
Brain Derived Neurotrophin Factor (BDNF) Level in Aged Sprague
Dawley Rats Brain after the Treatment of Centella Asiatica Leaf
Extracts
Indah Fitriani
*1
, Nathaniel Aditya
1
, Adisti Dwijayanti
2,3
, Desak Gede Budi Krisnamurti
2,3
, Erni
Hernawati Purwaningsih
2,3
, and Rani Wardani Hakim
2,3
1
Department of Medical Pharmacy, Faculty of Medicine, Universitas Indonesia
2
Undergraduate Student, Faculty of Medicine, Universitas Indonesia
3
Drug Development Research Cluster, Indonesian Medical Education and Research Institute (IMERI), Jakarta
Keywords: Centella asiatica, BDNF, Sprague-Dawley Rats, Aging
Abstract: Functional decrease in learning and memory is one of the characteristics of the aging process. It has been
known that a lower concentration of Brain Derived Neurotrophin Factor (BDNF) found on the brain, plays
a role in the phenomenon. This study was designed to determine whether a herbal plant, Centella asiatica
(CA), would increase the BDNF level on the aging brain tissue. 27 Male Sprague-Dawley rats aged 20-24
months and 4 weeks which were used in the study were divided into: negative control (were given
aquadest), positive control (supplementation of Vitamin E), young rats as a comparison (4 weeks old), and
treatment groups, which were given ethanol extract of CA leaves administered orally (300 mg/kg BW/week)
for 28 days. At the end of the treatment, the rats were terminated and the brain BDNF levels were assessed.
The data were analyzed by using a one-way ANOVA. The results showed a mean concentration of BDNF
for negative control, positive control group, young and treatment groups were 44.09±3.854, 43.09±11.99,
65.88±13.46, and 30.2±12.33 mmol/ml, respectively (p<0.05 vs control group). The treatment group
showed a higher BDNF level compared to all the groups. Interestingly, the BDNF level showed in the
positive control group were found to be lower than the treatment group. This result showed that the
supplementation of CA was effective in increasing BDNF brain level, thus raising the potential of having a
neuroprotective effect. These results implied the need of further research to find out the mechanism of
neuroprotective function exerted by CA.
1 INTRODUCTION
The global population is currently going through a
phenomenon called epidemiological transition. This
transition showed a pattern of population shifting
from high mortality and fertility pattern of
population to low mortality and fertility.The life
expectancy of population aged 65 years old and
above will continue to increase. In 2020, for the first
time in history, the population aged 60 years or more
would be higher than the children population aged
below 5 years. This shows that in the future, our
population would be constructed with more elderly
than before. (United Nations, 2017; WHO, 2011)
However, living longer does not mean living
healthier. Almost a quarter (23%) of the global
burden on morbidity and mortality of disease are
from the age group of people aged 60 years and
more. Elders are also more prone to many
communicable and non-communicable diseases.
Therefore, with the predicament of the increase of
this particular group, the global healthcare system
should answer accordingly. Beside of the increasing
age-span, ageing well should also be the goal of
future global healthcare system.(WHO, 2011)
One of the most aging-influenced aspect of
human life is cognitive health. Cognitive health
concerns about the brain function in ensuring the
independence of one individual, including the ability
of learning, intuition, judgment, language, and
remembering. A decline in cognitive function could
mean a loss of independence in older individuals,
causing burden to self and others. This decline
results from impaired neuronal plasticity (Tapia A et
al., 2008)
Fitriani, I., Aditya, N., Dwijayanti, A., Budi Krisnamurti, D., Purwaningsih, E. and Hakim, R.
Brain Derived Neurotrophin Factor (BDNF) Level in Aged Sprague Dawley Rats Brain after the Treatment of Centella asiatica Leaf Extracts.
