
Estimation of Battery Capacity using Voltammetry Method of Lead
Acid and Nickel Cadmium Battery based LMNN at Jember Electric
Substation
Lori Kusuma Dewi and Bambang Sri Kaloko
Electrical Engineering, Faculty of Engineering, Universityof Jember, Jl. Kalimantan 37, Jember, Indonesia 68121
Keywords: Lead Acid Battery, Neural Network, Nickel Cadmium Battery, Voltammetry cyclic.
Abstract: The reliable battery holds a very important role. Therefore, this refers to study the characterization of lead
acid batteries and nickel cadmium, this is a secondary battery of the most developed and the lead acid
batteries are widely used in the automotive field. The lead acid and nickel cadmium battery capacity is
determined by the amount of electrical charge that is obtained from the battery and the amount depends on
the active ingredient contained in the plate. To determine the characterization and capacity lead acid battery
and nickel cadmium battery are suitable for use, this study used two methods. With voltammetry analysis
and development the lead acid battery model design based on neural network method. In the
electrochemical field the voltammetry cyclic is a condition when the current is measured during a sweep
potential from the beginning to the end potential and then back again. It is also called sweeping or scanning
and can be reversed after the reduction takes. So the anodic and cathodic current can be measured. Then the
design of the model development lead acid battery based on neural network in this study using inputs
specifically the voltage as input, and the current as target. So the accuracy testing of the forecasting system
using neural network algorithm will be better and more efficient than the experiment data manually.
1 INTRODUCTION
This Lead acid batteries are composed of lead
dioxide as the cathode, a metal sponge as the anode
lead and sulfuric acid as the electrolyte. Each cell has
a voltage of 2 volts. Advantages of the use of lead
acid batteries which are robust, inexpensive, reliable,
tolerant of excess charging, low internal impedance.
While the lack of a battery of this type which are
very heavy, have low energy efficiency of about 70
%, dangerous overheating during charging, have a
lower cycle time is 300-500 cycles, and materials are
harmful to the environment (Al-Atas, H. M., 2015).
Then nickel cadmium battery This type of battery
has a cell voltage of 1.2 Volt per cell with twice the
energy density of lead acid batteries. As a cathode,
this battery uses nickel hydroxide Ni(OH)
2
and
cadmium Cd as anode possessed by alkaline
potassium hydroxide as its electrolyte. Nickel
cadmium batteries have a resistance. Which is very
small and allows for the charge and discharge with a
high level (Husain, I., 2003). Meanwhile, according
to SK520 PLN nickel cadmium batteries or Ni-Cd is
alkali battery with a capacity of 1.2 volts per cell and
is often used in PT. PLN as a DC provider of
protection systems, SCADA and PLC (KEPDIR
0520-2 K DIR, 2014)
With these descriptions, the authors will discuss
about Development Voltammetry Based
LavenbergMarquadtNeural Network (LMNN)
Method. The purpose of this research is to determine
the capacity of a lead acid battery and nickel
cadmium battery and also to know how to increase
the capacity of lead acid batteries with a constant
voltage, so we could get more efficient lead acid
battery.
2 EXPERIMENTAL
The lead acid batteries will be measured and
appliedby using Pulse Voltammetry cyclic method.
In this voltammetry measurement, the working
electrode use a pure Pb sheet. In order to remove the
oxide layer of the Pb electrode, Voltammetry cycles.
H
2
SO
4
were carried out for several times. It was
directly used as the negative electrode. As the
Dewi, L. and Kaloko, B.
Estimation of Battery Capacity using Voltammetry Method of Lead Acid and Nickel Cadmium Battery based LMNN at Jember Electric Substation.
DOI: 10.5220/0009012204030408
In Proceedings of the 7th Engineering International Conference on Education, Concept and Application on Green Technology (EIC 2018), pages 403-408
ISBN: 978-989-758-411-4
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
403

positive electrode, were carried out to form the PbO
4
layer. The counter electrode was a piece of the Pb or
PbO
2
electrode cut from the practical battery (Ikeda,
S., et al. 2003). Cyclic Voltammetry was carried out
in H
2
SO
4
solutions using a conventional potentiostat
and a function for negative electrodes positive
electrodes. Nickel-Cadmium or NiCd batteries will
be measured and applied to the voltammetry method
using a potentiostat. In this voltammetry
measurement, the working electrode uses a 2 x 2 cm
nickel sheet and a 2 x 2 cm Cadmium. In order to
remove the oxide layer from the Ni electrode, the
KOH voltammetry cycle is performed several times.
