Effect of Substrate Concentration Variation on Scleroglucan
Production using Aerobic Fermentation
Nancy Siti Djenar and Bintang Iwhan Moehady
Chemical Engineering Department, Politeknik Negeri Bandung, Jln.Gegerkalong Hilir Ds. Ciwaruga,Bandung, Indonesia
Keywords: S.rolfsii, scleroglucan, liquid sugar, substrate
Abstract: Sclerotium rolfsii is known as one of the pathogenic fungi that causes some diseases in plants. In Indonesia
the various studies that have been conducted on the fungus are still limited to prevention and control to
reduce its pathogenic aspect. Scleroglucan is a biopolymer produced from Sclerotium rolfsii fermentation
and has been used in several industries in various developed countries as both a thickener and emulsifier. In
this study scleroglucan was produced from liquid sugar substrate using S.rolfsii InaCC F-05. The use of
liquid sugar substrate with varying concentration can increase dry cell weight, yield of scleroglucan and
conversion of 73.36%, 3.45% and 3.46% respectively. The acquisition of scleroglucan viscosity was
1.8500 to 2.5713 cP. While low, it can still be applied in chemical industries as a mixture for toothpaste
and mouthrinse formulation.
1 INTRODUCTION
These days exopolysaccharides that were produced
from varieties of microorganisms are widely used in
some industries as emulsifers, gelling, and
thickening agents. Scleroglucan one of the
exopolysaccharides is produced from fermentation
of Sclerotium rolfsii fungal (Survase, 2007). The
properties and characteristics of scleroglucan are
similar to xanthan gum. The difference is that in
Indonesia scleroglucan is not yet well known, while
xanthan gum has been widely used even though it
still has to be imported.
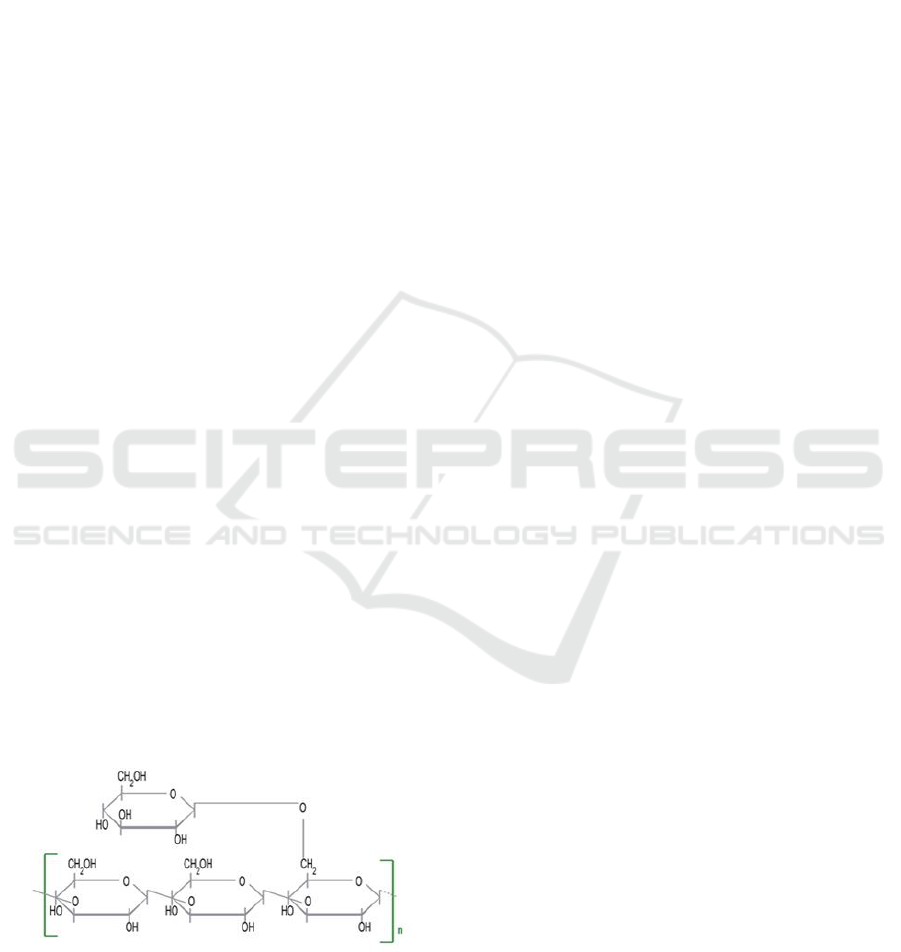
Chemical structure of scleroglucan is depicted in
Figure 1.