DOI: 10.5220/0009842400002406
In Proceedings of BROMO Conference (BROMO 2018) - Symposium on Natural Product and Biodiversity, page 1
ISBN: 978-989-758-347-6
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
1

The pathogenesis of such neurodegenerative
condition still has not been established definitely and
involves multiple factors that influences several
systems, however it was known to be related to the
declining level of BDNF responsible for the loss of
the neuron function and structure.(Erickson et al.,
2012) BDNF has been known to have an important
roles in proliferation, differentiation, target
innervation, and survival of neurons of the central
and peripheral neuron system.(Tapia-Arancibia et al.,
2008). Lower levels of BDNF were also associated
with poorer memory. (Cunha, 2010)
Centella asiatica (CA), a small annual herb from
the family Apiaceae and native to Indonesia, India,
and many part of Asia has always been used widely
as a traditional medicines such as Aryuvedic
medicine, Chinese medicine, and many Southeast
Asian countries traditional medicine. (Lokanathan et
al., 2016). The CA, also known as pegagan in
Indonesia or gotu kola in India, have a significant
number of reviews on their medicinal uses along
with their supportive evidences (Gohil, Patel, &
Gajjar, 2010; Lokanathan et al., 2016; Orhan, 2012;
Rajakumari, 2010). Indicating the strong potential of
the plant in medicinal sector.
The primary active components of CA are
saponins (also called triterpenoid). (Singh & Rastogi,
1969) Saponins include asiaticoside, a trisaccharide
with aglycone asiatic acid, madecosside, and
madasiatic acid. These components are responsible
for some of the CA medicinal effects such as wound
healing and vascular effects. (Gohil et al., 2010)
CNS, cognitive, and antioxidant actions of CA
has been studied in many research and maybe due to
its brahmoside and brahminoside components, but
are yet to be confirmed by clinical studies. (Gohil et
al., 2010; Khotimah, Sumitro, Ali, & Widodo, 2015).
Khotimah et al also found that methanolic extract of
CA could increase the BDNF level in neuronal tissue
of Rattus noevegicus strain Wistar that were exposed
to lipopolysaccharide. (Khotimah, Riawan, &
Kalsum, 2009)
However, there has been a gap in the current
research, in which study of the neuroprotective
effects of the CA has never been demonstrated on a
subject with aging. Using 20-24 months old Sprague
Dawley rats, we hypothesized that treatment with
CA extract could effectively induce a
neuroprotective effect on old age rats, hence this
study aims to expand the knowledge in CA
specifically its effectiveness to the level of BDNF
found on the brain tissue of aged rats.
2 METHODS
2.1 Study Design & Subjects
The Sprague-Dawley (SD) young (2-3-month-old)
and aged (20-24-month-old) male rats, were obtained
from Research and Development Department of the
Ministry of Health. In total, there were 27 rats that
were used in this study. SD is a strain of albino rat
which is used in many researches because its
calmness and ease of handling.
Before the experiment, the rats were acclimatized
for 1 week with lighting of 12 hours (light on from
06:00 p.m. to 06:00 a.m.), constant room temperature
of 24
o
C, were given standard food and ad libitum
drink. The rats were divided into 4 groups: the aged
rats with no treatment as negative control, the aged
rats with treatment of CA extract (300mg/kgBW),
the aged rats with Vitamin E treatment of 6 IU and
the young rats. The Vitamin E treatment were used
as a positive control, whereas the young rats were
used to provide comparation between aged and
young rats. The animals were kept for 28 days under
the same environment where the rats underwent
acclimatization. The treatment were given once
every 7 days.
2.1.1 Daily Nutrients
The rats were fed with a type of pellets made from a
mixture of cornmeal, rice bran powder, fishmeal,
soybean, coconut, meat and bone meal, oat, ground
nut, canola, skimmed milk, and fish pellet with brand
names of SPA-Z and FF999. This standard pellets
contained 18.5%-20.5% protein, 4% fat, 6% fiber,
8% ash, 0,9% calcium, 0,7% phosphor and has
metabolized energy of 3100-3200 kcal/kg. The rats
were given water, ad libitum.
2.2 Extraction of CA
The CA leaves were dried on a drying racks or
sundried until all the water content evaporated. After
being dried, the CA leaves were then grinded until
the leaves become powdery. Then, the grinded CA
leaves were extracted/macerated with an ethanol
solvent, until all the active constituents were
dissoluted into the solvent. This extraction process
were performed for 24-48 hours and then proceeded
with a separation of the active component from the
solvent. This was achieved by evaporation using
rotary evaporator. At last, the gravimetry analysis
were performed to analyze the water content so that
the solute percentage can be determined.