It is directly used as a negative electrode. For a
positive electrode, it is performed to form a Cd(OH)
2
layer. The counter electrode is a piece of Ni or
Cd(OH)
2
electrode cut from a practical battery.
Cyclic voltammetry is carried out in Electrolytes of
Potassium Hydroxide or KOH using conventional
potentiostat and function for negative electrode of
positive electrode.
Figure 1: Voltammetry cell.
Then, the data of measurement will be applied in
to neural network method. Design of the model
development lead acid battery based on neural
network in this study using current and voltage. So
the accuracy testing of the forecasting system using
neural network algorithm will be better and more
efficient than the experiment data manually.
3 RESULTS AND DISCUSSION
Voltammetry Cyclic is technical analysis used in
qualitative analysis of electrochemical reactions. This
technique is able to provide information on the
oxidation reduction process of thermodynamics and
kinetics of electron transfer which occurs on the
surface of the electrode. (Kaloko, B. S., et al.,
2016), (Kaloko, B. S., et al., 2011). This potential
voltammetry in the technique given in a cycle
between two values, the potential difference at the
start of a potential increase in the maximum and then
being down linearly with a slope of the same value
back to the initial potential. In this study used Lead
Acid batteries or battery lead acid will be tested using
the method voltammetry. This battery has two
electrodes, namely sized PbO
2
and Pb 2 x 3 cm
which serves as the cathode and the anode. In
voltammetry there are three independent test
electrodes, electrode, electrode pliable, and
determinants of the electrode. On testing this using
Pb as a working electrode which is the measured
electrode, and electrode as a determinant of PbO
2
namely electrodes that have fixed. And for battery
nickel cadmium NiCd battery or scars that will be
tested using the method voltammetry. This battery
has two electrodes, namely and Ni which serves as
the cathode and the anode. In voltammetry there are
three independent test electrodes, electrode, electrode
pliable, and determinants of the electrode. This test
using Pb as a work electrode which is measurable,
and the Cd as the electrode an electrode which has a
fixed value.
Voltammetry cyclic is a technique used to obtain
qualitative information about the electrochemical
reaction. Voltammetry cyclic is a method to measure
the potential waveforms potential electrochemical
potential is used in electrochemical analysis is a form
of linear waves, the potential continues to change as
linear functions at that time. The results of
measurements of the cyclic voltammetry can be used
to determine the thermodynamic properties of redox
processes, kinetic properties of the electron transfer
reaction and adsorption reactions (Ikeda, S., et al.
2003).
Voltammetry cell consists of an electrode, an
electrode and a reference electrode is a complement,
soaked in the cell where the three voltammetry
contains the sample solution. Beyond the potential
(V) is applied between the electrodes, the working
and the reference electrode to produce current. In this
study used as the working electrode PbO
2
and Pb in
comparison with electrode H
2
SO
4
as electrolyte. In
this experiment does not use the complementary
electrodes because electrode PbO
2
and Pb easily
react. So the measurement electrodes PbO
2
and Pb
using cyclic voltammetry with the method
potentiostat shows a graph like Figure 2.
