
HACCP Plan Implementation for Food Safety for Startup Business:
Fruit Combining
Kartiko Setiyadi
1
,Tantri Yanuar Rahmat Syah
1
, Semerdanta Pusaka
1
and H. S. Darmansyah
1
1
Master of Management, Faculty of Economics and Business, Esa Unggul University, Jakarta
Keywords: Hazard Analysis, Critical Control Point, Food Production.
Abstract: HACCP is a tool to assess hazards and establish control systems that focus on preventative measures rather
than relying mainly on end-product testing. Seven basic principles underline the concept. These principles
include an assessment of the inherent risk that may be present from harvest through ultimate consumption.
Six hazard characteristics and a ranking schematic are used to identify those points throughout the food
production and distribution system whereby control must be exercised in order to reduce or eliminate
potential risks. A guide for HACCP plan development and critical control point (CCP) identification are
noted. Further, the document points out the additional areas that are to be included in the HACCP plan: the
need to establish critical limits that must be met at each CCP, appropriate monitoring procedures, corrective
action procedures to takes if a deviation is encountered, record keeping, and verification activities.
1 INTRODUCTION
Aware of a large number of deficiencies or
absence of food safety assurance is obtained from
conventional inspection and testing as well as
examples of many products. PT Redceri Indonesia
applies the concept of HACCP (Hazard Analysis
Critical Control Point), i.e. food safety assurance
system based upon a realization that hazard (hazard)
potentially arising at various points or stage
production, and must be controlled to prevent the
occurrence of such hazards. HACCP focuses on
hazards in a food commodity that if not controlled
could affect public health and food product design,
processing, commercialization, provision and the
conditions controlling the hazards.
HACCP systems is not a food safety assurance
systems without the risk or zero-risk. However,
HACCP is designed for minimizing the risk of food
safety hazards in the food production process.
HACCP systems also is a risk management tool that
is used to protect food supply chains and production
processes towards contamination hazards, chemical
and physical purity.
The benefits of the application of the HACCP
system for PT Redceri Indonesia is as follows:
Prevent or detect raw materials or unsafe
ingredient before entering the production system.
Keep the issue not be great and handled by
implementing early detection.
Be aware of the presence of contamination at
facilities that are used together for various
products.
Reduce the detention of products internally and
the destruction of the products.
Prevent dependence testing against a final
product that can cause the issue of unsafe
products.
Application of HACCP in the food industry are
specific for each type of product, every process,
every factory. Besides the basic prerequisite is
required in the form of application of GMP (Good
Manufacturing Practice) and SSOP (Sanitation
Standard Operating Procedures). An important
factor for the success of the application of HACCP
in the food industry is largely determined by the
commitment of management to provide safe food.
In the implementation of HACCP, PT Redceri
Indonesia implementing measures systematically in
the 12 steps, which consists of five initial steps of
preparation followed by seven the next step which is
the seven HACCP principles. As for the stages of
these steps are:
Stage 1 : Drafting HACCP team
Stage 2 : Description of products
Stage 3 : Identifying the purpose of the use of
Setiyadi, K., Yanuar Rahmat Syah, T., Pusaka, S. and Darmansyah, H.
HACCP Plan Implementation for Food Safety for Startup Business: Fruit Combining.
DOI: 10.5220/0009953828652872
In Proceedings of the 1st International Conference on Recent Innovations (ICRI 2018), pages 2865-2872
ISBN: 978-989-758-458-9
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
2865
Stage 4 : Compiling flowchart
Stage 5 : Confirm the flowchart in roomy
Stage 6 : Conduct a hazard analysis
Stage 7 : Determine critical control points
(CCP)
Stage 8 : Determine the critical limits for each
CCP
Stage 9 : Specify a monitoring or monitoring
system for each CCP
Stage 10 : Specify the action correction if there is
a deviation from the limit of critical
Stage 11 : Specify the verification procedure
Stage 12 : Specify the system documentation and
record keeping systems or recording
2 THE FORMATION OF THE
HACCP TEAM
The first step in the preparation of HACCP is
forming a team of several members with the
educational background or extensive work
experience (multidisciplinary). The number of
HACCP Team consisting of people from various
parts of 5-6 or academic backgrounds such as
microbiology, sanitation experts, chemists,
engineers, part purchase, part of the QA/QC. People
who are involved in the ideal team is included: (1)
Staff of Quality Assurance or Quality Control Staff;
(2) personnel Production Section (understand the
raw materials and the production process); and (3)
personnel of the technical/Engineering Section; and
(4) Microbiological Experts. One Member is chosen
as the next Chairman of the team. The Chairman of
the team should already understand the preparation
of HACCP plans or between teams already exist that
follow HACCP training and/or auditing HACCP.
The team formed in charge of drawing up an
HACCP plan. For it, teams should meet regularly to
conduct discussions and brainstorming in the
HACCP plan.
