
Characterization of Activated Carbon Prepare from Low-rank Coal
of East Kalimantan by using Acid and Base Activation
Yuli Patmawati
1
, Alwathan
1
and Nurkholis Hadi Ramadani
1
1
Department of Chemical Engineering, Politeknik Negeri Samarinda
Jl.Dr.Cipto Mangunkusumo, Kampus Gn.Lipan Po.Box 1341 Samarinda, Kalimantan Timur - Indonesia
Keywords: Activated Carbon, Activation, Low Rank Coal.
Abstract: Indonesia is one of the countries that has large coal reserves in the world. Low rank coal in Indonesia
reached 28.4% of coal reserves, 67,198,300,021 tons/year of low-rank coal production in East Kalimantan.
Despite their vast reserves, low-rank coals are considered undesirable because has less economic value;
their high moisture content, entails high transportation costs, potential safety hazards in transportation
and
storage and the low thermal efficiency obtained in combustion of
such coals. Besides improving its
economic and usage values, processing low-rank coal into activated carbon becomes alternative way of
utilization of abundant amount of low-rank coal. The aim of this research is to determine the characteristics
of activated carbon produced from low rank coal of East Kalimantan by using acid and base activation with
different activators : HCl,
H
3
PO
4
and NaOH. Low rank coal which has been prepared -100 +120 mesh is
carbonized at 600
0
C for 3 h, then after cold it was activated using 2.5 M concentration of HCl, H
3
PO
4
and
NaOH for 8 h. Furthermore proximate analysis were used to
investigate the characteristics of activated
carbon produced such as moisture content, ash content, volatile matter and fixed carbon. Meanwhile, the
adsorption capacity of activated carbon is determined through the iodine adsorption number. The quality
requirements for activated carbon refers to Indonesian National Standard (SNI 06-3730-1995). The best
results were obtained by using HCl with activated carbon characteristics such as a moisture content, ash
content, volatile matter, fixed carbon and iodine absorption number respectively as follows 5.23%, 11.72%,
8.85%, 74.2% and 660.40 mg/g. Activated carbon
is one of the mostly widely used adsorbents
.
In the
treatment
of wastewater, it is usually employed for purification, decolorization and the removal
of
toxic heavy metal ions and organic pollutants.
1 INTRODUCTION
Coal was formed by the decomposition of plant
matter , and
it is a complex substance that can be
found in many forms. Coal is divided into four
classes: lignite/low-rank coal, sub-bituminous,
bituminous and anthracite. Low-rank coal in
Indonesia reached 28.4% of coal reserves,
67,198,300,021 tons/year of low-rank coal
production in East Kalimantan (Demirbas, 2007).
Activated carbon is mainly composed of
carbonaceous material with various
porous
structures and it is one of the mostly widely used
adsorbents
(Bilal, 2016). Activated carbons have
been widely employed in water and wastewater treat-
ment processes for removing organic contaminants,
because they generally have
large adsorption
capacity (Tsai, 2001). This activated carbon has a
specific affinity for non-polar
compounds, such as
organics (Dong Su Kim, 2004).
Activated carbon
can be produced from different sources, such as
lignocellulosic materials, coal, bagasse ash, asphalt
and oil, waste tyre rubber, activated sludge and
others (Shawabkeh and Ghamdi, 2014).
Coal has the
potenti al as a raw material to produce activated
carbon because it has a high carbon content
(Speight, 1994).
The production process of activated carbon
mainly consists of three steps:
dehydration,
carbonization, and activation. Dehydration is a
drying process to
remove moisture content
from the raw material. During carbonization,
organics
contained in the raw material are
converted into primary carbon, which is a
mixture of amorphous, crystalline carbon, tar,
and ash. Activation is the main step in the
178
Patmawati, Y., Alwathan, . and Ramadani, N.
Characterization of Activated Carbon Prepare from Low-rank Coal of East Kalimantan by using Acid and Base Activation.
DOI: 10.5220/0010021900002905
In Proceedings of the 8th Annual Southeast Asian International Seminar (ASAIS 2019), pages 178-181
ISBN: 978-989-758-468-8
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
whole process and is usually carried out in two
ways:
physical and chemical activation
.