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
2
2.3 BDNF Measurement by ELISA
After 28 days of treatment, the rats were sacrificed
by an intraperitoneal injection of ketamine and
xylacine. Consequently, the rats were dissected and
the tissue were obtained. The brain tissues were then
isolated and contained in an alcohol solution in
separate tubes. The tubes then were stored in a
refrigerated storage.
BDNF ELISA kit were used to evaluate the
BDNF level in the brain tissue. The ELISA kit that
were used were from ELabScience
®
. This ELISA kit
utilizes a Sandwich-ELISA method. The ELISA kit
has micro plates precoated with antibodies specific
to the rat BDNF. The samples were then added to the
micro plate wells and combined with the antibody.
Next, added to the well were biotinylated antibodies
specific for rat BDNF and Avidin-Horseradish
Peroxidase (HRP) conjugates. The microplates were
then incubated. After incubation, the free
components were washed away. The next step was to
add a substrate reagent to each wells, those wells that
contained rat BDNF, biotinylated detection antibody,
and Avidin-HRP conjugate would appear blue. The
termination of the enzyme-substrate reaction was
achieved by adding Stop Solution, changing the
samples appearance yellow in color. A
spectrophotometry were then used to measure the
optical density (OD) of the samples at the 450nm ± 2
nm wavelength. The OD value would be
proportional to the BDNF level. A comparation of
the samples OD to the standard curve would show
the level of BDNF in the samples. Here, we
measured every each of the subjects brain tissue
BDNF level in a grouped manner.
2.4 Statistical Analysis
All the grouped data from the ELISA tests were then
analyzed with an ordinary one-way analysis of
variance (ANOVA) using the Graphpad Software,
Inc statistical analyzer, Prism 7 for Windows. The
results obtained from this analysis were presented as
mean data ± SD. P values of less than 0.05 were
considered indicating statistical significance.
2.3 Ethical Consideration
The Healths Research Ethical Committee, Faculty of
Medicine, Universitas Indonesia – Cipto
Mangunkusumo Hospital approved the study
protocol and the usage of animal sibjects (December
2016). The ethic registration number is
1016/UN2.F1/ETIK/2016 and
402/UN2.F1/ETIK/IV/2018.
3 RESULTS & DISCUSSION
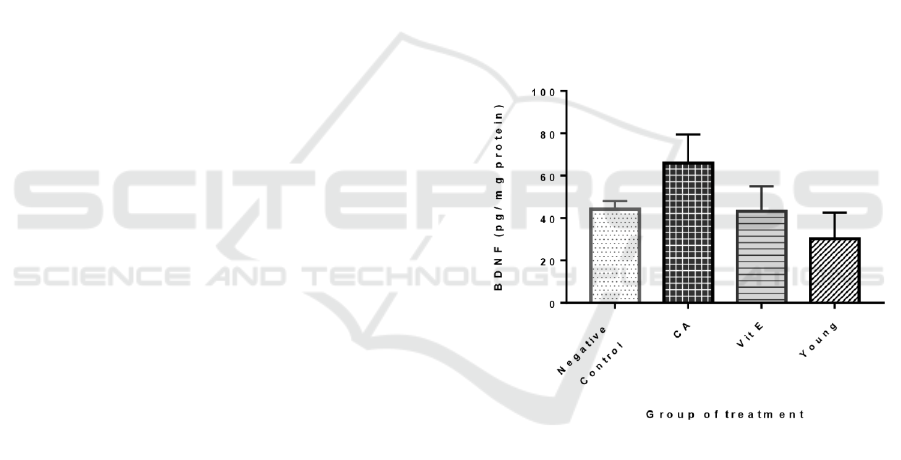
The results showed the BDNF level mean ± SD for
the negative control, the treatment groups, the Vit E
group, and the young group were 44.09±3.854,
65.88±13.46, 43.09±11.99, and 30.2±12.33
mmol/ml, respectively. In the rats treated with C.