For PT Redceri Indonesia, HACCP team consists
of Section Head of Research and Development,
Production Supervisor, QA/QC Supervisor (as
Chairman), Section Head of operations, Purchasing
Staff and some of the employees as members.
3 PRODUCT DESCRIPTION
The Second Step in the preparation of HACCP
plans are describing the product. HACCP team
should choose which products to be made its
HACCP plan if you have more than one product
type.
The information must exist at the time described
the product include composition, characteristics of
finished products, processing methods are applied to
the product (aw, pH, moisture content), while
preserving the method applied to such products,
primary packaging, packaging for transportation,
storage conditions, method of distribution, the
recommended shelf life, special labeling, usage
instructions, special supervision in the distribution
and where the product will be sold.
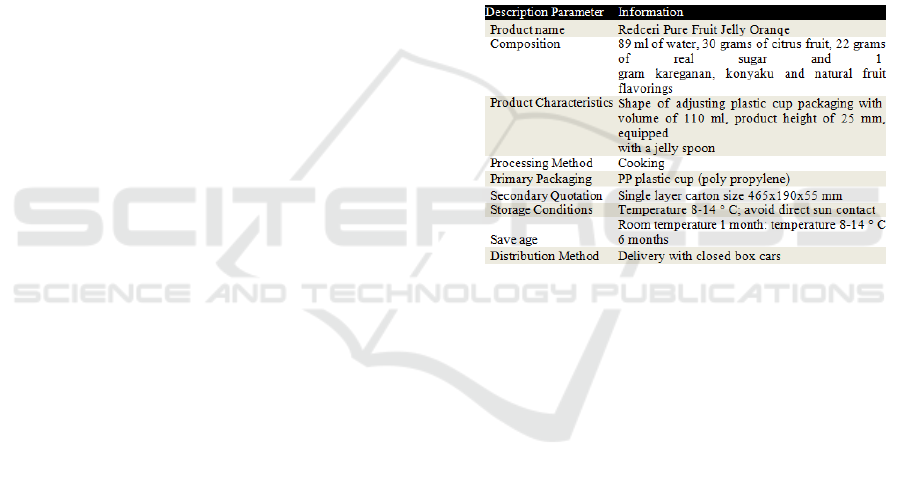
One example of PT Redceri Indonesia product
description for product Redceri Puree Fruit Jelly
Orange can be seen in the following table:
Table 1. Description of Product
Redceri Orange
4 THE DETERMINATION OF
THE USE OF THE PRODUCT
At this stage, the team identifies how to use
HACCP products by consumers, serving, as well as
a group of consumers who consume the products.
Important to know whether the product will be
directly consumed (ready to eat) or be cooked
beforehand by the consumer. It must be remembered
there are high-risk consumer groups which include
infants, the elderly, immuno compromised groups
(pregnant women, sick people, people who are
undergoing chemotherapy, AIDS patients).
For product Redceri Puree Fruit Jelly Orange,
descriptions of the users of its products is as follows:
can be in direct consumption by consumers from all
circles of society.
ICRI 2018 - International Conference Recent Innovation
2866

5 PRODUCT FLOW DIAGRAM
Process flow diagram was drawn up with the aim
to describe the entire process of production.
Flowchart of this process in addition to beneficial to
assist in performing the HACCP teamwork, can also
serve as a guideline for other person or institution
who would like to understand the process and
verification.
Flowchart should be covered all the stages in the
process are clearly concerning:
The details of the whole process of activities
including inspection, transportation, storage
and a delay in the process,
The materials to be included in such a process
of raw materials, packaging materials, water,
air and chemicals,
The output of the process such as waste:
packaging, raw material, product, product
reprocess in progress (rework), and products
that are disposed of (rejected).
6 VERIFY THE FLOWCHART IN
PLACE
In order for a process flowchart is made more
complete, and by the implementation on the ground,
then the HACCP team should review the operations
to test and prove the accuracy as well as the
perfection of the process flow diagram. When it
turns out that the process flow diagram is not right
or less than perfect, then to do modifications.
Flowchart of the process that have been made must
be documented and verified.
Flowchart process verified available, it can be done
by:
Observe the flow of the process.
Sampling Activities.
Interview.
Observe routine operations/non-routine.
7 THE ANALYSIS DANGER
Hazard Analysis include activity:
Identify hazards.
Determine the significance.
Identify precautions.
8 IDENTIFICATION OF DANGER
By referring to the flowchart process, HACCP
team lists all dangers real or potential that might be
worth is estimated to occur at each stage of the
process. Such dangers include the danger of
biological or chemical purity, dangers and physical
danger.
Study of the risk (the significance of) the dangers
a. The possibility of danger will occur
This is usually called the chance of danger will
occur. HACCP team needs to consider the likelihood
(odds) for any hazards that have been identified.
