
Magnetic and Physical Properties Modification using Sintering
Temperature Variations in the Process of Making Barium
Hexaferrite Permanent Magnet
Wibowo, R Lullus L. G. Hidajat, Sony Wijaya, Eko Surojo
Mechanical Engineering Department, Sebelas Maret University, Surakarta 57128, Indonesia
Keywords: Hard-Magnetic, Barium Hexaferrite, Magnetic Properties, Mechanical Alloying, Sintering.
Abstract: This paper discusses the modification of magnetic hysteresis and particle size of barium hexaferrite
permanent magnet using sintering temperature variatons. The materials used were Barium Carbonate
(BaCO3) and Hematite (Fe2O3) with a stochiometric ratio of (1: 6), obtained by the dry miling mixing
process for 6 hours. Then this material was calcined at 1100°Celsius for 30 minutes and sieved to pass
through the 200 mesh filter. The sample is compacted by 3wt% additive shellac and 5 Ton pressing to form
a pellet with diameter of 5 mm. The next process is samples were sintered at temperatures of 900°C, 1000°C
and 1100°C for 30 minutes. The magnetic hysteresis of this sample were then determined using VSM and
microstructure analysis was determined using SEM. The results showed that the average Hmax and particle
size increase for temperatur of 900°Cto 1000°C and decrease from 1000°C to 1100°C. At sintering
temperature of 1000° C a granullar fusion was formed while new phase had not been formed, whereas at a
sintering temperature of 1100°C a new phase had been formed which causes coercivity decreased. It was
concluded that the optimal modification of magnetic properties and particle size was obtained at sintering
temperature of 1000°C.
1 INTRODUCTION
Permanent magnets are the one of primary
component for modern machinery equipment in
various fields such as automotive machinery,
electronic equipment and energy. Industrial
applications require permanent magnet components
with certain specifications to run the machining
system. Because Indonesia as a developing industrial
country, permanent magnets is demanded so high
that it have to import such permanent magnet
components. Thus, the local magnet industry is
needed to meet domestic magnetic demand (P.
Sardjono et all.,2012).
In electric machinery, a permanent magnet is a
passive component in producing a magnetic field,
which allows work without electric current supplied
to coil or solenoid to maintain the magnetic field.
The induced magnetic in the permanent magnet
material will be maintained, so that when the electric
current is terminated the magnetic field of the
permanent magnet material remain stored (D. Jiles,
1991). Ceramic permanent magnets replace
electromagnets in many applications and widely
used as permanent magnets in electric motors,
generators and speakers (S. Collocot, 2007).
One of the materials to produce ceramic type of
permanent magnet is barium. Barium is a silvery
white metal formed in nature in various forms
commonly in compound forms. This material is
found in nature in two forms of material, namely
barium sulfate and barium carbonate which are
deposits deposited on earth mantle (Clement
International Corporation, 1992). The chemical
properties of barium material i.e. melting point at
720°C, boiling point 1,640ºC, and density of 3,51
gcm
3
(Sunarya, S. A. 2009). This magnetic proper-
ties of the material after magnetization is permanent
(M. I. Alif, 2012,), mechanical properties are very
strong and not easily corroded (Snoek, 1947). In
addition, mix of barium carbonate and oxide ferrite
produces a permanent magnet barium hexaferrite
(Priyono, 2001). The use of M.hexaferit-based
barium magnets, i.e., as a microwave absorber in the
aircraft cabin (D. P. Efhana et all, 2013), and a
permanent magnet based on Ba/Sr-ferrite are used as
measuring instruments on water meters (I. Yusan, et
all,2012). Although it is very potential as a mineral
material to produce magnets, in Indonesia, this
72
Wibowo, ., Hidajat, R., Wijaya, S. and Surojo, E.
Magnetic and Physical Properties Modification using Sintering Temperature Variations in the Process of Making Barium Hexaferrite Permanent Magnet.
DOI: 10.5220/0009006600720076
In Proceedings of the 7th Engineering International Conference on Education, Concept and Application on Green Technology (EIC 2018), pages 72-76
ISBN: 978-989-758-411-4
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

material is a steel industry waste that has not been
managed optimally.
In this research, barium ferrite permanent magnet
manufacturing was performed by using powder
metallurgy method. The raw material are Barium
Carbonate (BaCO
3
) and Oxide Ferrite (Fe
2
O
3
) with a
stoichiometric ratio of 1:6, using the milling process.