asiatica (300mg/kgBW), the BDNF level were found
to be significantly increased (p<0.05 vs control
group). However, in the group treated with Vitamin
E, there was no significant change in the BDNF level
compared to the negative control. There were also no
significant difference of the BDNF level found
between the young and negative control group. The
highest concentration of BDNF was found on the CA
treatment group, meanwhile the lowest was found on
the Vitamin E group. (Figure 1)
O n e – w a y A N O V A d a t a
Figure 1: The BDNF levels (pg/mg protein) in 4 groups of
Sprague Dawley rats. The data are shown in mean ± SD (P
= 0.0014)
BDNF, a neurotrophin that functions in
maintaining the integrity of neuron system, have
long been thought to play a role in brain aging. Here
in this study we tried to demonstrate the effect of CA
on the brain BDNF level in an effort to reverse the
effect of declining BDNF level on the aging brain.
Our findings demonstrated that the ethanol extract of
CA succeeded in increasing the BDNF level in the
aged rat brain tissue compared to the negative
control. This results was consistent with previous
study that used LPS-induced rats (Khotimah et al.,
2012). Khotimah et al. also stated CA extract
Brain Derived Neurotrophin Factor (BDNF) Level in Aged Sprague Dawley Rats Brain after the Treatment of Centella asiatica Leaf Extracts
3

elevated BDNF level through induction on the
expression of BDNF in brain tissue. Moreover,
another study also showed the antioxidant and
antiinflammatory properties of CA play a role in the
neuroprotective effect of CA. (Gohil et al., 2010).
Studies about CA have showed its remarkable effects
on brain aging (Orhan, 2012). This effects have been
generally attributed to its tripertene saponosides
which covered many substances. However, the main
active component varies between studies. Some of
the tripertenes components that have been showed
responsible for CA neuroprotective effects are
asiaticoside, madecassoside, brahmoside, and
brahminoside. Unfortunately, the current studies
results are limited to answer this question. (Gohil et
al., 2010; Orhan, 2012)
In the results, it is apparent that the BDNF level
on the CA group was increased significantly but not
on the Vitamin E group. This contradicted our
former idea of the vitamin E activity as an
antioxidant was expected to enhance the BDNF
levels. However, study from Sakr et al. conformed
with this results (Sakr et al., 2015). According to
Sakr, this result might be due to the different
between CA and Vitamin E mechanism of action on
the BDNF. This previous research showed that CA
extract increased the expression of BDNF, while
Vitamin E treatment did not exert the same effect.
Hence suggestedly the absence of the BDNF level
increase in VIt E group. This could be the reason
why there was a difference shown by the 2 groups.
Still however, CA and Vitamin E both also achieved
their neuroprotective effect through antioxidant and
anti-inflammatory activities. (Khotimah et al., 2009;
Sakr et al., 2015)
Meanwhile, the young rats group had lower level
of BDNF than the control group (young:
44.09±3.854, control: 43.09±11.99 pg/mg protein)
although it was not significantly different. According
to the studies about the BDNF level during aging it
was shown that the BDNF expression could also be
induced by the process of neuron degeneration to
increase the growth of neuron, which happened
naturally in aged rats (Katoh-Semba et al., 1998).
Moreover, in accordance to this study by Katoh-
Semba et al., young and very young rats will have
lower BDNF. However, another research by Cunha
et al. showed that this phenomenon differs in rats
with neurodegenerative condition. In rats with
neurodegenerative condition, the BDNF level is
expected to be low beacuse of the defect on the
BDNF regulation, causing the compensation
mechanism to not occur. This would be manifested
in the clinical symptoms of the neurodegenerative
diseases (Cunha, 2010).
4 CONCLUSIONS
The results demonstrated that the BDNF levels were
increased with CA extract treatment. In addition, this
study also found evidence that Vitamin E treatment
attenuated the oxidative stress thus decreasing the
BDNF expression. Whereas, in young rats the BDNF
was lower than expected due to less oxidative stress
as the BDNF expression trigger. These results call
for further studies especially to determine the
molecular mechanism of how CA influences BDNF.