This inspection can be based on knowledge of
HACCP team; the literature on food microbiology,
HACCP, food products, and food processing,
scientific research papers; the journal; supplier; food
producers or processors; information regarding the
withdrawal of products; consumer complaints; the
areas of process, raw materials, or product that has
been identified is problematic. The possibility of
harm occurring in a simple can be rated as high,
medium, or low.
b. The level of seriousness of the Danger
Level of the seriousness of the danger can be
grouped as follows:
The seriousness of the hazard can be established
by looking at its effect on the health of the
consumer and also impact on the reputation of
the business.
The seriousness of the danger can also be
assessed: low, medium or high.
By combining opportunities with heavy and light
danger will be able to set the level of risk (the
SIGNIFICANCE of) the danger of being revealed as
high, medium or low. Such an approach can be used
to specify the type of control measures a must-have
in place and the higher the risk of danger, then the
higher the specified monitoring frequency
Thus the danger that there may also be classified
based on their significance, as shown in the table
below. The significance of the danger can be
decided by the team with the opportunity to consider
the occurrence of (reasonably likely to occur) and
severity (severity) of a danger.
c. Determination of Precautions
The next step after analyzing the dangers is to
identify the possible precautions to control any
hazards. The team then had to consider whether
precautionary measures, if any, can be applied to
any danger.
HACCP Plan Implementation for Food Safety for Startup Business: Fruit Combining
2867

LMH
L LL ML HL
M LM MM HM*
H LH MH* HH*
Peluang Terjadi
(
Reasonably like to occur
)
Tingkat Keparahan
(
Severity
)
Table 2. Determination Of The Significance Of Risk
Or Hazard Categories
Remarks:
L = Low, M = Medium, H = High
* Generally considered significant and will be considered
in the determination of the CCP
Precautions are all activities and activities that
are needed to eliminate hazards or minimize its
effects or its existence at an acceptable level. More
than one precautionary measures may be needed to
control the specific hazards, and more than one
hazard may be controlled by specific precautions.
Precautionary measures may include actions
which are chemical, physical or other controlling
food safety hazards. Precautionary measures in
tackling the danger can be more than one if needed.
This stage is an important stage after analysis of the
danger. Precautions are defined as any action that
may inhibit the incidence of danger into products
and refers to operating procedures are applied at
each stage of processing. Due to the nature of the
HACCP concept of prevention, then the HACCP
system in designing precautionary measures should
always be a concern. Here are a few examples of
precautions:
The separation of raw materials with a finished
product in storage.
Use a water source that already has security
requirements.
Calibration of the scales and gauges of
temperature.
using trucks that offer temperature control, etc.
Hazard analysis results poured in the table
analysis of hazards. In the case of the production of
Redceri Pure Fruit Jelly danger analysis table can be
seen in the following table :
Table 3. Hazard Production Analysis
OPPORTUNITY SEVERITY SIGNIFICANCE
AcceptanceofRawMateri al s B:Destructivemicrobes Storage StorageSOP
(peeledfruit,sugar, (Amylolytic)
carrageenan,flavoring) K:Heavymetals Takenfromsuppl ie r
L
L
TN Supplierguarantee
F:Gravel,Insects,Frui t Takenfromsuppli er Supplierguarantee