The next process is calcination, compaction, and
sintering treatment. The heat treatment process has
generally been known to have a negative impact on
magnetic properties, but this process cannot be
avoided in the process of metallurgy powder in order
to make a strong magnet that can be utilized in
machinery. Sintering is a heat treatment process that
is employed to produce a dense material by
adjusting the treatment for components of metal or
ceramic powder. For this reason, a study of sintering
conditions is needed to obtain high quality
permanent magnet materials.
The next phase of this research is analysis using
a scanning electron microscope (SEM) method to
determine the microstructure. While the magnetic
properties of the sample was observed through
hysteresis curve analysis using Vibrating Sample
Magnetometer (VSM).
2 RESEARCH METHODOLOGY
The powder metallurgy method is used in this
research The stoichiometric ratio for barium
carbonate and oxide ferrite is (1: 6) was determined.
The first process of making samples is the materials
were scaled using digital scales. After weighing
according to the desired composition the material
was mixed using ball milling in a wet state, so that
the mixture obtained had a high homogeneous level.
The next processeswere calcination, compaction,
and sintering heat treatment. Powder material before
calcination is shown in Figure 1 (a). Figure 1 (b) is a
ball milling device for mixing for 6 hours. The
calcination process was then carried out to form the
crystalline phase of barium hexaferrite with a
temperature of 1100ºC with a holding time of 30
minutes. Figure 1 (c) shows the material after
calcination process.
Powder material produced from the calcination
process is then sieved using 200 mesh size filter in
order to obatin homogen particle size powder. Then
2% additive material was added as a binder. At the
compaction stage the material that was initially in
the form of granulars, was compacted to form a solid
specimen as pellets with a pressure of 5 tons. The
specimen was shown in Figure 2. Furthermore the
specimens were subject to sintering heat treatment
with variations in temperature of 900°C, 1000°C,
and 1100°C with a holding time of 30 minutes.
The sintering treatment was conducted to
perform granule fusion and to reduce porosity.
Heating variations is for temperature of 900°C,
1000°C, and 1100°C with a holding time of 30
minutes. It was expected that a bonding process
between magnetic fragments had been occured
without changes ofmagnetic phase, so that a solid
and hard magnetic material is obtained (Strant,
Wahlfarth, et al, 1952). Holding time 30 minutes
after sintering process was performed to eliminate
residual stress so that the material does not crack
easily and then improves its coercivity. Furthermore,
test of magnetic properties using a vibrating
magnetometer sample (VSM) was conducted to
obtain hysteresis curve of each sample. The
microstructure of sample was investigate susing
scanning electron microscopic (SEM) test.
Figure 2: Specimens after compaction.
(a) (b) (c)
Figure 1: (a) powder before calcination; (b) ball milling machine; (c) after
calcination.
Magnetic and Physical Properties Modification using Sintering Temperature Variations in the Process of Making Barium Hexaferrite
Permanent Magnet
73
3 RESULTS AND DISCUSSION
Results shows the effect of sintering temperatures on
magnetic properties and microstructure of the
magnetic samples. Temperature variation results in
changes in the properties of magnetic materials both
magnetic properties and physical properties. These
changes include coercivity, maximum energy pro-
ducts of magnetic properties, and microstruc-ture.
3.1 Characterization of Magnetic
Properties
Characterization of magnetic properties was based
on values contained in the hysteresis curves of are
shown in fig. 3. Figure 3 shows the hysteresis curve
produced from barium hexaferrite material for three
sintering temperature variations, namely: 900°C,
1000°C, and 1100°C and holding time 30 minutes.