EIC 2018 - The 7th Engineering International Conference (EIC), Engineering International Conference on Education, Concept and
Application on Green Technology
404
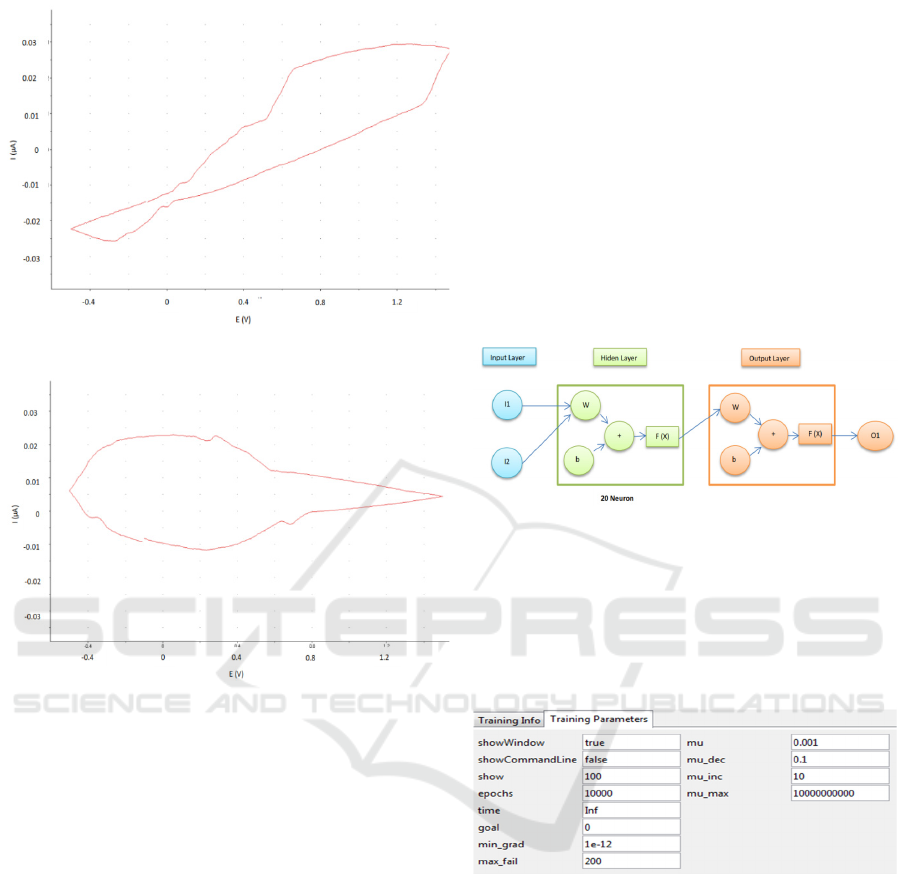
(a)
(b)
Figure 2: Measurement of (a) Lead acid and (b) NiCd with
cyclic voltammetry..
After experimenting with using the method
voltammetry it will be known to the data flow and
voltage which is owned by the battery Of data that
has been retrieved is then converted on testing LM-
NN or Lavenberg Marquardt Neural Network
(Potocnik, P., 2012). By using the neural network
toolbox on MATLAB software, artificial neural
network structures created as in Figure 2 that has two
inputs and one output with two layers. The first layer
is hidden layer neurons of fruit with 20 and the
second layer is the layer with a single output neuron.
On this structure using the input (I) in the form of
voltage and battery capacity, while for the target (T)
using the results of the multiplication capacity with
voltage so that it becomes total energy in batteries.
Then on the Neuron, Neurons in this section consists
of two parts. The first part is a part of the process of
the incorporation of the entire input or inputs from
neurons in the image symbolized by the Sigma (∑),
then the second part is a part of activation neurons
that later in this section information is thrown to the
output to be certain information or still thrown into
other networks for value input from a network.
Mathematically, the workings of the system
formulated by the formula (Kusumadewi, S., 2003).
∑
∙
(1)
Where in
i
is Summation weights and input to the
unit i anda
j
is value activation of unit j, w
j,i
is the
weights of the units to the unit iand j, then g is
function activation and a
i
is the value of the
activation of the unit i as Figure 3.
Figure 3: Design of Neural Network Structure
.
Where in
i
is Summation weights and input to the
unit i anda
j
is value activation of unit j, w
j,i
is the
weights of the units to the unit iand j, then g is
function activation and a
i
is the value of the
activation of the unit i as Figure 3.
Figure 4: The parameter Traininglm(Training Lavenberg
Marquardt) in the lead acid and NiCd battery
.