rotten,fruitsize
B:Coliform,E.Coli Factorywatersource M H N Tre atmentofWaterSanitation
K:Heavymetals Factorywatersource M
L
TN WaterAnalysis
F:Gravel,Insects,Objects Factoryenvironment TreatmentofWaterSanitation
foreign
ReceptionSupplie s (cup B:Microbes Storage SOPforStorageand
plastic,spoonjelly,lid, Supplierguarantee
cardboard) K:Heavymetals,migration Takenfromsupplier
L
L
TN Supplierguarantee
F:Brokenness,Clarity, Takenfromsuppli er Supplierguarantee
Perforated,cut
B:Microbe s,sporebacteria Directcontactofworker H H N GMP,SSOP(workerhygiene)
K:Chemicalcontamination, Air,workenvironment GMP,SSOP(areahygiene
Dust [work)
F:Gravel,Insects,Objects Air,workenvironment GMP,SSOP(areahygiene
foreign,rottenfruit [work)
B:Bacteria,Coliform,E.Coli Temperatureandcookingtime GMP,
SSOP(hygiene
notenough worker),CookingSOP
K:Dust,dirt Air,workenvironment GMP,SSOP(areahygiene
[work)
F:Mix er,Piping ToolsandPlantInstallation
L
MN[GMP,Mai ntenance
B:Bacteria,Molds Dirtycontainer [GMP,SSOP(areahygiene
[work)
K:Dirt,Dust Air,workenvironment GMP,SSOP(areahygiene
[work)
F:Rottenfruit,roomtemperature ToolsandPlantInstallation [GMP,SSOP,Timecontrol
>18°C,Insects andtemperature
F:Forei gnobjects Dirtyscales, Scal e ssani tation
Environment
|B:Bacteria(Salmonella) Insuff icie nttemperatureand
cookingtime|
HHNGMP,SSOP
(workerhygiene),
CookingSOP
|K:Dust,Dirt Air,workenvironment
L
L
TN GMP,SSOP(workareahygiene)j
F:Mix er,Piping ToolsandPlantInstallation
L
MNGMP,Mainte nance
B:Bacteria,Coliform,E.Coli,^
Salmonella
Dirtymachine H H N GMP,SSOP(workhygiene),SOP
Filling
|K:Dust,Dirt Air,workenvironment
L
L
TN GMP,SSOP(workareahygiene)j
F:Piping,Presssealing ToolsandPlantInstallation
L
MNGMP,Mainte nance
Dirtyscales,
L
L
TN Sanitationscales
Environment
11 Packing Packing F:Foreignbodycontamination,Leaking Worker L L TN GM P,SSOP(workerhygiene)
12 Storage Logistics F:Pests,Roomtemperature|>18°C,
Insects
Workers,factoryenvironment L M N GMP,SSOP,Timeandtemperature
control
8
Filling9
Weighing10
HAZARDIDENTIFICATION PREVENTIONACT
DANGERSIGNIFICANCE
JUSTIFICATIONOFCAUSES
JellyCooking Productio
n
F:Forei gnobjects
Preparati
on
SugarWaterCooking5
AREAINPUT/PROCESSSTAGENO
Fruitpreservation6
Weighing7
L
H
L
L
H
Warehou
se
1
Inputofcleanwater2
3
FruitSort4
TNL
M
L
L
H
L
L
L
L
LLTN
TN
H
LTN
N
HN
NM
LTN
TNL
LTN
LTN
TNL
LTN
ICRI 2018 - International Conference Recent Innovation
2868
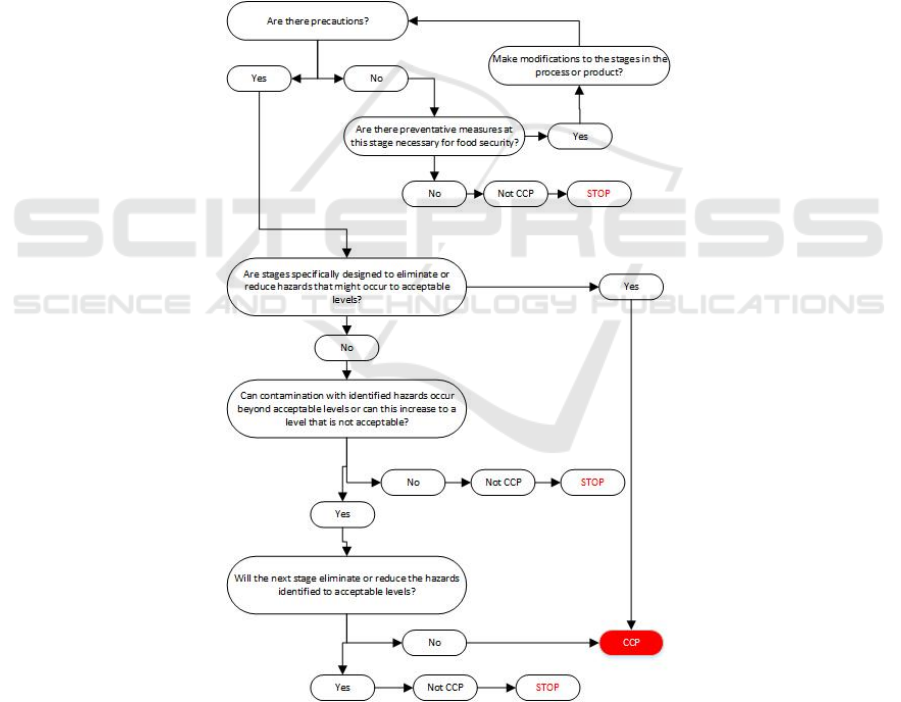
Remarks :
B (biological hazards), K (chemical hazards), F (physical
hazards), L (low), M (medium), H (high), TN (not real
danger), N (real / significant hazard).
9 DETERMINATION OF
CRITICAL CONTROL POINTS
OR CCP
For each significant hazard then it must be
specified whether or not included in the Critical
Control Point or not. A critical control point is a
stage or procedure where control can be applied and
a food safety hazard can be prevented, eliminated or
reduced to an acceptable level so that the risks can
be minimized. In this stage can not controlled then it
can cause hazard food safety HACCP team establish
where the dangers are high risks can be controlled.
CCP can be identified by using knowledge of the
production process and all potential hazards and
dangers of an analysis of the hazards and
precautions. To help find where it should be true,
CCP can use decision tree Diagram of CCP (CCP
Decision Tree).
Decision tree diagram is a logical question series
asking every danger. The answer to each question
will facilitate HACCP team and bring the logically
decide whether CCP or not.
In addition to decision tree diagram process, to help
set can also be used decision tree CCP for raw
materials and formulations.