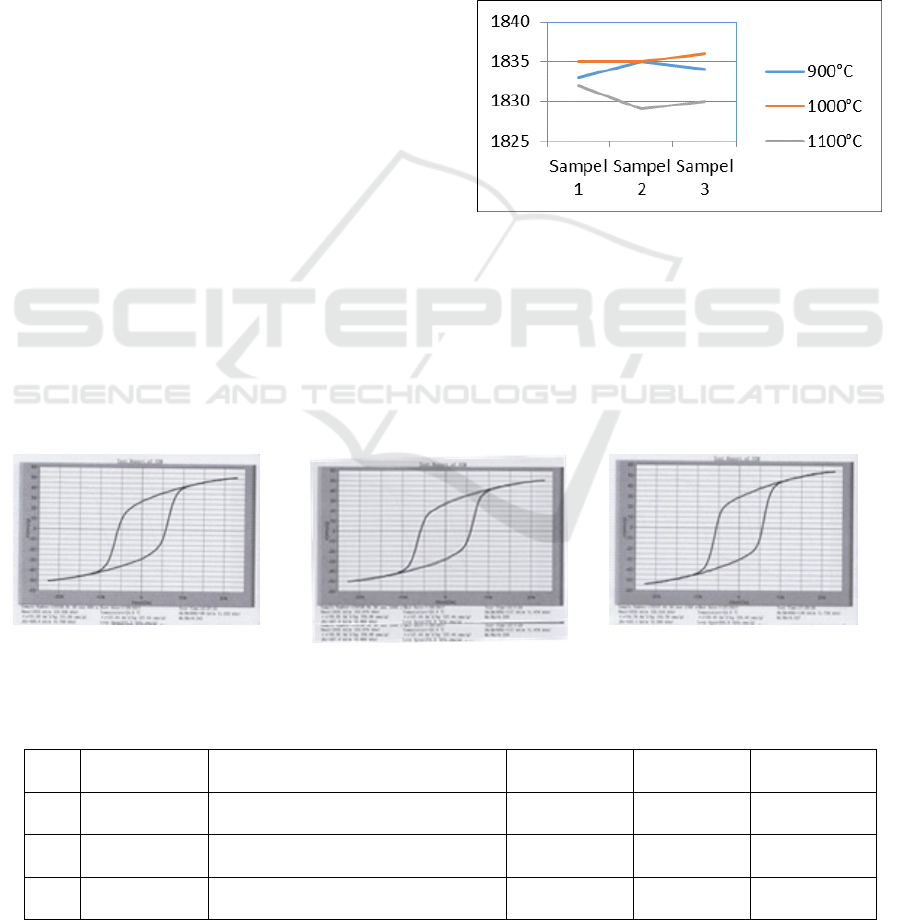
Tabel 2 shows that at temperature of 900°C the
H
max
value of 1835kA/m (23.058 kOe) at test
temperature of 25.3°C, Hc 449.8kA/m (5,653 kOe)
and 53,10emu/g of magnetic saturation were
obtained. While at temperature 1000°C H
max
value
of 1836kA/m (23.076 kOe) at test temperature of
25.0°C. Hc 467.9 kA/m (5,880 kOe)were obtained
and at 1100°C Hmax value of 1832 kA/m (23.016
kOe) at test temperature 24.9°C. Hc 443.1 kA/m
(5,569 kOe) were obtained. The results of Hmax for
each samples is shown graphically in fig. 4. Figure 4
shows graphs of VSM test results.
Figure 4. shows the effect of sinters temperatures
on magnetic coercivity. It was find that from the
hysteresis curve, the effect of sintering temperature
on magnetic coercivity at sintering temperature
1100
O
C has the smallest value of 1832 kA/m
(23,016 kOe), while at 1000
O
C has value of 1836
kA/m (23,076 kOe). The highest value from
hysteresis curve is for sintering temperature of
1000
O
C. In addition, during the sintering process
morphological of the particles are possibly changes.
Morphological changes in particles is not only
change the density between granules but also
microstructure changes.
Figure 4: Graph of H
max
values.
3.2 Microstructures
Microstructure pictures from the SEM process are
shown in fig. 5. The magnification used is 5000
times for each samples with sintering temperature of
900°C, 1000°C, and 1100°C and a holding time of
30 minutes.
(a) Sinterin
g
of 900°C (b) Sinterin
g
of 1000°C (c) Sinterin
g
of 1100°C
Figure 3: Hysteresis curves.
Table 2: Magnetic properties of barium ferrite magnets for temperature variations of sinterings.
No
Heat
Temperature
Material composition Hmax Hc
Magnetic
Saturation
1 900
O
C barium carbonate : oxide ferrite (1:6)
1835kA/m
(23.058 kOe)
449,8kA/m
(5,653 kOe)
53,10emu/g
2 1000
O
C barium carbonate : oxide ferrite (1:6)
1836kA/m
(23.076 kOe)
467,9 kA/m
(5,880 kOe)
53,10emu/g
3 1100
O
C barium carbonate : oxide ferrite (1:6)
1832kA/m
(23.016 kOe)
443.1 kA/m
(5,569 kOe).