Physical
activation usually involves the carbonization of
pre-cursor followed by the gasification of the
resulting char or direct CO
2
/steam activation of
the starting material. Chemical activation involves
the impregnation of the given precursor with
activation agent such as phosphoric acid (H
3
PO
4
),
chloric acid (HCl), nitrit acid
(HNO
3
), zinc
chloride (ZnCl
2
), and alkaline
metal compounds
(Dong-Su Kim, 2004).
The
adsorption performance of activated carbon, to a
large extent, depends on the choice of activators
(Fen Li, Bo Yan and Tao Lei, 2014).
The adsorption capacity of activated carbon is
very important because this property determines
how much of the substance can be absorbed per
gram of carbon (Demirbas, 2007). This capacity is
related to the pore structure and chemical nature of
the carbon
surface in connection with preparation
conditions (Pehlivan and Cetin, 2008). The quality
requirements for activated carbon refers to
Indonesian National Standard (SNI 06-3730-1995)
with max.15% moisture content, max.10% ash
content, max. 25% volatile matter, min. 65% fixed
carbon and min. 750 mg/g iodine absorption number
(Departemen Perindustrian and Perdagangan, 2003).
Lots of research has been
reported on the
preparation of activated carbon from different
sources and on the effects of diferrent preparation
condition on the characteristics of the activated
carbon. Research conducted by Maulana et.all
(2017) “Activation Process of Candlenut Shell Use
Different Activators (
H
3
PO
4
, CaCl
2
, NaOH) a n d
Concentration”
The best result were obtained at 15%
concentration of NaOH, produce activated carbon
with characteristics of moisture content, ash content,
volatile matter, fixed carbon and iodine absorption
number respectively as follows 5.55%, 7.65%,
65.54%, 27.80% a n d 663.82 mg/g. Another study
was making of activated carbon from sub-
bituminous coal with chemical activation using
H
3
PO
4
and combination of
H
3
PO
4
-NH
4
HCO
3
activators
.
Sub-bituminous is carbonized at 600
o
C
for 3 h, continued by chemical activation for 8 h and
drying process at 600
o
C for 2 h. The best result were
obtained on the concentration
H
3
PO
4
-NH
4
HCO
3
2.5
M with moisture content of 7.4%, ash content of
10%, volatile matter of 39.1%, fixed carbon of 43.5
% and iodine absorption number of 1238.554 mg/g
(Kusdarini and Ghafarunnisa, 2017).
The aim of this research is to determine the
characteristics of activated carbon produced from
low rank coal of East Kalimantan by using acid and
base activation with different activators such as HCl,
H
3
PO
4
and NaOH.
2 METHODOLOGY
Low rank coal which has been prepared -100
+120 mesh is carbonized at 600
0
C for 3 h, then after
cold it was activated using 2.5 M concentration of
HCl,
H
3
PO
4
and NaOH for 8 h. Furthermore, an
analysis of activated carbon refers to Indonesian
National Standard SNI 06-3730-1995 was carried
out including moisture content, ash content, volatile
matter, fixed carbon and iodine absorption number.
Coal to be processed into activated carbon is
determined by parameters of moisture content, ash
content, volatile matter, fixed carbon, iodine
absorption number and calorific value respectively
as follows 37.86%, 5.53%, 25.06%, 31.55%, 215.75
mg/g and 3665 cal/g.
3 RESULTS AND DISCUSSION
Table 1 summarizes the results of activation low-
rank coal using acid and base activators.