Figure 1. Decision Tree Diagram Process CCP
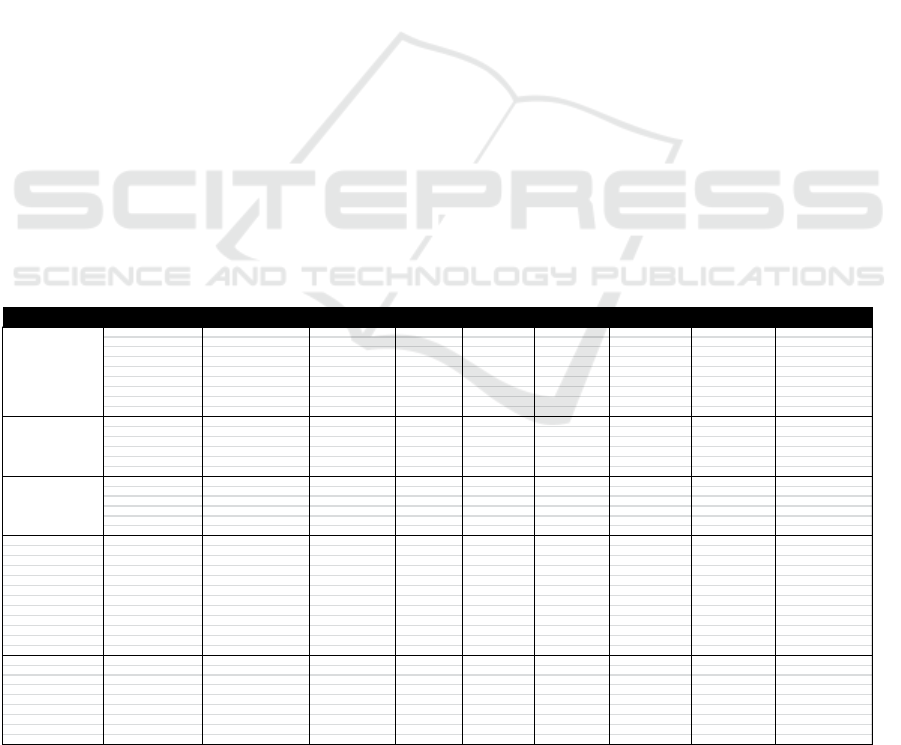
Examples of the results of the determination of
the CCP by the HACCP team PT Redceri Indonesia
Redceri on the production of Pure Fruit Jelly can be
seen in the following table:
HACCP Plan Implementation for Food Safety for Startup Business: Fruit Combining
2869

Table 4. The result of the determination CCP
10 THE DETERMINATION OF
CRITICAL LIMITS
For each CCP identified critical limits should be
determined then. Critical limit shows the difference
between products that are safe and not safe so that
the production process can be managed in a secure
level. These critical limits should not be passed to
ensure that the CCP is effectively in control of the
dangers of purity, chemical and physical. The
common criteria is used to determine the critical
limit is the physical criteria such as temperature,
time, humidity levels, and viscosity, as well as
chemical criteria such as pH, free chlorine residual,
acid levels. Microbiological criteria are not used as a
critical limit due to these measures take a long time.
In addition to physical and chemical measurements
can be used as indicators of measurement or control
of purity.
To set a critical limit can use data sources from
articles in the journal, regulations and Government
documentation, the guidelines of the Association,
publications of research at universities,
manufacturers, consultants and maker of the
equipment used.
11 SETTING PROCEDURE
MONITORING
Monitoring procedures (Monitoring) is a stage of
the observation or measurement of critical limits are
planned generate the proper recording and is
intended to ensure that the critical limit was able to
maintain the security of the product. HACCP team
set a series of monitoring procedure for each critical
limits are set that covers the what, who, where, when
and how the monitoring was done.
What question is answered with what should be
monitored, that is based on a critical limit is defined
as the temperature, time, size and so on. Answered
the question why the reason that if it not monitored
and the critical limit will cause certain dangers and
not allow cause insecurity products. The question
which should be answered at which point or at a
location where the monitoring should be done. The
question of how the ask method of monitoring,
whether in remote, chemistry or specific
measurements. Next is the question of when doing
monitoring, ideally a minimum which occurs in the
flow of production interruptions, or a lot, or other
data which establishes a period of monitoring. Last
is the question of who is doing the monitoring,
which ideally is the personnel who have access to a
very easy on the CCP, have the skills and knowledge
of the CCP and ways of monitoring, highly trained
and experienced.
By setting the critical limit is then obtained data
and information for the underlying decisions, got
early warning if there are any irregularities, to
prevent or minimize loss of product, indicating the
reasons for the problem and provide a document that
the product has been produced in accordance with
the HACCP plan. All documents related to record-
keeping and monitoring the CCP must be signed by
a person who does the monitoring and by the person
in charge.
12 SET THE ACTION OF THE
CORRECTIONS
Act Corrections is all the action taken if the
monitoring results on CCP deviations of critical
limits (losing control) because if control is lost, then
the product is not eligible. In practice, there are two
levels of correction actions, namely:
immediate action (Immediate Action), i.e. the
adjustment process to be controlled again and
deal with the suspected products affected by
the irregularities.
the precautionary measures (Preventive
Action), i.e., accountability for the action
recording and action correction.