55,20emu/g
EIC 2018 - The 7th Engineering International Conference (EIC), Engineering International Conference on Education, Concept and
Application on Green Technology
74

(a) Temperature of 900°C (b) Temperature of 1000°C (c) Temperature of 1100°C
Fi
g
ure 5: Microstructure photo
g
raphs.
The magnetic analysis and microstructure
analysis of barium hexaferrite material were
perform-ed. It was obtain that H
max
value is 1835
kA/m (23,058 kOe) at 900°C, and increasesto 1836
kA/m (23,076 kOe) at a temperature of 1000°C, then
decreases to 1832 kA/m (23,016 kOe).
Microstructure analysis was conducted the
microstructure photographs for samples with sinter
temperature of 900°C is shown in fig. 5. (a), sinter
temperature of 1000°C is shown in fig. 5(b), and
sinter temperature of 1100°C is shown in fig. 5(c). It
can be seen that grain size increasesfor temperature
900°C to 1000°C than it decrease in grain size for
temperature 1100°C. At sintering temperature of
1000°C, formation of a structure of granules fusion
was indicated and then the magnetic coercivity
increases. These results are in accordance with the
reference journal which states that at high sintering
temperature then coercivity increases and at
temperature of 1100°C it decreases (Shi, T, S. &
Grile. D, 2012,).
4 CONCLUSION
From the discussion of the research, it can be
conclude that VSM test results shows that sintering
temperature increases than Hmax values increases.
The highest Hmax value is obtain-ed at sinter
temperature of 1000°C and decreases at a
temperature of 1100ºC. Therefore the optimum
modification of hysteresis magnetic was at sinter
temperature 1000ºC. SEM test results show that
sintering temperature increases than the grain size
increase. The largest grain size is achieved at a
temperature of 1000°C. Therefore the optimum
modification of grain size was at sinter temperatur of
of 1000°C.
REFERENCES
Clement International Corporation, 1992. “Toxi-
cological Profile for Barium”. July,
D. Jiles, 1991. Introduce to MAgnetism and
Magentic Materials. Springer-Science +
Business Media, B. V.
D. P. Efhana, A. D. E. Septyani, V. Dita, Fitriana, A.
Setiawan, & M. Zainuri, 2013.
“PEMBUATAN PELAPIS PENYERAP GE-
LOMBANG MIKRO BERBASIS M-
HEXAFERRITE BaFe12-xZnxO19 Dari Pasir
Alam Pada Kabin Pesawat". Prosiding
Elektronik Pimnas, DIKTI
I. Yusan, C. Kurniawan, A. P. Tetuko, & P.
Sardjono, 2012. “Pengembangan MagNet
Permanen Berbasis Ferrite Untuk Produk
Meter Air PT Multi Instrumentasi, Bandung”,
Prosiding Pertemuan dan Presentasi Ilmiah
KIM LIPI ke-38, pp 98-107. LIPI
M. I. Alif, 2012. “Komposit Barium Ferrit Dengan
Pengikat Kaca Cult,” Unnes Phys. J.
P. Sardjono et al.,2012, “Inovasi teknologi
pembuatan magnet permanen untuk
membangun industri magnet nasional,” Pros.
InSINas, pp. 102–108.
Priyono, A. Wibowo, & M. Nur, 2001, “Preparasi
Serbuk Barium Ferrite Untuk Menghasilkan
Medan Koersive Tinggi : Tinjauan Pada proses
sintering,” Jurnal Berkala Fisika vol. 4, no. 2,
pp. 45–48.
Magnetic and Physical Properties Modification using Sintering Temperature Variations in the Process of Making Barium Hexaferrite
Permanent Magnet
75

S. Collocot, 2007.“Rare-Earth Per-Manent
Magnets: New Magnet Materials And
Applications,” pp. 1–8.
Shi, T, S. & Grile. D, 2012, “Pengembangan Bahan
Magnetik Barium Heksaferite Dari Mine-Ral
Yarosit Alam Dan Karakter-Isasinya.” Jurnal
Bionatura-Jurnal Ilmu-ilmu Hayati dan Fisik,
vol. 14, no. 2, pp. 156–163.
Sunarya, S. A. 2009, Pusat Perbukuan Departemen
Pendidikan Nasional. Depertemen Pendidikan
Nasional,
EIC 2018 - The 7th Engineering International Conference (EIC), Engineering International Conference on Education, Concept and
Application on Green Technology
76