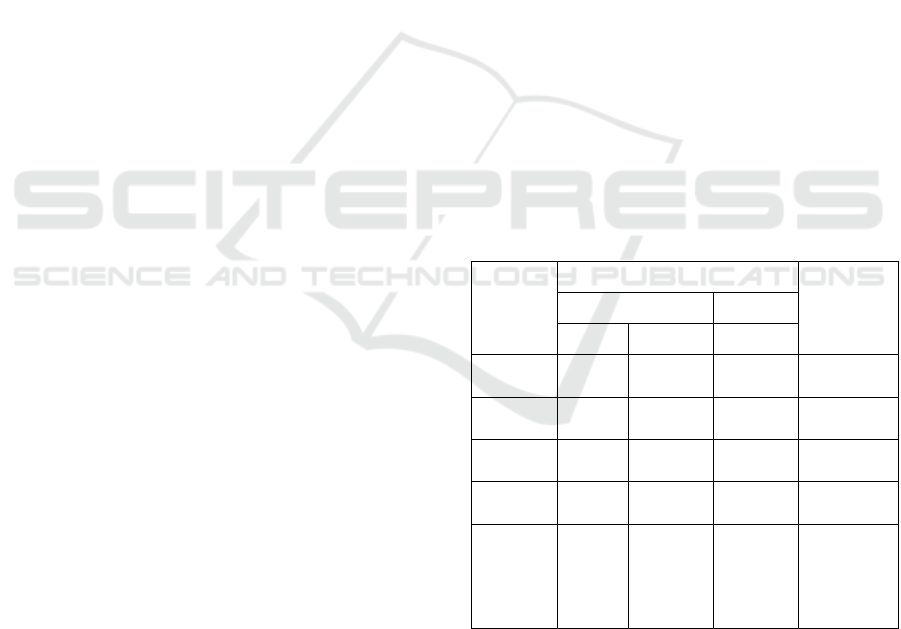
Table 1: Characteristics of Activated Carbon after
Activation Low-Rank Coal.
Parame
ter, %
Activators
SNI
Standard
Acid Base
HCl H
3
PO
4
NaOH
Moist.
Content
5.23 3.42 4.67 Max. 15%
Ash
Content
11.72 13.25 13.64 Max. 10%
Volatile
Matter
8.85 10.13 10.61 Max. 25%
Fixed
Carbon
74.20 73.20 71.08 Min. 65%
Iodine
Adsorpti
on
Number,
mg/g
660.4 469.53 479.11 Min. 750
Characterization of Activated Carbon Prepare from Low-rank Coal of East Kalimantan by using Acid and Base Activation
179
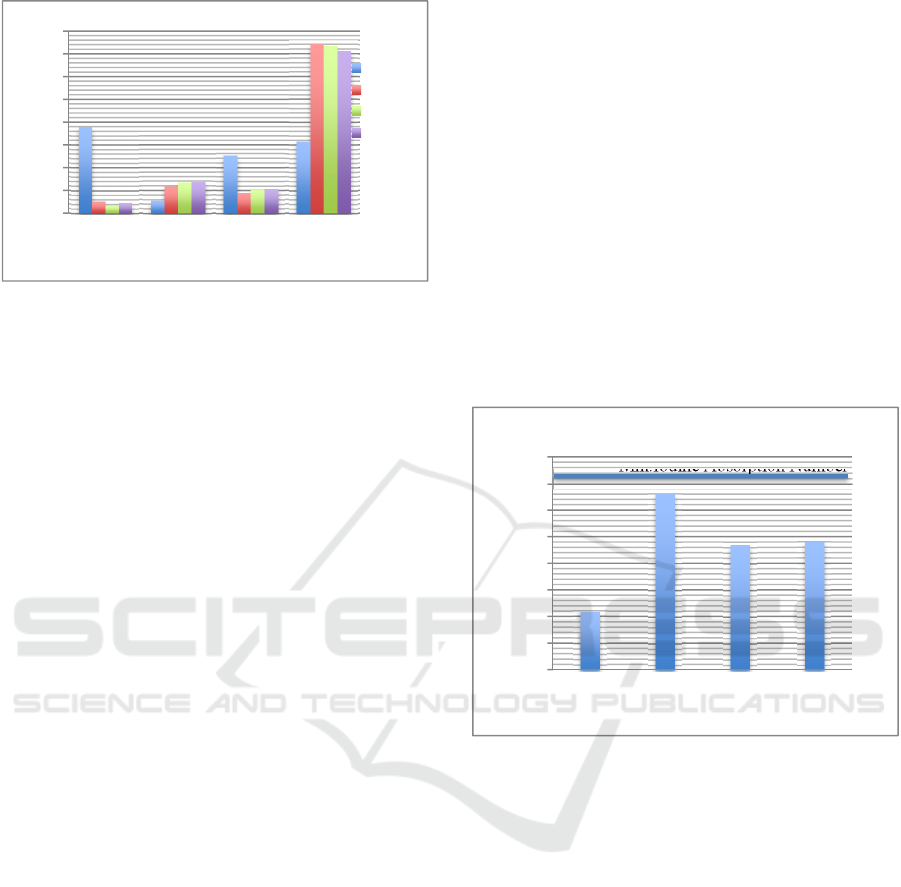
Figure 1: Characteristic of Low Rank Coal at Initial
Condition and After the Activation Process with Different
Activators.