INPUT/PROCESSSTAGE HAZARDIDENTIFICATION P1 P2 P3 P4 STATUS
Destructivemicrobes
Y
Y
CCP
Gravel,Insects,Fruitrotten,fruitsize
Y
Y
CCP
Inputofcleanwater Coliform,E.Coli
Y
NN NOTCCP
ReceptionSupplies(cup Mi crobes
Y
Y
CCP
plastic,spoonjelly,
lid,cardboard)
Brokenness,Clarity,Perforate d,cut Y N N NOTCCP
Microbes,sporebacteria
Y
Y
CCP
Dust
Y
N
Y
Y
NOTCCP
Gravel,Insects,Objects,foreign,
rottenfruit
Y Y CCP
Bacteria(Salmonella)
Y
Y
CCP
Dust,dirt
Y
N
Y
Y
NOTCCP
Mix er, Piping
Y
N
Y
Y
NOTCCP
Bacteria,Mol ds
Y
Y
CCP
Dirt,Dust
Y
N
Y
Y
NOTCCP
Rottenfruit,roomtemperature
Y
N
Y
N CCP
Bacteria,Coliform,E.Coli,Salmonella
Y
Y
CCP
Dust,Dirt
Y
N
Y
Y
NOTCCP
Piping,Pressseali ng
Y
N
Y
Y
NOTCCP
Bacteria(Salmonella)
Y
Y
CCP
Dust,Dirt
Y
N
Y
Y
NOTCCP
Mix er, Piping
Y
N
Y
Y
NOTCCP
Weighing Foreignobjects
Y
NN NOTCCP
Packing Foreignbodycontamination,Leaking
Y
N
Y
Y
NOTCCP
Storage Pests,Roomtemperature
Y
N
Y
N CCP
AcceptanceofRaw
Mate ri als(peeledfruit,
JellyCooking
Filling
Fruitpreservation
SugarWaterCooking
FruitSort
ICRI 2018 - International Conference Recent Innovation
2870
13 SET OF PROCEDURES
VERIFICATION
HACCP Team devised a procedure to assure that
the HACCP plan is already valid and that the
HACCP plan drawn up has been implemented as
planned. Verification is the application of a method,
procedures, tests or other evaluation to determine the
suitability of implementation with the HACCP plan.
Verification gives assurances that the HACCP plan
has complies with daily operations and will result in
the product Redceri Puree Fruit Jelly with good
quality and/or safe to consume. Specifically, the
verification procedure must ensure that:
The HACCP plan are applied absolutely right to
prevent the danger of the process and product
hazards.
Monitoring Procedures and corrective actions
still applied.
Internal audit, microbiology or chemical testing
on the final product.
14 DOCUMENTATION AND
RECORDINGS
Either documents or data records is written
evidence that an action has been performed. These
documents can be used (1) for inspection and (2) to
the study of lapses that resulted in the damage and
find the appropriate correction action. Type of
document (data records) that must be present in the
preparation of HACCP plans are:
HACCP plan and all supporting material.
Document monitoring.
Document Action correction.
Document verification.
He arranges with the system documentation, then
it was the preparation of HACCP plans the
production of PT Redceri Indonesia. HACCP plans
are subject to change in case of a change in raw
materials, the layout of the factory, equipment
replacement, cleaning or sanitation program
changes, the application of the new procedures,
changes in consumer products group and the
presence of new information about a hazard.
Determination of the CCP, the determination of
critical limits, designation procedures monitoring,
the setting of the correction action, determination of
procedures verification and documentation is good
next pour in the HACCP Plan table.