Figure 1: Chemical structure of scleroglucan
(https://de.wikipedia.org/wiki/Scleroglucan).
Scleroglucan is composed of (1-3)- β-linked
glucopyranosyl backbone with single (1-6)-β-linked
glucopyranosyl branches on every third subunit.
In Indonesia Sclerotium rolfsii is better known as
one of the pathogenic fungal that causes a number of
diseases in plants such as peanuts, potatoes,
tomatoes, soybeans, cabbage, onions, celery, corn
and lettuce. This limits various studies that have
been carried out regarding the fungal to prevention,
control, and characterization in reducing the
pathogenic properties of Sclerotium rolfsii (Yana,
2011 & Pudjihartati, 2007). The S. rolfsii
metabolism process produces several enzymes
including cellulases, phosphatidase, arabinase,
exogalactanase, polygalacturanase, galactosidase
and exomanase. The enzymes then convert raw
materials into scleroglucan through this fermentation
process (Castillo et.al, 2015).
In an industry that utilizes the fermentation
process, the role of its medium is very important in
increasing the microbial growth rate. PDB (Potato
Dextrose Broth) is one of the medium commonly
used for the growth of S.rolfsii. To support cell
regeneration and productivity, the modified PDB is
one of the best growth medium for S.rolfsii because
it contains beef extract , K
2
HPO
4
and KH
2
PO
4
,
which function as a nitrogen source and buffer
solution to maintain the pH of fermentation broth
(Bhagat.I, 2011).
Djenar, N. and Moehady, B.
Effect of Substrate Concentration Variation on Scleroglucan Production using Aerobic Fermentation.
DOI: 10.5220/0009012504250429
In Proceedings of the 7th Engineering International Conference on Education, Concept and Application on Green Technology (EIC 2018), pages 425-429
ISBN: 978-989-758-411-4
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
425

Temperature is a very important parameter that
affect both culture growth and exopolysaccharide
production. In this case the optimum temperature for
scleroglucan production is in the range of 20-37°C
and at 28
0
C for culture growth. At less than 28°C,
oxalic acid will gradually form which has an adverse
effect on the production of scleroglucan (Survase,
2007).
pH affects the physiology of microorganism such
as its solubility, nutrition and enzyme activity. The
optimum pH for exopolysaccharide production can
differ from the pH for culture growth. Generally the
optimum pH for scleroglucan synthesis is between
4.0 and 5.5 (Castillo et al, 2015). Aeration and
agitation optimization are the most important factors
for controlling cell growth and scleroglucan
production because it can increase the rate of
formation of metabolites and oxygen from liquid
medium to cells.
Substrates used in scleroglucan production are
for example sucrose, condensed corn solubles,
coconut water, molasses, sugar cane juice, and
glucose (J.I Farina.1998, Fosmer.2010). Liquid
sugar syrup produced from tapioca flour can be used
as a substrate because it can shorten the scleroglucan
production chain. Liquid sugar is easy to obtain
even if its utilization is limited only as a raw
material for the food and beverage industry, thus it is
expected a diversification step will emerge from
liquid sugar syrup which is made from local raw
materials and spread throughout Indonesia (Djenar
et al, 2017).