Then do training or training on artificial neural
network with the use of trainlm and give the
parameters of the Train such as in Figure 3 this
function so that the structure of the network become
increasingly better at recognizing, saving the dam
convey information. Each Training have different
results, thus the process of training done repeatedly
in order to get the best results.
Estimation of Battery Capacity using Voltammetry Method of Lead Acid and Nickel Cadmium Battery based LMNN at Jember Electric
Substation
405
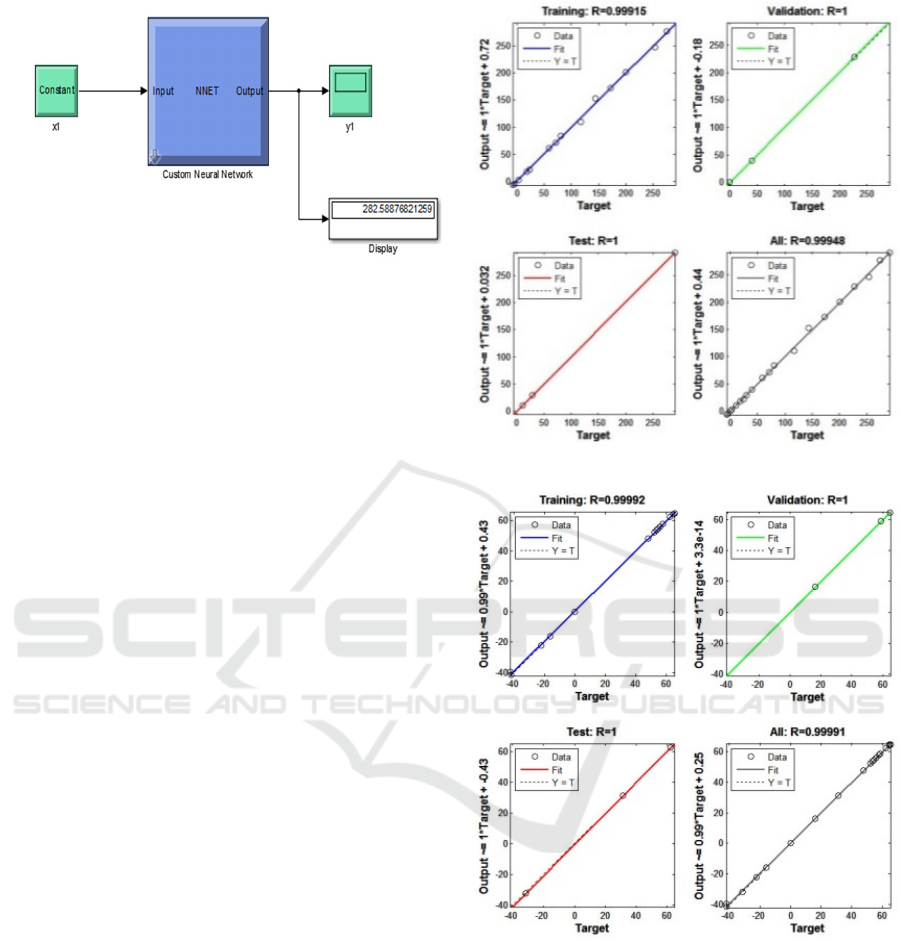
Figure 5: Simulink Diagram NN Lead Acid and NiCd
Battery
.
Following the result of the test condition t (time)
of 20s and E (voltage) of 1.3 V, with a target of total
energy battery is 274.8096 Wh. In Figure 5 shows an
output energy of 276.809 Wh. Those values very
have approached target with error of 1.9994. This is
because the value of the Regression (R) generated
during the processing of 0.99915 only training and
the level of Validation of 1 as shown Figure 6. The
value obtained when the epoch reached 200 iterations
within 2 seconds.
The value of Regression determine the correlation
between the output and the target. If the value of the
Regression approaches 1 as Figure 6, it will indicate
the proximity of correlation Regression approach and
vice versa if the number 0, then it could be said the
output still far from the desired target. Validation is
used to measure the network generalization and to
stop the process of training. While the epoch is the
maximum number of iterations that were made
during the process of training.