In Figure 1 can be seen that moisture content of
low rank coal has decreased from 37.86% to 3.42 –
5.23%. The lowest moisture content produced by
coal activated by H
3
PO
4
is 3.42% while the highest
moisture content produced by coal activated by HCl
is 5.23%. Acid activators cause complex damage to
oxygen during the activation process so that the
moisture content in activated carbon is less than base
activators (Erawati and Fernando, 2018). But it does
not happen to HCl activators because the pH
produced is so small that it requires more water in
the neutralization process. This causes activated
carbon to absorb more water. Refers to Indonesian
National Standard (SNI 06-3730-1995), allowable
moisture content max. of 15%.
The ash content of the activated carbon has
increased from 5.53% to 11.72 – 13.64%. The
increase in ash content was due to the carbonization
process at high temperatures cause the oxidation of
most volatile substances including carbon. Whereas
ash is not oxidized because it is not a volatile
substance. Based on Figure 1, the use of acid
activators produces lower ash content compared to
alkaline/base activators. This is because the acid
activators of HCl and H
3
PO
4
bind together with the
alkaline elements in activated carbon and form salts
that dissolve easily in water. Conversely, the base
activators such as NaOH, contain the mineral
element (sodium, Na) will be absorbed in the pores
of activated carbon thereby increasing ash content in
activated carbon (Rahim and Indriyani, 2010). Based
on Indonesian National Standar (SNI 06-3730-
1995), allowable ash content max. of 10%.
The use of activators in the activation process is
able to reduce component of non-carbon compounds
found on the surface of activated carbon and enlarge
the surface pores of activated carbon (Maulina and
Iriansyah, 2018). Figure 1 shows acid and base
activators able to degrade organic matter that is
present on the surface of carbon and also release
volatile materials. The lowest volatile matter
produced by coal activated by HCl is 8.85% while
the highest volatile matter produced by coal
activated by NaOH is 10.61%. Refers to Indonesian
National Standard (SNI 06-3730-1995), max. 25%
volatile matter allowed.
The fixed carbon of low-rank coal was 31.55%,
it has increased after the activation process to 71.08
– 74.2%. This can be seen in Figure 1. The increased
in fixed carbon was due to decrease in moisture
content and volatile matter of activated carbon.
Refers to Indonesian National Standard (SNI 06-
3730-1995), allowable fixed carbon of min. 65%.
Figure 2: Iodine Absorption Number of Low-Rank Coal at
Initial Condition and After the Activation Process with
Different Activators.
The iodine adsorption number reflected the
adsorption per-formance of activated carbon, as
shown in Figure 2. It tends to increase from 215.75
mg/g to 469.53 mg/g – 660.40 mg/g. The iodine
absorption number of H
3
PO
4
and NaOH activators is
lower than HCl because some of minerals element
such as sodium (Na) in NaOH is absorbed in the
pores of activated carbon. It causes the micropore
structure that has been formed to be covered again
by the Na element, thereby reducing the absorption
capacity of activated carbon. The highest iodine
absorption number were obtained by using HCl
activators was 660.40 mg/g. Refers to Indonesian
National Standard (SNI 06-3730-1995), allowable
iodine adsorption number of min.750 mg/g. The
iodine absorption number of activated carbon in this
research still below the standards referred to.
0
10
20
30
40
50
60
70
80
Persentage, %
Parameters
Initial
HCl
H3PO4
NaOH
Moisture Ash Vol.Matter Fixed Carbon
0
100
200
300
400
500
600
700
800
Initial HCl H3PO4 NaOH
Iodine Absorption Number, mg/g
Initial and with Activators
Min.Iodine Absorption Number
ASAIS 2019 - Annual Southeast Asian International Seminar
180

4 CONCLUSIONS
1. Activation of low-rank coal of East
Kalimantan was evaluated by taking
different activators. The best result
were
obtained by using HCl with activated
carbon characteristics such as a moisture
content, ash content, volatile matter, fixed
carbon and iodine absorption number
respectively as follows 5.23%, 11.72%,
8.85%, 74.2% and 660.40 mg/g.
2.
Ash content and iodine absorption number
still below the standards referred to
Indonesian National Standard (SNI 06-
3730-1995).