2 EXPERIMENTAL DETAILS
2.1 Microbial Preparation
At this stage regeneration of fungal S.rolfsii on :
a) Potato dextrose Agar (PDA) medium
containing potato, dextrose and agar ,
incubated at 28°C for 5-7 days.
b) Modified PDA medium containing potato,
dextrose, beef extract, KH
2
PO
4
, K
2
HPO
4
and
agar, incubated 28°C for 5-7 days.
2.2 Production of S.rolfsii Inoculum
2.2.1 Production of S.rolfsii inoculum in
Potato Dextrose Broth (PDB)
Potato Dextrose Broth medium was prepared by
dissolving 1.0 g potato, 0.1 g dextrose, 0.1 mg
CaCO
3
, 0.1 mg MgSO
4
7H
2
O in erlenmeyer
containing 5 mL of distilled water. The medium then
sterilized for 20 minutes at 121°C and 1.4 atm. The
stock culture S.rolfsii in PDA was inoculated to the
PDB medium and incubated at 28°C for 48 hours
and stirred at 150 rpm. Furthermore, this active
inoculum will be used to make S. rolfsii growth
medium.
2.2.2 Production of S.rolfsii inoculum in
modified Potato Dextrose Broth (PDB)
Potato Dextrose Broth medium was prepared by
dissolving 1.0 g potato, 0.1 g dextrose, 0.1 mg
CaCO
3
, 0.1 mg MgSO
4
7H
2
O, 0.0115 g K
2
HPO
4
,
0.0591 g KH
2
PO
4
and 0.005 g beef extract in
erlenmeyer containing 5 mL of distilled water. The
medium then sterilized for 20 minutes at 121°C and
1.4 atm. The stock culture S.rolfsii in PDA was
inoculated to the PDB medium and incubated at
28°C for 48 hours and stirred at 150 rpm.
Furthermore, this active inoculum will be used to
make S. rolfsii growth medium.
2.3 Experimental Work
In this step, aerobic fermentation was done in
variation of growth medium at 170 rpm, pH 5, at 30-
35 °C using 5% and 7% liquid sugar as substrate
(Table 1)
Table 1: Variation of growth medium and substrate
concentration on scleroglucan production.
No
Growth
medium
Substrate
concentration
1
PDB
5% Liquid sugar
2
PDB
7% Liquid sugar
3
Modified PDB
5% Liquid sugar
4
Modifiied PDB
7% Liquid sugar
Pasteurization. The pasteurization was done at 90°C
for 25 minutes. The pasteurized fermentation was
separated between scleroglucan solution and its
cells using centrifugation.
Purification and precipitation. Precipitation of
scleroglucan was done by adding an organic solvent
of isopropyl alcohol (IPA) with a ratio of 3: 1 (v / v)
to the supernatant. The obtained precipitate was
then dried in an oven at 50°C, weighed, and the
yield and % conversion were calculated.
EIC 2018 - The 7th Engineering International Conference (EIC), Engineering International Conference on Education, Concept and
Application on Green Technology
426
Scleroglucan testing is done by measuring its
viscosity. While the functional groups analysis uses
FT-IR.
3 RESULT AND DISCUSSION
In this batch-based S.rolfsii fermentation the oxygen
was produced from air which exists in the
bioreactor by using a ratio of 1:5 between
fermentation medium and the bioreactor (v/v)
(Standbury P.F et al, 2000). At this stage curves
were made to determine the production time of
scleroglucan for this process, with pH between 4.0-
5.5, temperature 30-35
o
C with stirred at 170 rpm.
The time needed to produce the maximum amount
scleroglucan is shown in Table 2 below.
Table 2: The time needed to produce scleroglucan using
liquid sugar substrate.
Growth
medium
Substrate
The time needed to
produce
scleroglucan (hour)
PDB
5% liquid
sugar
57
PDB
7% liquid
sugar
68
Modified
PDB
5% liquid
sugar
76
Modified
PDB
7% liquid
sugar
65
In Table 3 It was shown that S.rolsii grown in
PDB produced both low yield and conversion. The
PDB only contains potatoes and dextrose which
functions as carbon source. However, to increase the
productivity, nitrogen source and others are
required. In this PDB, an increase in substrate
concentration causes a decrease both of yield and
conversion. This happened as a result of the
fermentation that was carried out in batch. This
condition causes substrate repression to often occurs.
