
Utilization of Red Onion Skin Waste as Natural Dyes
Rudi Firyanto,
Soebiyono, and Dian Anung Putri
Department of Chemical Engineering, Universitas 17 Agustus 1945 Semarang, Indonesia
Keywords: Onion skin, Anthocyanin, Experimental design.
Abstract: Red onion skin waste can be used as food coloring. Red onion skin is one source of brownish orange color
derived from anthocyanin compounds and is used as natural dyes for traditional foods. The purpose of this
study was to determine the most influential variables of reaction time, material size, and ratio weight
material to the volume solvent in red onion skin extraction. The research method used is an experimental
design method, where this method means a set of differences designed to obtain evidence of a hypothesis
data. This research was carried out at a temperature of 80
o
C and using 80% ethanol solvent. The results
showed that the most influential was the ratio of weight material to volume solvent. The optimum results
were obtained at a ratio of 1 gr: 14 ml with an extraction time of 2 hours and a size of 60-80 mesh.
1 INTRODUCTION
The addition of food additives, especially food
coloring, aims to provide a more attractive color,
sometimes the use of food coloring agents does not
pay attention to their effects on health. Some parties
use harmful dyes to produce attractive and selling
food products to get the maximum profit.
Dyes commonly used are natural coloring agents
and synthetic coloring agents. Natural coloring
agents, made from extracts of certain plant parts.
Synthetic dyes, made from chemicals. Compared to
natural dyes, synthetic dyes have several advantages,
namely more color choices, easy to store and long
lasting. Some of the weaknesses of synthetic dyes
include carcinogenic and toxic properties (Winarno,
1997).
The use of natural dyes has been widely used by
the community, among others, the yellow color of
turmeric, the green color of the suji leaf, the purple
color of purple sweet potato, the black color of the
straw and others. Onion skin is one of the sources of
brownish orange color derived from anthocyanin
compounds and is used as a coloring agent for
traditional foods (Cahyadi, 2009)
Oancea (2013) conducted a study that found the
highest total Anthocyanin 99.66 mg/100 g of
anthocyanin extract ingredients from the outer skin
part of dried onion grown in Romania. The solvents
used were ethanol/acetic acid/water (50/8/42),
ethanol/acetic acid / water (70/4/26), ethanol/acetic
acid/water (80/1/19); 50% ethanol (v/v); ethanol
70% (v/v), and ethanol 80% (v/v). From the results
of the study, the best type of solvent is 80% ethanol.
Red onion also have high levels of flavonoids,
especially in the form of quercetin. Quercetin is a
flavonoid compound that can reduce blood pressure
and prevent plaque in arteries that can cause strokes.
The content of flavonoids in 1 kg of onion (Allium
ascalonicum) is approximately 415-1917 mg. The
onion skin has more antioxidants than the onion
itself.
Concerns about the safety of the use of synthetic
dyes encourage the development of natural dyes as
food coloring ingredients. The use of synthetic dyes
can be replaced with natural dyes. Red onion skin
can be used as a natural food coloring because it has
a color pigment, namely anthocyanin compounds.
These compounds play a role in the onion skin
coloring (Jackman, 1996).
Red onion skin is commonly found as household
waste and has been underutilized optimally and it's
useless . To be able to utilize the onion skin waste, it
can be used as food coloring. So that the onion skin
waste can be something more economical and has a
selling value.
Hussein and Alhassanen extracted the onion skin
as a dye using the reflux process, this is done so that
the anthocyanin compounds found in the onion skin
are easier to extract. The extraction process is
carried out for 40 minutes. So in this study
variations will be made with the smallest extraction
Firyanto, R., Soebiyono, . and Anung Putri, D.
Utilization of Red Onion Skin Waste as Natural Dyes.
DOI: 10.5220/0009012704350438
In Proceedings of the 7th Engineering International Conference on Education, Concept and Application on Green Technology (EIC 2018), pages 435-438
ISBN: 978-989-758-411-4
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
435
time of 40 minutes and the largest is 120 minutes to
determine the effect of the extraction time. In
addition, variations in material size and comparison
between ingredients and solvents were added to see
which conditions were the most optimum in the
process of taking dyes on the onion skin.
There are a number of extraction methods, the
simplest is cold extraction, in this way the dried
material produced by the mill is extracted at room
temperature in a row with the solvent with the higher
polarity. The advantage of this method is that the
extraction method is easy because the extract is not
heated so it is less possibility that the natural
material will decompose.
The use of solvents with increased polarity of
natural materials will separate natural ingredients
based on solubility. This makes the isolation process
becomes easier (Rodrigues et al., 2003).
