal larynx and an
age range from 20 – 80 years old. All subjects
recorded 28 tasks which included 12 sustained
different vowel sounds with a duration of
approximated 2 seconds. This database also included
full words and spoken sentences from the speakers.
For this study, the sustained vowel ‘aa’ was taken
from this database to be consistent with the
Parkinsonian database.
2.1.3 Glottal Estimation from Database
For this experiment all speech recordings with a
sustained vowel ‘a’ were investigated. As all speech
recordings were of various durations, a window of
500ms of continuous voiced speech was extracted
from the centre section of each recording. This also
ensured there was no transient effects included for the
glottal analysis. 5 of the 22 speech files from the
source (Hanratty et al., 2016) were excluded as they
did not meet the protocol for requirements of 500 ms
of continuous speech. The recorded sustained vowel
‘o’ from the source (Sakar et al., 2013) were excluded
as the ‘a’ recordings were only considered for this
experiment. The overall database included 52 healthy
speech files and 44 Parkinsonian speech files of a
sustained ‘a’ sound from each speaker for a duration
of 500ms.
For this experiment, the GIF methods chosen were
closed phase methods and iterative methods as they
have shown to be robust in extracting the glottal
source in varying phonations. Spectral decomposition
methods were not selected as they consider the closed
phase of the glottal signal to be zero (Alku, 2011) and
this would not be appropriate for Parkinsonian speech
knowing the vocal fold disorders attributed to the
disease. The closed phase technique chosen was
quasi-closed phase (QCP) inverse filtering
(Airaksinen et al., 2014) and the iterative method
chosen was iterative and adaptive inverse filtering
(IAIF) (Alku, 1992). The QCP method needs
identification of GCIs and GOIs and these were
computed by the SEDREAMS algorithm (Drugman
and Dutoit., 2009). The glottal signal was estimated
from each speech recording by both methods.
Algorithms for these methods were implemented
from the sources (Degottex et al., 2014; Alku et al.,
2017).
For each glottal estimate from all speakers, the
selected five glottal parameters were measured on
every pitch period across the 500ms window. The
median value of the parameter was computed to
represent the value for the parameter on each file. The
median value was selected to remove any outliers and
to represent a true single value of one parameter from
the glottal signal. This single value for each of the five
glottal parameters was used to create ROC curves to
show the performance of distinguishing between
healthy and Parkinsonian speech. A value for the
AUC and the SE was computed to illustrate the
performance of the parameters. Glottal parameters
were extracted twice, using the IAIF and QCP
methods to indicate if one is producing a better
performance.
3 RESULTS
The performance of the separation of healthy and PD
speech for each parameter for the IAIF method is
shown in Figure 3. This is presented as individual
ROC curves for each parameter in different colours,
where the dashed line represents the value 0.5. It can
be seen from the curves that three of the tested glottal
parameters produce a good performance; QOQ, PSP
and H1-H2. The parameter QOQ shows the highest
performance for separating healthy and PD speech for
the IAIF method.
The performance of the separation of healthy and
PD speech for each parameter for the QCP method is
shown in Figure 4. This is presented as individual
ROC curves for each parameter in different colours,
where the dashed line represents the value 0.5. It can
be seen from the graph that the performance of all the
glottal parameters is slightly above the 0.5 line with
no single parameter showing an excellent
performance of separating PD and healthy speech.
BIOSIGNALS 2019 - 12th International Conference on Bio-inspired Systems and Signal Processing
120
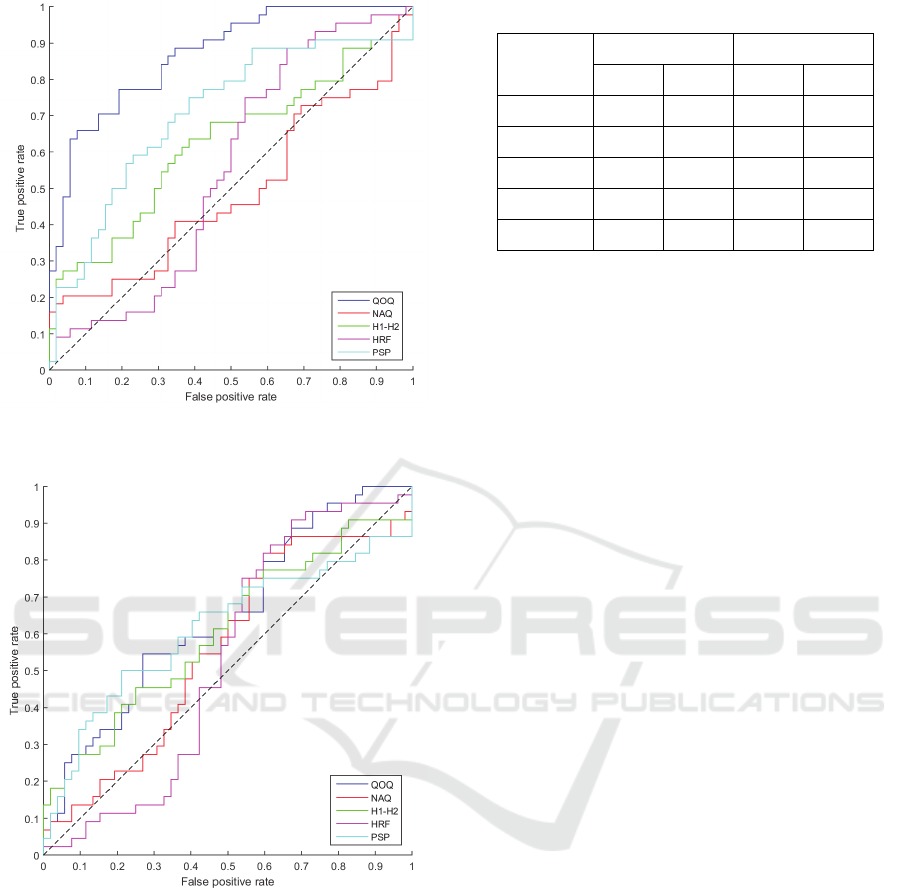
Figure 3: ROC curves for glottal parameters tested by IAIF
method.