In this experiment, 7% liquid sugar substrate
repressed S.rolfsii metabolism, thus disrupting the
enzyme synthesis (Egli, 2009).
A modified PDB is different in that changes in
liquid sugar substrate with 5% to 7% concentration
can increase yield and conversion about 3.45% and
3.46% respectively. Overall, when compared to
PDB, there is an increase in yield and conversion of
17-18 g/L and 35-36% respectively. This is because
the modified PDB contains beef extract as a source
of nitrogen and K
2
HPO
4
and KH
2
PO
4
. Nitrogen is a
major component of amino acids, and these amino
acids will form the proteins needed for cell
metabolism – namely the growth and synthesis of
enzymes, so as to increase the production of
biopolymers (Survase,2007). Phosphorus is an
important element for secondary cell metabolism. In
fermentation medium, phosphorus is in the form of
phosphate salts such as K
2
HPO
4
or KH
2
PO
4
which
serve as a pH buffer. Potassium is the main
inorganic cation in cells and is usually added as an
inorganic salt. Potassium is a cofactor for several
enzymes needed for carbohydrate metabolism. The
results of scleroglucan production are as shown in
the following Table 3.
As shown in Table 3 the value of scleroglucan
viscosity was still low which was around 0.9585 -
2.5713 cP. However, according to Castillo, N. A., et
al, (2015), scleroglucan with viscosity of 1.5-3 cP
can still be applied in the chemical industries as a
mixture for toothpaste and mouthrinse formulation.
The viscosity of an exopolysaccharide is affected
by fermentation conditions such as temperature, pH,
and oxygen content (Garcia-Ochoa, 2000).
According to Castillo.A.N et al. (2015) and Survase
S.A, et al. (2007), the rate of aeration used is usually
at 2 vvm at a stirring speed of 180-600 rpm using air
bubbles. In this study aeration was carried out by
utilizing oxygen in the bioreactor with a ratio
between fermentation medium with bioreactor of 1:5
(v/v) (Standbury,2000).
Table 3: Scleroglucan research result.
No
Growth Medium
Substrate
Concentration
Fermentat
ion time
(hour)
Scleroglucan
produced (g)
Yield
(g/L)
Conversion
(%)
Viscocit
y (cP)
1
PDB
5% liquid
sugar
57
0.3785
1.893
3.785
0.9585
2
PDB
7% liquid
sugar
68
0.2892
1.446
2.066
1.9358
3
Modified PDB
5% liquid
sugar
76
3.5017
17.505