ACKNOWLEDGEMENTS
We would like to show our gratitude to Publikasi
Terindeks Internasional Untuk Tugas Akhir
Mahasiswa UI (PITTA) Grant by Universitas
Indonesia that give us opportunity to publish this
study.
REFERENCES
Cunha. (2010). A simple role for BDNF in learning and
memory? Frontiers in Molecular Neuroscience.
doi:10.3389/neuro.02.001.2010
Erickson, K. I., Miller, D. L., & Roecklein, K. A. (2012).
The Aging Hippocampus: Interactions between
Exercise, Depression, and BDNF. The
Neuroscientist, 18, 82–97.
Gohil, K., Patel, J., & Gajjar, A. (2010). Pharmacological
review on Centella asiatica: A potential herbal cure-all.
Indian Journal of Pharmaceutical Sciences, 72, 546.
Khotimah, H., Riawan, W., & Kalsum, U. (2009). Efek
Neuroprotektif Ekstrak Pegagan (Centella asiatica)
terhadap BDNF, TNFaR, NFkB, dan Apoptosis pada
Kultur Sel Syaraf yang Diinduksi LPS.
Khotimah, H., Riawan, W., & Kalsum, U. (2012).
Neurostimulant and Neuroprotective Effect of Centella
asiatica : In Vitro and In Vivo Studies. International
Symposium of Austronesian Humanities and Custom
Medicine.
Khotimah, H., Sumitro, S. B., Ali, M., & Widodo, M. A.
(2015). Standardized Centella Asiatica Increased
Brain- Derived Neurotrophic Factor and Decreased
Apoptosis of Dopaminergic Neuron in Rotenone-
Induced Zebrafish. GSTF Journal of Psychology
(JPsych), 2, 4.
Lokanathan, Y., Omar, N., Ahmad Puzi, N. N., Saim, A.,
& Hj Idrus, R. (2016). Recent Updates in
Neuroprotective and Neuroregenerative Potential of
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
4

Centella asiatica. The Malaysian Journal of Medical
Sciences: MJMS, 23, 4–14.
Orhan, I. E. (2012). Centella asiatica (L.) Urban: From
Traditional Medicine to Modern Medicine with
Neuroprotective Potential. Evidence-Based
Complementary and Alternative Medicine, 2012,1–8.
Patterson, S. L., Grover, L. M., Schwartzkroin, P. A., &
Bothwell, M. (1992). Neurotrophin expression in rat
hippocampal slices: a stimulus paradigm inducing LTP
in CA1 evokes increases in BDNF and NT-3 mRNAs.
Neuron, 9, 1081–1088.
Rajakumari, S. (2010). Enhancement of memory in rats
with Centella asiatica (Vol. 21).
Sakr, H. F., Abbas, A. M., & El Samanoudy, A. Z. (2015).
Effect of vitamin E on cerebral cortical oxidative stress
and brain-derived neurotrophic factor gene expression
induced by hypoxia and exercise in rats. Journal of
Physiology and Pharmacology: An Official Journal of
the Polish Physiological Society, 66, 191–202.
Singh, B., & Rastogi, R. P. (1969). A reinvestigation of the
triterpenes of Centella asiatica. Phytochemistry, 8,
917–921.
Tanaka, J.-I., Horiike, Y., Matsuzaki, M., Miyazaki, T.,
Ellis-Davies, G. C. R., & Kasai, H. (2008). Protein
synthesis and neurotrophin-dependent structural
plasticity of single dendritic spines. Science (New
York, N.Y.), 319, 1683–1687.
Tapia-Arancibia, L., Aliaga, E., Silhol, M., & Arancibia, S.
(2008). New insights into brain BDNF function in
normal aging and Alzheimer disease. Brain Research
Reviews, 59, 201–220.
United Nations: Department of Economic and Social
Affairs, Population Division. (2017). The end of high
fertility is near. (No. Population Facts No. 2017/3).
United Nations.
World Health Organization, & National Institute of Aging.
(2011). Global Health and Aging. Geneva.
Brain Derived Neurotrophin Factor (BDNF) Level in Aged Sprague Dawley Rats Brain after the Treatment of Centella asiatica Leaf Extracts
5