Figure 4: ROC curves for glottal parameters tested by QCP
method.
The ROC curves for the glottal parameters are
shown in Figure 3 and Figure 4. For further analysis
of the performance, the AUC was computed for each
parameter for both estimation techniques. The
obtained values from this experiment are presented in
Table 1. The results are presented in terms of AUC
and SE, with each individual glottal parameter result
from the two glottal estimation techniques, IAIF and
QCP.
Table 1: Results obtained for glottal parameters tested.
Glottal
Parameter
IAIF QCP
AUC SE AUC SE
QOQ 0.857 0.040 0.631 0.057
NAQ 0.467 0.059 0.547 0.059
H1H2 0.613 0.058 0.593 0.058
HRF 0.533 0.059 0.520 0.059
PSP 0.708 0.053 0.619 0.058
According to the results obtained from the IAIF
estimation algorithm the glottal parameter, QOQ,
computed an AUC value of over 0.857 which
indicates this parameter was different in healthy and
PD speech. PSP was found to have an AUC value
exceeding 0.71 which again indicates a good
separation between healthy and PD. NAQ was found
to have the lowest performance for the IAIF method
scoring an AUC value of 0.47 indicating this
parameter could not distinguish between healthy and
PD speech from this database.
The results for the QCP method show that the
parameters QOQ and PSP perform the highest at
separating healthy and PD speech for this technique
with both obtaining AUC values exceeding 0.61.
HRF has the lowest performance with an AUC value
of 0.52 indicating this does not perform well at
distinguishing between healthy and PD speech
signals.
The performance of the two estimation
techniques, IAIF and QCP, was analysed by
comparing the AUC and SE values of the parameters
obtained by both. The estimation technique that had
higher AUC values was considered to perform better
at separating healthy and Parkinsonian speech. IAIF
obtained higher values for the AUC in all parameters
except one, NAQ. For the parameter QOQ, IAIF
scored a higher AUC value, obtaining 0.857
(SE=0.040) with QCP obtaining 0.631 (SE=0.057).
The frequency domain parameter, PSP, also scored a
higher value using the IAIF method. This indicates
that overall, with these parameters, the IAIF method
performs better at discriminating between healthy and
Parkinsonian speech from the speech files in this
database.
Laryngoscope studies on PD patients reported that
vocal fold disorders are evident in Parkinsonian
speech. It would be expected that vocal fold disorders
could lead to pathological features in the glottal
signal. The results found in this study suggest that the
glottal flow exhibits different characteristics in
Parkinsonian speech when compared with healthy
Glottal Flow Analysis in Parkinsonian Speech
121

speech. Hanratty et al. (2016) reported that the
parameter QOQ scored a performance of over 90% at
separating healthy and PD speech files when the
glottal source was estimated by the IAIF method. In
this study, the database was increased to include more
PD speech files and QOQ still produced a high AUC
value of 0.857 when distinguishing between healthy
and PD.
4 CONCLUSIONS
Speech impairments are a common occurrence in PD
patients and this could be related to the vocal fold
abnormalities found in the patients. The results in this
study indicate that different behaviour is evident in
the glottal flow signal, with two glottal parameters
showing separation between PD and healthy speech
recordings from the test database.
The results indicate that the timing based
parameter, QOQ, and the frequency domain
parameter, PSP, show significant results when tasked
with distinguishing between healthy and PD speech.