35.017
1.8500
4
Modified PDB
7% liquid
sugar
65
3.6228
18.114
36.228
2.5713
Effect of Substrate Concentration Variation on Scleroglucan Production using Aerobic Fermentation
427
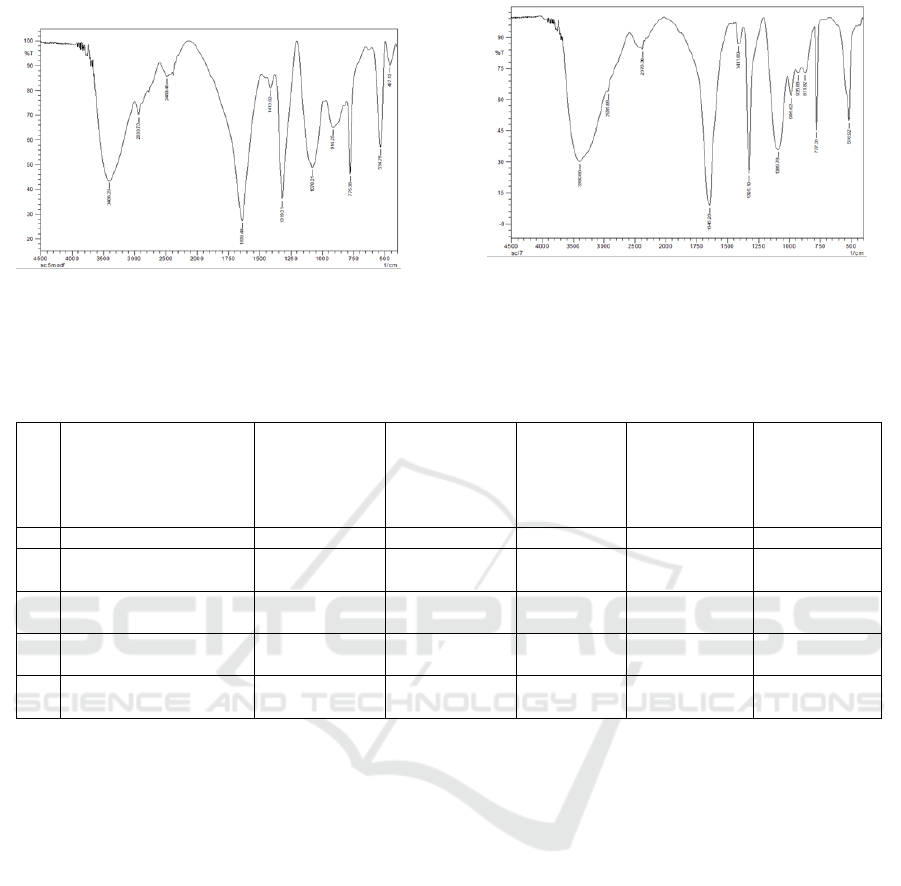
Figure 2: FTIR spectrum of scleroglucan from S.rolfsii
fermentation using a modified PDB medium with a
substrate of 5% liquid sugar.
Figure 3. FTIR spectrum of scleroglucan from S.rolfsii
fermentation using a modified PDB medium with a
substrate of 7% liquid sugar.
Table 4: The comparison of functional groups between literature scleroglucan obtained using two types of substrates
concentration.
No
Annotation
OH Stretching
and Bending
(cm
-1
)
CH Stretching
and Bending
(cm
-1
)
CH (cm
-1
)
CH Bending
(cm
-1
)
GOC
Glycosidic
and CCOH
Stretching
(cm
-1
)
1
Farina, J.I. et. al, 2015
3400
2937
-
1475 – 1250
1000 – 1200
2
Casadei, M.A. et al,
2007
3422
-
1636
1420 – 1395
1079
3
Moehady, B. I et. al,
2016
3392
2933
-
1409 – 1382
1026 – 1153
4
Scleroglucan (using 5%
liquid sugar)
3406
-
1639
1413 – 1319
1078
5
Scleroglucan (using7%
liquid sugar)
3390
2935
1645
1411 –1325
1089
Based on the results of FTIR analysis, the
presence of functional groups in the scleroglucan
obtained from various fermentation process
conditions can be recognized. Figure 2 and 3 showed
the FTIR spectrum of scleroglucan using modified
PDB with 5 and 7% liquid sugar substrate
respectively.
The compatibility of functional groups between
literature scleroglucan and scleroglucan of the study
is shown in Table 4 below. Overall, this suggests
that the fermentation of S.rolfsii using two types of
the liquid sugar substrate concentration can produce
scleroglucan.
4 CONCLUSION
The FTIR analysis, showed the compatibility of
functional groups between literature scleroglucan
and scleroglucan of the study. This suggests that the
fermentation of S.rolfsii using two types of the liquid
sugar substrate concentration will produce
scleroglucan. The use both of 5% and 7% liquid
sugar in modified PDB can increase yield and
conversion about 3.45% and 3.46% respectively.
The acquisition of scleroglucan viscosity was 0.
9585 to 2.5713 cP. While low, it can still be
applied in chemical industries as a mixture for
toothpaste and mouthrinse formulation.
.REFERENCES
Anisa, Yana, Nawangsih, & Abdjad Asih, 2011.