2 MATERIALS AND METHOD
2.1 Material
In this study the material used is dry onion skin on
the market regardless of its type. Another supporting
material used in this study is 80% ethanol as a
solvent
2.2 Experimental
2.2.1 Sample Preparation
Red onion skin is cleaned from dirt by rinsing use
running water. Dry under the sun and oven at 50°C
until dry. After drying and mashed with blending
and then sieved with a 40-60 mesh and 80-100 mesh
sieve.
Research methodology used is Experimental
design. Experimental design is a set of data designed
to obtain concrete data to prove a hypothesis.
Experimental design method with two-level factorial
design, low level (-) and high level (+) is used for
reasons because a little run for each variable is
investigated, so it can save time, cost, and material.
In this study using a fixed variable: temperature
80
o
C and 80% ethanol solvent, while the variable
changed: extraction time (t) 40 and 120 minutes, the
ratio of ingredients solvent (R) was 1:10 and 1:15,
the size of the material (N) was 40-60 and 80-100
mesh.
The tool used is an extraction tool (reflux)
consisting of three neck flasks, condensor, hot
plates, magnetic stirrers, ovens, thermometers, water
bath.
2.2.2 Extraction Process
The onion skin is weighed as needed, put in a three-
neck flask. 80% ethanol is put into a three-neck
flask, then assemble the appliance and attach a hose
that connects the condenser and water tap as shown
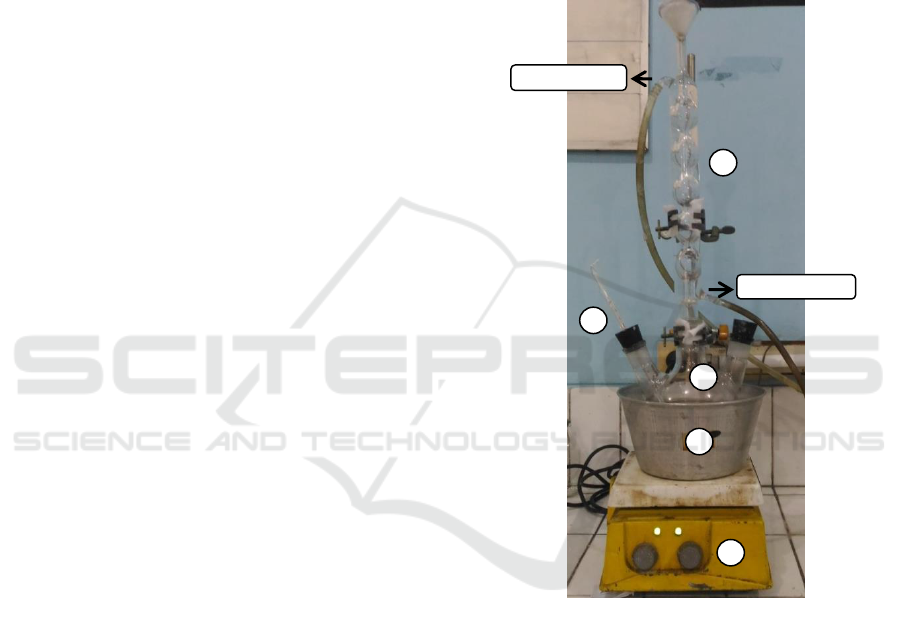
in Figure 1.
(1) Condensor, (2) three neck flasks, (3)
thermometers, (4) water bath, (5) hot plate
Figure 1: Extraction tool.
Extraction is done by varying the extraction time,
material:solvent ratio and material size. The
extraction results obtained were then concentrated in
a waterbath at 50
o
C. Then weighed and calculated
the resulting dyes content.
Anthocyanin testing was carried out by 2 mL red
onion skin extract added with 2 mL HCl 2 M. Then
it is heated at 100 ° C for 5 minutes, if it appears red
then the result is positive.
5
Water
inlet
Water
outlet
4
3
2
1
EIC 2018 - The 7th Engineering International Conference (EIC), Engineering International Conference on Education, Concept and
Application on Green Technology
436
3 RESULTS AND DISCUSSION
The results of the research that have been carried out
are obtained shown in Table 1.
Table 1: Results observations of yield.
t
(minutes)
R
(rasio)
N
(mesh)
Yield
(%)
40
1 : 10
40-60
5.52
120
1 : 10
40-60
5.68
40
1 : 15
40-60
6.63
120
1 : 15
40-60
7.38
40
1 : 10
80-100
6.66
120
1 : 10
80-100
6.80
40
1 : 15
80-100
8.92
120
1 : 15
80-100
10.61
The yield results from Table 1 are included in the
calculation formula for the effect, to find the most
influential variables using chart % P vs Z (Normal
Probability Curve) and % P vs I.