The value that appears in the output is the result
of addition of the multiplication of each input value
weights or Weigh. To be able to know the
information the weighting of each Dendrite then can
be done by opening a section in the block layer 1 or
layer 2. In this structure, using a sigmoidal activation
function because the function is more easily applied
and have good results or close to target. There is a
wide range of functions for example, sigmoid
activation purelin, ramp, linear, and the step function.
After going through the activation of the function, the
value of the output of the hidden layer called layer 1
activation value added with value validation at layer
2 or output layer, consisting of the sum of the values
of the weights (w) and bias (b) using the sigmoid
activation function.
(a)
(
b
)
Figure 6: Plot Regression (a) Lead Acid Battery and (b)
NiCd Battery.
EIC 2018 - The 7th Engineering International Conference (EIC), Engineering International Conference on Education, Concept and
Application on Green Technology
406
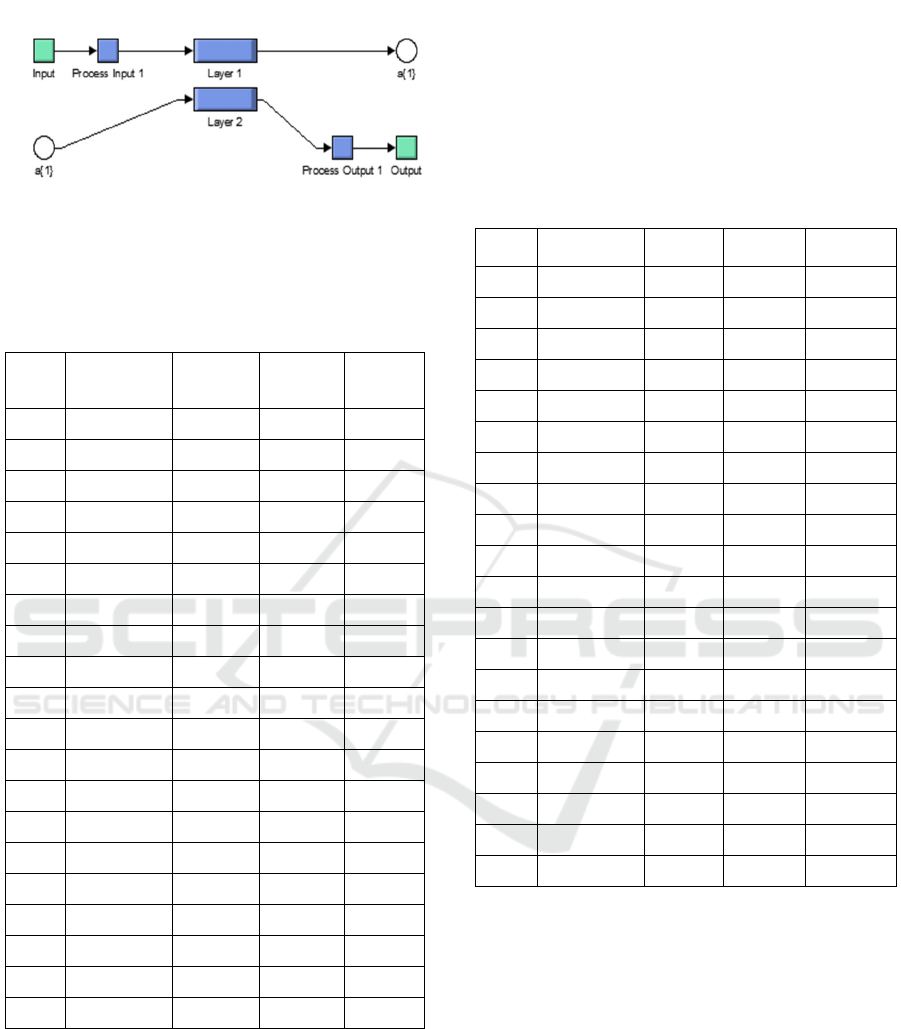
Figure 7: Block Layer 1 and Layer 2 Lead Acid and NiCd
Battery.
Table 1:Voltammetry test data results and Neural Network
on Lead Acid Battery.