Pengaruh mulsa dan PGPR terhadap insidensi
penyakit busuk pangkal batang (S.rolfsii) pada
tanaman kedelai (glysine max (L) Merill). Institut
Pertanian Bogor, Bogor
Bhagat, I., 2011. “Factors influencing mycelial growth of
Sclerotium rolfsii”, Nepalese Journal of Biosciences,
Vol. 1, pp. 26-31.
Casadei, M.A., Matricardi P , Fabrizi G, Feeney M , &
Paolicelli P, 2007. “Physical gels of a carboxymethyl
derivative of scleroglucan: synthesis and
characterization”, European Journal Of
EIC 2018 - The 7th Engineering International Conference (EIC), Engineering International Conference on Education, Concept and
Application on Green Technology
428

Pharmaceutics and Biopharmaceutics, Vol. 67, pp.
682-689.
Castillo, N. A., Valdez, A. L., & Fariña, J. I., 2015.
“Microbial production of scleroglucan and
downstream processing”, Frontiers In Microbiology,
Vol. 6, pp. 1106.
Djenar N.S and Wahyu. E., 2017. “The Effect of
Substrate Modification on Xanthan Gum Production
by Aerobic Fermentation Method”. Advanced Science
Letters, Vol. 23, No. 6, pp. 5678-5680
Egli, T., 2009. “Growth kinetics, bacterial”. In M.
Schaechter (Ed.), Encyclopedia of Microbiology, pp.
180-193.
Fariña, J.I., Siñeriz, F., Molina, O.E. & Perotti, N.I.
1998. “High scleroglucan production by Sclerotium
rolfsii: Influence of medium composition”. Journal Of
Biotechnology Letters, Vol. 20, Issue 9, pp 825–831.
Fariña, J.I., Viñarta, S.C., Cattaneo, M., & Figueroa,
L.I.C., 2008. “Structural stability of Sclerotium rolfsii
ATCC 201126 β‐glucan with fermentation time: a
chemical, infrared spectroscopic and enzymatic
approach”. Journal Of Applied Microbiology, Vol.106,
pp. 221-232.
Fosmer, A, Gibbons, W. R. & Heisel, N. 2010.
“Reducing the Cost of Scleroglucan Production by
Use of a Condensed Corn Solubles Medium,” Journal
of Biotechnology Research, Vol. 2, 2010, pp. 131-143.
García-Ochoa F, Santos VE, Casas JA, & Gómez E.,
2000. “Xanthan gum: production, recovery, and
properties”. Biotechnology Advances, Vol. 18, pp.
549-579.
https://de.wikipedia.org/wiki/Scleroglucan.
Moehady, B. I., Djenar, N. S., Widyanti, E. M., 2016.
“Produksi scleroglucan dari S.rolfsii menggunakan
media gula cair”, Prosiding Seminar Nasional
Teknik Industri, Universitas Gajah Mada, pp M2-M9.
Peter F Stanbury, P. F., Whitaker, A, Hall, S., J. 2000.
Principles of Fermentation. Butterworth-Heinemann,
Oxford, Melboune.
Prasetyoningrum, Adnan, T, & Muin, A., 2012. “Potensi
seduhan kompos untuk pengendalian penyakit layu
S.rolfsii pada tanaman kedelai”. Institut Pertanian
Bogor, Bogor.
Pudjihartati, Sudarsono, E., Ilyas, Satriyas, & S,
Siswanto, R., 2007. “Ketahanan kacang tanah dan
tembakau terhadap infeksi S.rolfsii Sacc. dengan
ekspresi enzim kitinase tinggi”, Pusat Pelatihan
Agrobisnis, Bioteknologi dan Sarana Industri, Bogor.
Survase, S. A., Saudagar, P. S., Bajaj, I. B., & Singhal,
R. S. 2007. “Scleroglucan: Fermentative Production,
Downstream Processing and Applications”, Food
Technol.Biotechnol. Vol. 45, No. 2, pp. 107-118.
Effect of Substrate Concentration Variation on Scleroglucan Production using Aerobic Fermentation
429