Calculation of the main effects:
I
0
= ⅛ (Y
1
+ Y
2
+ Y
3
+ Y
4
+ Y
5
+ Y
6
+ Y
7
+ Y
8
)
I
t
= ¼ (-Y
1
+ Y
2
– Y
3
+ Y
4
– Y
5
+ Y
6
– Y
7
+ Y
8
)
I
R
= ¼ (-Y
1
– Y
2
+ Y
3
+ Y
4
– Y
5
– Y
6
+ Y
7
+ Y
8
)
I
N
= ¼ (-Y
1
– Y
2
–Y
3
– Y
4
+ Y
5
+ Y
6
+ Y
7
+ Y
8
)
Calculation of interaction effects:
I
t.R
= ¼ (Y
1
– Y
2
– Y
3
+ Y
4
+ Y
5
– Y
6
– Y
7
+ Y
8
)
I
t.N
= ¼ (Y
1
– Y
2
+ Y
3
– Y
4
– Y
5
+ Y
6
– Y
7
+ Y
8
)
I
R.N
= ¼ (Y
1
+ Y
2
– Y
3
– Y
4
– Y
5
– Y
6
+ Y
7
+ Y
8
)
I
t.R.N
= ¼ (-Y
1
+ Y
2
+ Y
3
– Y
4
+ Y
5
– Y
6
– Y
7
+ Y
8
)
The results of the calculation of effects are
presented in Table 2.
Table 2: The results of the calculation of the effect value.
No.
Effect
Efffect
Identity
%P=
%100
5
n
i
1
0,2281
I
t.N
7,14
2
0,2381
I
t.R.N
21,43
3
0,5367
I
t.R
35,71
4
0,6867
I
t
50,00
5
0,8127
I
R.N
64,28
6
1,9427
I
N
78,57
7
2,2189
I
R
92,86
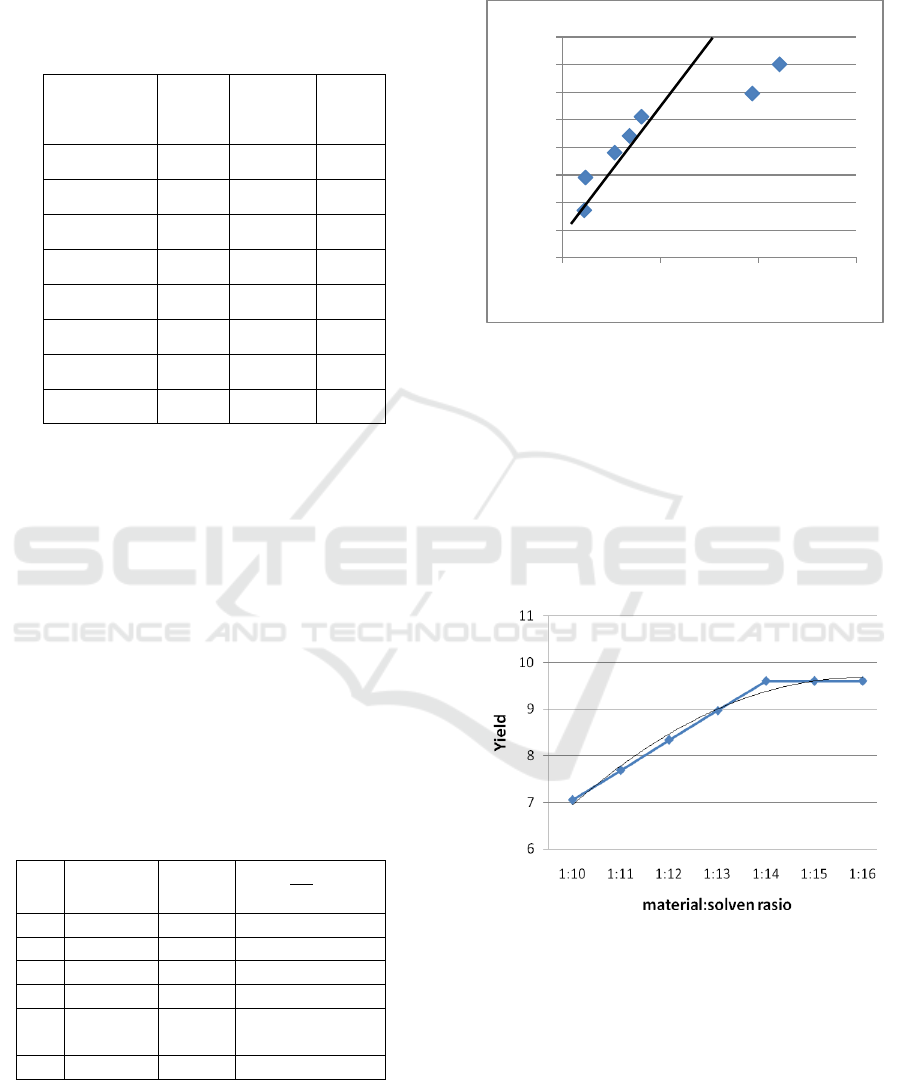
Based on the chart % P vs Z and % P vs I, the
most influential variable is I
R
(variable ratio of
materials and solvents). This can be seen from chart
%P vs I (Figure 2), which is the farthest point from
the „approach line‟
Figure 2: %P vs I.