According to the results from this experiment the
estimation technique IAIF outperformed the QCP
method with the selected glottal parameters. IAIF
obtained higher AUC values for all parameters except
one, indicating it is the appropriate method for
estimating the glottal source from Parkinsonian
speech
This experiment selected five glottal parameters
and two glottal estimation techniques but note that
many more possibilities exist that were not
considered in this study. Estimating the glottal flow
from speech signals can be a challenging task and is
particularly difficult for pathological speech, such as
that found in PD. Sources of variations exist in the
test dataset, which include different recording
protocols, severity of the disease in the PD group and
age of participants not matched to the control group
of healthy speech. Based on these critiques, there
must be caution on drawing broader conclusions.
Future work with an improved database with more
participants will be considered to fully understand
how parameters behave in the glottal flow of
Parkinsonian speech.
ACKNOWLEDGEMENTS
The authors would like to acknowledge
Technological University Dublin for funding this
research project.
The authors would also like to acknowledge
participants and staff that contributed to this study
from St. Mary’s Hospital, Dublin.
REFERENCES
Airaksinen, M. et al. (2014) Quasi Closed Phase Glottal
Inverse Filtering Analysis with Weighted Linear
Prediction. IEEE/ACM Transactions on Audio, Speech
and Language Processing (TASLP). 22 (3), pp. 596-
607.
Airas, M. (2008) TKK Aparat: An Environment for Voice
Inverse Filtering and Parameterization. Logopedics
Phoniatrics Vocology. 33 (1), pp. 49-64.
Alku, P. (2011) Glottal Inverse Filtering Analysis of
Human Voice production—a Review of Estimation and
Parameterization Methods of the Glottal Excitation and
their Applications. Sadhana. 36 (5), pp. 623-650.
Alku, P. (1992) Glottal Wave Analysis with Pitch
Synchronous Iterative Adaptive Inverse
Filtering. Speech Communication. 11 (2-3), pp. 109-
118.
Alku, P., Bäckström, T. & Vilkman, E. (2002) Normalized
Amplitude Quotient for Parametrization of the Glottal
Flow. The Journal of the Acoustical Society of
America. 112 (2), pp. 701-710.
Alku, P., Pohjalainen, H. & Airaksinen, M. (2017) Aalto
Aparat–A Freely Available Tool for Glottal Inverse
Filtering and Voice Source Parameterization
In Subsidia: Tools and Resources for Speech Sciences.
Alku, P., Strik, H. & Vilkman, E. (1997) Parabolic Spectral
Parameter-A New Method for Quantification of the
Glottal Flow.
Belalcázar-Bolaños, E.A., Orozco-Arroyave, J.R., Vargas-
Bonilla, J.F., Haderlein, T. & Nöth, E. (2016) Glottal
Flow Patterns Analyses for Parkinson’s Disease
Detection: Acoustic and Nonlinear
Approaches. International Conference on Text, Speech,
and Dialogue. : Springer, pp. 400.
Chien, Y. et al. (2017) Evaluation of Glottal Inverse
Filtering Algorithms using a Physiologically Based
Articulatory Speech Synthesizer. IEEE/ACM
Transactions on Audio, Speech, and Language
Processing. 25 (8), pp. 1718-1730.
Childers, D.G. (1999) Speech Processing. : John Wiley &
Sons, Inc.
Childers, D.G. & Lee, C. (1991) Vocal Quality Factors:
Analysis, Synthesis, and Perception. The Journal of the
Acoustical Society of America. 90 (5), pp. 2394-2410.
Degottex, G., Kane, J., Drugman, T., Raitio, T. & Scherer,
S. (2014) COVAREP—A Collaborative Voice
Analysis Repository for Speech
Technologies. Acoustics, Speech and Signal
Processing (ICASSP), 2014 IEEE International
Conference on. : IEEE, pp. 960.
Dorsey, E.R. et al. (2007) Projected Number of People with
Parkinson Disease in the most Populous Nations, 2005
through 2030. Neurology. 68 (5), pp. 384-386.
BIOSIGNALS 2019 - 12th International Conference on Bio-inspired Systems and Signal Processing
122

Doval, B., d'Alessandro, C. & Henrich, N. (2006) The
Spectrum of Glottal Flow Models. Acta Acustica
United with Acustica. 92 (6), pp. 1026-1046.
Drugman, T., Bozkurt, B. & Dutoit, T. (2012) A
Comparative Study of Glottal Source Estimation
Techniques. Computer Speech & Language. 26 (1), pp.
20-34.
Drugman, T. & Dutoit, T. (2009) Glottal Closure and
Opening Instant Detection from Speech Signals. Tenth
Annual Conference of the International Speech
Communication Association.
Fant, G. (1995) The LF-Model Revisited. Transformations
and Frequency Domain Analysis. Speech
Trans.Lab.Q.Rep., Royal Inst.of Tech.Stockholm. 2 (3),
pp. 40.