As for the HACCP Plan table for the production of
Redceri Pure Fruit Jelly was as follows:
Table 5. HACCP Plan PT. Redceri Indonesia
PROCESSSTAGE DOCUMENTATION
OFCCP WHAT HOW WHERE WHO WHEN ANDRECORD
Thereisnodirt Physicalconditionoffrui t Do Theplace Empl oye e Every 1.Contactstaff Reviewtheform recordof
(forei gnmaterial), peeland examination reception warehouse arrivaland QC/QAanddecide everyreceipt rawmaterialacceptance
standardsize, Certificateof visualand rawmaterial rece ption acceptedor
rejected month
notrottenand Analysis(CoA) check 2.Complainingto
smelling,guarantee supplierguaran tee supplier
supplier(CoA throughCoA
basedonSNI
3165:2009or
4230:2009)
Thereisnodirt Physicalconditionand Do Theplace Employe e Every 1.Contactstaff Reviewtheform recordof
(forei gnmaterial), Certificateof examination reception warehouse arri valand
QC/QAanddecide everyreceipt materialacceptance
supplierguarantee Analysis(CoA) visualand rawmateri al reception acceptedorrejected month raw
(CoAbasedonSNI) check 2.Complainingto
supplierguarantee supplier
throughCoA
Thereisnodirt Physicalconditionand Do Theplace Employe e Every 1.Contactstaff Reviewtheform recordof
(forei gnmaterial), Certificate
of examination reception warehouse arrivaland QC/QAanddecide everyreceipt receptionsuppl ies
contamination,NG Analysis(CoA) visualand rawmateri al reception acceptedorrejected month
product,guarantee check 2.Komplainto
supplier(CoA supplierguarantee supplier
basedonSNI) throughCoA
Inputofcleanwater 1.Clarity,color, Physicalconditi onofwater 1.Do Bak QA/
QCinspector 1.Everystart 1.Contactstaff Reviewtheform recordof
odorandcontaminati on input examination shelter Spv. productionprocess QC/QAanddecide rawwaterchecking rawwaterchecking
(attribute) visual rawwater Mai ntenance 2.Periodic6 qualify daily
2.Standardvariable 2.Periodictest onceamonth forprocess
cleanwater watercontent productionornot
input 2.Contacttheteam
maintenancefor
checkingconditions
watertreatment
factory
3.Complainingto
supplier
Sortfruit Thereisnodirt Physicalconditionoffruit Do Fruitsortingarea QA/QCinspector Everyprocess 1.Contactstaff Reviewthe sortform Sortingrecord
(forei gnmaterial), pee l and examination Spv.Production production QC/QAanddecide
dailyfruit fruit
notcontaminated, suitability visualand takeplace qualify
standardsize, internalstandard suitability forprocess
notrottenand standard productionornot
smells 2.Complaintsand
returntosection
warehouse
3.Accost
VERIFICATION
MONITORINGPROCEDURE
CORRECTION
MEASURES
Sugar,carrageenanand
flavori ngacceptance
Cup,plastic,jelly
spoon,lidandcarton
acceptance
Peelfruitacceptance
CRITICALLIMITS
HACCP Plan Implementation for Food Safety for Startup Business: Fruit Combining
2871
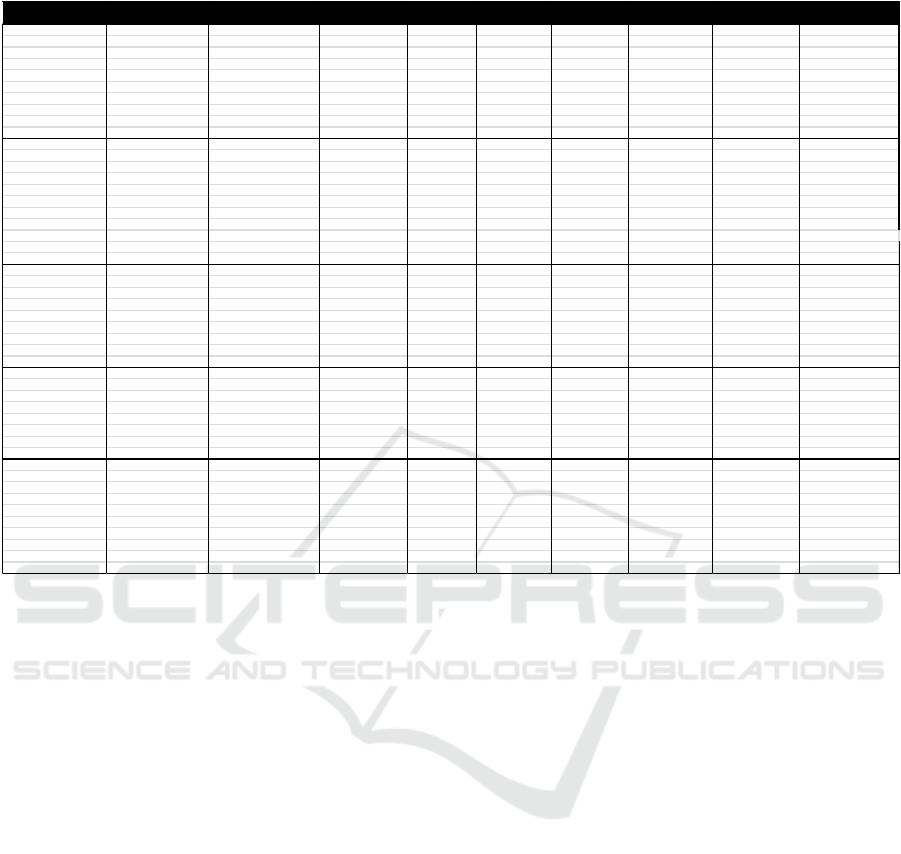
Table 5. HACCP Plan PT. Redceri Indonesia (Continued)
REFERENCES
Graham Friend and Stefan Zehle, Guide To Business
Planning, The Economist Newspaper
Porter, Micheal E. (1980). Competitive Strategy. New
York: Free Press.
Porter, Micheal E. (1985). Competitive Advantage. New
York: Free Press.