After analyzing using the factorial design
method, the material and solvent ratio (R) is the
most influential variable, because it is located
farthest from the approach line on the distribution
curve.
Next, optimization of various material:solvent
ratios under operating conditions 120 minutes (t) and
material size 80-100 mesh (N) as shown in Figure 2.
Figure 3: Optimization material:solven ratio.
Figure 3 shows clearly that the greater the
amount of solvent than the amount of material, the
greater yield will be produced. The greater dyes
yield is obtained because the solvent's solubility
ability to extract dyestuffs in the onion skin is better
and provides greater opportunities between solvents
and ingredients to touch.
0
10
20
30
40
50
60
70
80
0.0 1.0 2.0 3.0
%P
I (Efect Interaction)
IR
IN
It
It,R
It,N
It,R,
N
IR,
N
Utilization of Red Onion Skin Waste as Natural Dyes
437

The solubility of dyes will continue to increase
with the amount of the material ratio and the
extraction solvent until saturation occurs in the
solvent. Material and solvent ratio of 1:14 (50
grams: 700 ml), yield has not increased again. This
is due to saturation of the solvent concentration so
that the diffusion process between the material and
the solvent occurs very slowly. So that the addition
of a larger solvent will not add to the extraction
power of the dyes in the extraction process.
Another factor that influences the high and low
yield produced in this extraction process is the size
of the material. In this study using the material size
of 40-60 and 80-100 mesh, after the experiment it
was seen that the use of material sizes with 80-100
mesh gives higher yields compared to the use of
material sizes with 40-60 mesh. This is because the
surface area of the extracted material is getting
larger so the greater the chance for the material to
interact with the solvent, so that the solvent can
extract more.
4 CONCLUSIONS
The results of the study and observations concluded
that the variables that most influence the yield of
dyes from onion skin waste are the ratio of materials
and solvents. The greater the ratio of materials and
solvents, the greater the yield value. The optimum
ratio value is 1:14 with extraction time of 120
minutes, particle size of 80-100 mesh and a yield of
9.6%.
REFERENCES
Cahyadi, W., 2009. Analisis & Aspek Kesehatan Bahan
Tambahan Pangan. Bumi Aksar. Jakarta, Edisi Kedua.
Hussein, I, & Alhassanen Y, 2013. Protection of Humans
From Ultraviolet Radiation (UVR) Through the Use of
Cotton Clothes Dyed With Aqueous Extracts of Onion
Skin as the Natural Colorant, Minouviya University:.
Egypt.
Jackman, R. L., & Smith, J. L. 1996 Anthocyanin and
Batalains. Natural Foods Colourants. Blackie
Academic and Professionals. London, Second Edition.
Oancea, S. & Drághici O., 2013. “pH and Thermal
Stability of Anthocyanin-Based Optimised Extracts of
Romanian Red Onion Cultivars” Czech J. Food Sci.,
Vol. 31, pp. 283–291.
Rodrigues, A., S., Fogliano V., Graziani G., Mendes, S.,
Vale, A. & Goncalves, C., 2003., “Nutrition Value of
Onion Regional Varieties in Northwest Portugal”,
Electronic Journal of Environmental, Agricultural and
Food Chemistry, Vol. 2, No. 4, pp. 519-524.
Winarno, F., G., 1997. Kimia Pangan dan Gizi, PT.
Gramedia. Jakarta.
EIC 2018 - The 7th Engineering International Conference (EIC), Engineering International Conference on Education, Concept and
Application on Green Technology
438