E
(Volt)
Voltammetry
I(µA)
Capacity
(Ah)
Energy
(Wh)
Energy
NN
(Wh)
-0.5 -0.02228 -160.416 80.208 83.656
-0.4 -0.02022 -145.584 58.2336 61.788
-0.3 -0.01829 -131.688 39.5064 39.377
-0.2 -0.01651 -118.872 23.7744 22.011
-0.1 -0.01472 -105.984 10.5984 10.659
0 -0.01240 -89.28 0 0.058
0.1 -0.00928 -66.816 -6.6816 -6.11
0.2 -0.00333 -23.976 -4.7952 -4.822
0.3 0.00148 10.656 3.1968 3.172
0.4 0.00622 44.784 17.9136 18.372
0.5 0.00815 58.68 29.34 29.284
0.6 0.01657 119.304 71.5824 71.442
0.7 0.02328 167.616 117.3312 110.169
0.8 0.02502 180.144 144.1152 152.758
0.9 0.02662 191.664 172.4976 172.575
1 0.02781 200.232 200.232 200.885
1.1 0.02863 206.136 226.7496 228.541
1.2 0.02934 211.248 253.4976 248.676
1.3 0.02936 211.392 274.8096 276.809
1.4 0.02890 208.08 291.312 290.941
From table 1 above by giving potential from the
smallest to the largest value produces different
currents. On testing voltammetry and neural network
value flow reaches the point of maximum 0.02936
µA at the moment given the voltage of 1.3 volts.
When testing is done using LM-NN, the resulting
output value is not too far from the target or the value
of testing voltammetry. With the difference in
relative small like that then the error generated is also
getting a little bit. As for the calculation of the value
of the NN should be equal to the value of output
value calculation due to the NN is the value of the
calculation of the process of the formation of the
value of the output itself from the simulink diagram
as in Figure 5.
Table 2:
Voltammetry test data results and Neural Network
on NiCd Battery.
E
(Volt)
Voltammetry
I (µA)
Capacity
(Ah)
Energy
(Wh)
Energy
NN (Wh)
-0.5 0.00616 44.352 -22.176 -22.176
-0.4 0.01454 104.688 -41.8752 -41.295
-0.3 0.01954 140.688 -42.2064 -39.902
-0.2 0.02175 156.6 -31.32 -32.076
-0.1 0.02231 160.632 -16.0632 -16.0632
0 0.02261 162.792 0 -1.421
0.1 0.02272 163.584 16.3584 16.3584
0.2 0.02175 156.6 31.32 31.32
0.3 0.02221 159.912 47.9736 47.9736
0.4 0.01894 136.368 54.5472 54.5472
0.5 0.01549 111.528 55.764 55.764
0.6 0.01208 86.976 52.1856 52.1856
0.7 0.01166 83.952 58.7664 58.7664
0.8 0.01086 78.192 62.5536 62.5536
0.9 0.00997 71.784 64.6056 64.6056
1 0.00908 65.376 65.376 64.844
1.1 0.00816 58.752 64.6272 64.336
1.2 0.00721 51.912 62.2944 62,366
1.3 0.00616 44.352 57.6576 57.668
1.4 0.00534 38.448 53.8272 53.8272
The calculation of the value of the lead acid
battery capacity that has been through a chemical
reaction can be found in the following ways. The
number of free charge generated by the active
ingredients on the negative electrode and consumed
by the positive electrode is called the battery
capacity. Capacity is measured in Ah (1 Ah = 3600
C, or Coulomb, where 1 C is the charge transferred in
1 s by 1 A flow on the unit charge MKS). The
theoretical capacity of the battery (in C) is where x is
the number of moles of the limiting reactants
associated with release of the complete battery, n is
the number of electrons generated by the reaction to
the release of the negative electrode, and F = Le0.