Osterwalder, Alexander & Pigneur, Yves. (2010).
Business Model Generation. New Jersey: John Wiley
& Sons Inc.
Jacobs, F. Robert & Chase, Richard B. (2011). Operations
and Supply Chain Management. Singapore: McGraw-
Hill.
Welsc, Glenn A. Budgeting: Profit Planning And Control,
Fourth Edition, Prentice Hall Inc., New Jersey, 1976.
Mulyadi, Sistem Perencanaan dan Pengendalian
Manajemen, Edisi Ketiga, Salemba Empat, Jakarta,
2007
PROCESSSTAGE DOCUMENTATION
OFCCP WHAT HOW WHERE WHO WHEN ANDRECORD
Fruitpreservation Thereisnodirt Tubcondition 1.Examination Area QA/QCinspector Faithfulprocess 1.Contactstaff 1.Reviewtheform 1.Recording
(foreignmaterial), prese rvation, bakandroutine prese rvation Operator producti on QC/QAanddecide fruitpreservation fruitpreservation
notcontaminated, temperature temperature preservation takeplace qualify daily 2.Checklistrecord
standardsize, preservative
room, 2.Do forprocess 2.Reviewtheform 3.Checkl istrecord
notrotten,smelling, physical conditionoffruit standardandmedia producti onornot sanitationchecklist roomtempe rature
roomtemperature peeland preservation 2.Productthatfailedequipment
<18°C,cleanliness cleanliness 3.Observe annihilated preservation
preservative, theworker hygieneconditions 3.Re viewtheform
workersanitationand
theworker temperaturechecklist
GMPissatisf ying room
Sugarwatercooking Thereisnodirt Tubcondition 1.Examination Cookingarea QA/QCinspector Faithfulprocess 1.Contactstaff 1.Reviewtheform 1.Recording
andjelly (foreignmaterial), prese rvation, temperatureandtime sugarwate randj
e
Operator production QC/QAanddecide 2.Reviewtheform cookingsugarwate r
notcontaminated, temperatureandtime 2.Do cooking takeplace qualify sanitationchecklist andjelly
standardbucket cookingand 3.Examination forprocess cookingequipment 2.Checklistrecord
rawmaterial, cleanliness scales producti onornot 3.Reviewtheform 3.Checklistrecord
tubcleanliness theworker theingredients 2.
Productthatfailedtemperaturecheckli st roomtemperature
preservative,sanitation used annihilated cookingtime 4.Records
worker,temperature 3.Observe 3.Accost 4.Reviewtheform useofmaterials
90°C, time hygieneconditions useofmaterials
workersandGMP theworker
satisfying
Filling Sanitationworkers, cleanliness 1.Examination Areafilli ng QA/QCinspector Faithful process 1.Contactstaff 1.Reviewthe
form 1.Producti onrecord
sanitaryfillingmachines, environment, cleanlinessofthearea Spv.Production production QC/QAanddecide dailyproduction 2.Checkl istrecord
andGMP cleanliness filling takeplace qualify 2.Reviewtheform 3.Checklistrecord
satisfying fillingmachine, 2.Do forprocess sanitationchecklist
andcleanliness 3.Examination productionornot fillingmachine
theworker cleanliness 2.Accost 3.Reviewtheform
4.Observe 3.
Productthatfailed workersanitation
hygieneconditions annihilated
theworker
Packing Sanitationworkers, cleanliness 1.Examination Packingarea QA/QCinspector Faithfulprocess 1.Contactstaff 1.Reviewtheform 1.Productionrecord
sanitarypacki ngarea, environment, cl eanlinessof thearea Spv.Producti on producti on QC/QAanddecide dailyproduction 2.Checklistrecord
andGMP andcleanliness packing takeplace qualif y 2.
Reviewtheform 3.Checkl istrecord
satisfying theworker 2.Do forprocess sanitationchecklist roomtemperature
3.Observe productionornot packingarea
hygieneconditions 2.Accost 3.Reviewtheform
theworker 3.Productthatfailed workersanitation
annihilated
Storage Sanitationarea cleanliness 1.Examination Area Logisticsstaff Every 1.Contactstaff 1.Reviewtheform 1.Recording
storage, environment, cleanlinessofthe
area storage Spv.Logisti cs acceptanceof QC/QAanddecide FGreceipts FGreceipts
roomtemperature andtemperature storage QA/QCinspector finishe dgoods qualify daily 2.Checklistrecord
storageand 2.Do tobesentor 2.Re viewtheform storage
GMPissatisf ying 3.Observe not sanitationchecklist 3.Checkl istrecord
temperature 2.Accost storagearea roomtemperature
storage 3.Theproduct 3.Reviewthe
form
defe ctindicated tempe raturechecklist
destroyed,after room
reworked
CRITICALLIMITS
MONITORINGPROCEDURE
CORRECTION
MEASURES
VERIFICATION
ICRI 2018 - International Conference Recent Innovation
2872