Fant, G. (1971) Acoustic Theory of Speech Production:
With Calculations Based on X-Ray Studies of Russian
Articulations. : Walter de Gruyter.
Fawcett, T. (2006) An Introduction to ROC
Analysis. Pattern Recognition Letters. 27 (8), pp. 861-
874.
Hacki, T. (1989) Classification of Glottal Dysfunctions on
the Basis of Electroglottography. Folia Phoniatrica. 41
(1), pp. 43-48.
Hanratty, J., Deegan, C., Walsh, M. & Kirkpatrick, B.
(2016) Analysis of Glottal Source Parameters in
Parkinsonian Speech. Engineering in Medicine and
Biology Society (EMBC), 2016 IEEE 38th Annual
International Conference of the. : IEEE, pp. 3666.
Hanson, D.G., Gerratt, B.R. & Ward, P.H. (1984)
Cinegraphic Observations of Laryngeal Function in
Parkinson's Disease. The Laryngoscope. 94 (3), pp.
348-353.
Harel, B., Cannizzaro, M. & Snyder, P.J. (2004) Variability
in Fundamental Frequency during Speech in Prodromal
and Incipient Parkinson's Disease: A Longitudinal Case
Study. Brain and Cognition. 56 (1), pp. 24-29.
Jeancolas, L., Benali, H., Benkelfat, B., Mangone, G.,
Corvol, J., Vidailhet, M., Lehericy, S. & Petrovska-
Delacrétaz, D. (2017) Automatic Detection of Early
Stages of Parkinson's Disease through Acoustic Voice
Analysis with Mel-Frequency Cepstral
Coefficients. Advanced Technologies for Signal and
Image Processing (ATSIP), 2017 International
Conference on. : IEEE, pp. 1.
Kane, J. (2012) Tools for Analysing the Voice. PhD Diss.,
Trinity College, Dublin, Ireland.
Little, M.A. et al. (2009) Suitability of Dysphonia
Measurements for Telemonitoring of Parkinson's
Disease. IEEE Transactions on Biomedical
Engineering. 56 (4), pp. 1015-1022.
Logemann, J.A. et al. (1978) Frequency and Cooccurrence
of Vocal Tract Dysfunctions in the Speech of a Large
Sample of Parkinson Patients. Journal of Speech and
Hearing Disorders. 43 (1), pp. 47-57.
Perez, K.S. et al. (1996) The Parkinson Larynx: Tremor and
Videostroboscopic Findings. Journal of Voice. 10 (4),
pp. 354-361.
Plumpe, M.D., Quatieri, T.F. & Reynolds, D.A. (1999)
Modeling of the Glottal Flow Derivative Waveform
with Application to Speaker Identification. IEEE
Transactions on Speech and Audio Processing. 7 (5),
pp. 569-586.
Quatieri, T.F. (2006) Discrete-Time Speech Signal
Processing: Principles and Practice. : Pearson
Education India.
Sakar, B.E. et al. (2013) Collection and Analysis of a
Parkinson Speech Dataset with Multiple Types of
Sound Recordings. IEEE Journal of Biomedical and
Health Informatics. 17 (4), pp. 828-834.
Sharma, R. (2014) Early Detection of Parkinson's Disease
through Voice. Advances in Engineering and
Technology (ICAET), 2014 International Conference
on. : IEEE, pp. 1.
Smith, M.E. et al. (1995) Intensive Voice Treatment in
Parkinson Disease: Laryngostroboscopic
Findings. Journal of Voice. 9 (4), pp. 453-459.
Tsanas, A. et al. (2010) Accurate Telemonitoring of
Parkinson's Disease Progression by Noninvasive
Speech Tests. IEEE Transactions on Biomedical
Engineering. 57 (4), pp. 884-893.
Tsanas, A. et al. (2012) Novel Speech Signal Processing
Algorithms for High-Accuracy Classification of
Parkinson's Disease. IEEE Transactions on Biomedical
Engineering. 59 (5), pp. 1264-1271.
Tsuboi, T. et al. (2015) Characteristic Laryngoscopic
Findings in Parkinson’s Disease Patients After
Subthalamic Nucleus Deep Brain Stimulation and its
Correlation with Voice Disorder. Journal of Neural
Transmission. 122 (12), pp. 1663-1672.
Wong, D., Markel, J. & Gray, A. (1979) Least Squares
Glottal Inverse Filtering from the Acoustic Speech
Waveform. IEEE Transactions on Acoustics, Speech,
and Signal Processing. 27 (4), pp. 350-355.
Yücetürk, A. et al. (2002) Voice Analysis and
Videolaryngostroboscopy in Patients with Parkinson's
Disease. European Archives of Oto-Rhino-
Laryngology. 259 (6), pp. 290-293.
Glottal Flow Analysis in Parkinsonian Speech
123