ThenL is the number of molecules or atoms in a mole
Estimation of Battery Capacity using Voltammetry Method of Lead Acid and Nickel Cadmium Battery based LMNN at Jember Electric
Substation
407

(known as Avogadro's constants), and e
0
is the
electron charge. F is the Faraday constants (Kaloko,
B. S., et al., 2016), (Kaloko, B. S., et al., 2011)
6
. The
values for the constants are 6.022 10
,
1.601 10
and 96412.2/ .The
calculation of the capacity in Ah:
0.278
(2)
Where is the mass of the reactant which mR limit
(in kg), and Mm is the molar mass of the limiting
reactant (in g/mol). The cells in the battery typically
connected in series and the battery capacity is
determined by the capacity of the smallest cell.
Therefore, QT = QT battery cell. Then discharge rate
the current situation where the battery is depleted.
The discharge rate is expressed as Q/h rate, where Q
is the rated battery capacity, and h is the discharge
time in hours. For rechargeable battery has a capacity
of QT Ah and are depleted is divided with the
formula Q t/T then:
/
(3)
Lead Acid Battery secondary battery is often
used. In the calculation of the lead acid battery
capacity battery has a larger capacity than nickel
cadmium batteries. In the calculation of the lead acid
battery, capacity of 397.993 Ah in one battery and
nickel cadmium batteries while the capacity of the
battery in Ah one 306.001. Jember Substation had
nickel cadmium battery witch the most widely in use
as protection systems 84 battery and 40 batteries for
SCADA. Then 20 Lead acid batteries just for PLC
(Power Line Carrier). In the calculation of the lead
acid battery capacity battery has a larger capacity
than nickel cadmium batteries. In the calculation, the
lead acid battery capacity Ah 397.993 in one of the
battery and the battery required battery voltage with
as many as 121.358 v. Whereas nickel cadmium
battery has a capacity of 306.001 Ah in one and
batteries that are needed as much as 8 batteries with
voltage V 2.099.
4 CONCLUSION
Results of the two methods yield differences are not
far away. From the results of the comparison of
simulated neural network with cyclic voltammetry
test methods, then there is a difference, and that
difference is the error that occurred. If the value of
the error is smaller than the resulting data better.
On the battery capacity calculation of lead acid
batteries have a larger capacity than nickel cadmium
batteries. In the calculation, the lead acid battery
capacity Ah 397.993 in one of the battery and the
battery required battery voltage with as many as
202.099 V. While the batteries are nickel cadmium
has a capacity of 306.001 Ah in one and batteries
that are needed as much as 84 battery with voltage
1.358 V.
ACKNOWLEDGEMENTS
This research was supported by grant of Higher
Education Program University of Jember. I would
like to express my thanks to everyone who helped
me faithfully to finish the job. I would also like to
thank the reviewers who gave very useful
suggestions which help me improve the quality of
research.
REFERENCES
Al-Atas, H. M., 2015. Pengembangan Model
BateraiTimbalAsamBerbasis RBFNN. Universitas
Jember. Jember.
Husain, I., 2003. Electric and Hybrid Vehicles Design
Fundamentals. CRC PRESS. Washington, D.C.
KEPDIR 0520-2 K DIR, 2014. Buku Pedoman
Pemeliharaan Primer. PT PLN (Persero). Indonesia.
Ikeda, S., Oka, H., Mori, Y., Maeda, M., Ohta, M., Ono,
S., et al., 2003. Effects of Additives on Positive and
Negative Electrodes of Lead-Acid Batteries 1.
Kaloko, B. S., Loka, A. H., & Udin, M., 2016).
"Forecasting of Lead Acid Battery Capacity Based on
LVMNN". J.Theor. Appl. Inf. Technol., Vol. 89, No.
1, pp. 261-266.
Kaloko, B.S., Soebagio., & Purnomo, M.H., 2011.
"Mapping of Electrochemistry a Neural Network
Model for Lead Acid Battery". Indonesian J. Chem.,
Vol. 11, No. 2, pp. 140-147.
Potocnik, P., 2012. Neural Networks: MATLAB examples.
University of Ljubljana: MATLAB® 7.14. Slovania.
Kusumadewi, S., 2003. Artificial Intelligece
(TeknikdanAplikasinya). PT GrahaIlmu. Yogyakarta.
EIC 2018 - The 7th Engineering International Conference (EIC), Engineering International Conference on Education, Concept and
Application on Green Technology
408