ACKNOWLEDGEMENTS
The authors would like to acknowledge the
Department of Chemical Engineering, Polytechnic
State of Samarinda and PT.Sucofindo Samarinda for
the Proximate and Iodine Adsorption Number
Analysis.
REFERENCES
Bilal Khalid et.al., 2016. Effects of KOH Activation on
Surface Area, Porosity and Desalination
Performance
of Coconut Carbon Electrodes. Desalination and
Water Treatment Journal
57. pp. 2195–2202.
Demirbas, A., 2007. Utilization of Coal as a Source of
Chemical. Energy Sources : Part A : Recovery,
Utilization Environmental Effects. Sila Science,
Universite Mahallesi Trabzon, Turkey.
Dong-Su Kim, 2004.
Activated Carbon from Peach
Stones Using Phosphoric
Acid Activation at Medium
Temperatures.
Journal of Environmental Science
and Health Part A—Toxic/Hazardous Substances
& Environmental Engineering
Vol. A39. No. 5. pp.
1301–1318.
Department of Environmental Science
and Engineering
.
Ewha Womans University.
Korea.
Departemen Perindustrian dan Perdagangan, 2003. Syarat
Mutu dan Uji Arang Aktif SNI No. 06-3730-1995.
Balai Perindustrian dan Perdagangan.
Erawati and Fernando, 2018. Pengaruh Jenis Aktivator
dan Ukuran Karbon Aktif Terhadap Pembuatan
Adsorbent dari Serbuk Gergaji
kayu Sengon
(Paraserianthes Falcataria). Jurnal Integrasi Proses
Vol. 7. pp.
58-66. Unversitas Muhammadiyah
Surakarta.
Fen Li, Bo Yan, Yanping Zhang, Linhuan Zhang and Tao
Lei, 2014. Effect of activator on the structure and
desulphurization efficiency of sludge-activated carbon.
Environmental Technology. 35:20. pp. 2575-2581.
Kusdarini, E., Budianto, A. and Ghafarunnisa, 2017.
Produksi Karbon Aktif dari Batubara Bituminus
dengan Aktivasi Tunggal H
3
PO
4
, Kombinasi H
3
PO
4
-
NH
4
HCO
3
, dan Termal. Jurnal Reaktor UNDIP.
Maulana, G.G.R., Agustina, L. and Susi, S., 2017. Proses
Aktivasi Arang Aktif dari
Cangkang Kemiri
(Aleurites Moluccana) dengan Variasi Jenis dan
Konsentrasi Aktivator Kimia. Universitas Lambung
Mangkurat, Teknik
Industri Pertanian.
Maulina, S. and Iriansyah, M., 2018. Characteristics of
Activated Carbon Resulted From Pyrolysis of The Oil
Palm Fronds Powder. Universitas Sumatra Utara.
Teknik Kimia.
Muthusamy Karthikeyan, Wu Zhonghua and Arun S.
Mujumdar, 2009. Low-Rank Coal Drying
Technologies. Current Status and New Developments,
Drying Technology: An International Journal. 27:3. pp.
403-415.
Pehlivan, E. and Cetin, S., 2008. Application of Fly Ash
and Activated Carbon in the Removal of Cu
2+
and
Ni
2+
Ions from Aqueous Solutions. Energy Sources,
Part A: Recovery, Utilization, and Environmental
Effects. 30:13. pp. 1153-1165.
Rahim, M. and Indriyani, O.S., 2010. Pembuatan
Karbon Aktif dari Batubara Peringkat Rendah.
Jurnal Teknologi Media Perspektif. pp. 40-44.
Shawabkeh, R.A., Al-Harthi and Al-Ghamdi, 2014. The
Synthesis and Characterization of Microporous,
High
Surface Area Activated Carbon from Palm Seeds.
Energy Sources. Part A. 36:93–103.
Speight, J.G., 1994. The Chemistry and Technology of
Coal. Marcel Dekker. Inc. New York.
Tsai, et al., 2001. Characterization of Activated Carbons
Prepared From Sugarcane Bagasse By ZnCl
2
Activation. J.Environ.Sci.Health. B36(3). pp. 365 –
378.
Characterization of Activated Carbon Prepare from Low-rank Coal of East Kalimantan by using Acid and Base Activation
181
