
Antihiperlipidemic Activity of the Methanolic Extract of Parijoto
(Medinilla speciosa) on the Protein Profile of Hyperlipidemic Rats
Noor Nailis Sa’adah, Awik Puji Dyah Nurhayati
Department of Biology, Faculty of Science, Institut Teknologi Sepuluh Nopember, Surabaya, Indonesia
Keywords: Hyperlipidemia, parijoto (Medinilla speciosa), protein profile.
Abstract: High-fat diets and frequent feeding contributes to the onset of hyperlipidemia, a family of disorders that is
characterised by abnormally high levels of lipids in the blood. Hyperlipidemia is a major cause of
atherosclerosis, which is related to conditions such as coronary heart disease (CHD), ischemic cerebrovascular
disease, peripheral vascular disease and pancreatitis. Parijoto (M. speciosa) is an endemic plant in Asia with
a distribution center in Malaysia, Indonesia and Philippines that is generally consumed by pregnant women
and used to treat diarrhoea and cholesterol. The parijoto fruit contains a flavonoid compound which has been
suggested to decrease the risk of coronary heart disease, inflammatory process, and atherosclerosis through
their antioxidant activities. During pathological conditions, there are differences in the protein profiles that
indicate the presence of protein biomarkers. Different levels of protein expression serves as a biomarker of
disease progression. This study aims to determine the effects of the methanolic extract of parijoto (M.
speciosa) on the protein profile of hyperlipidemic rats. Rats were divided into five groups: normal rats,
hyperlipidemic rats, and hyperlipidemic rats that were given the methanolic extract of parijoto (M. speciosa)
at 500 mg ·kg
˗1
, 1000 mg · kg
˗1
, and 1500 mg · kg
˗1
body weight. Rats were terminated after the 30 day
treatment of the methanolic extract of parijoto (M. speciosa) and their blood collected. Protein profile was
determined by the Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) method.
Results showed that the proteins that appeared in each group were proteins with the molecular weight 160;
144; 131; 124; 117; 110; 93; 76; 59; 52; 49; 42; 33; 25 and 14 kDa, however the(control) protein 117 kDa
was not present in group I. Protein 117 kDa was presumed as the sterol regulatory element binding protein-
1c (His-SREBP-1c), the transcription factor that transduces the insulin signal.
1 INTRODUCTION
Hyperlipidemia is a family of disorders that is
characterised by abnormally high levels of lipid (fats)
in the blood (Verma, 2016). Hyperlipidemia
contributes to the occurrence of atherosclerosis, one
of the factors that triggers cardiovascular disease,
hypertension and coronary heart disease (Kumar,
2010). Cardiovascular disease is one of the health
problems in society and is one of the leading causes
of death worldwide. Based on data from the World
Health Organization (WHO), it is predicted that
23.300.000.000 people will die of cardiovascular
disease in 2030, while Basic Health Research
(Riskesdas) in 2013 showed the prevalence of heart
disease was 1.5 % on a national scale (Kemenkes RI,
2014).
Lipids are associated with blood plasma proteins
and remain in a dissolved state in the blood.
Hyperlipidemia may be classified as primarily caused
by specific genetic abnormalities and defects in lipid
metabolism which is caused by the defect in
lipoprotein lipase activity or the absence of the
surface Apoprotein C-II. Secondary hyperlipidemia
results from another underlying disorder that leads to
alterations in plasma lipid and lipoprotein metabolism
and environmental factors (Nirosha et al., 2014).
Puskas et al. (2004) reported that hyperlipidemia
causes an alteration of genes expression in the heart,
including procollagen type III, cofilin/destrin, tensin,
transcription repressor p66, synaptic vesicle protein
2B, Hsp86, chaperonin subunit 5ε, metallothionein,
glutathione S-transferase, protein kinase C inhibitor,
ATP synthase subunit c, creatine kinase, chloride
intracellular channel 4, NADH oxidoreductase and
dehydro-genase, fibronectin receptor β chain, CD81
antigen, farnesyltransferase, calreticulin, disintegrin,
p120 catenin, Smad7, etc. Some of these genes are
Sa’adah, N. and Nurhayati, A.
Antihiperlipidemic Activity of the Methanolic Extract of Parijoto (Medinilla speciosa) on the Protein Profile of Hyperlipidemic Rats.
DOI: 10.5220/0008907900002481
In Proceedings of the Built Environment, Science and Technology International Conference (BEST ICON 2018), pages 123-128
ISBN: 978-989-758-414-5
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
123

suspected to be related to cardiovascular diseases.
During pathological conditions, there are differences
in the protein profiles that indicate the presence of
protein biomarkers. Protein biomarkers are used for
the diagnosis and prognosis of various diseases.
Different levels of protein expression serves as
biomarkers of disease progression (Naz et al., 2009).
Many natural resources containing phytochemical
components have been used as anti-hyperlipidemic
drugs. The parijoto (M. speciosa) plant is a species
endemic to Indonesia but has not been fully explored
pharmacologically; it contains phytochemical
components such as flavonoids, saponins and
kardenolin (Tussantiet al., 2014).
The intake of flavonoids is negatively correlated
to coronary heart disease because of its potential as an
antioxidant; protects LDL oxidation, a process
involved in atherogenesis (Yang et al., 2008); and
also inhibits lipase enzyme activity (Martins et al.,
2010). Sa'adah et al., (2017) reported that the
methanol extract of parijoto (M. speciosa) reduced
total cholesterol, atherogenic index, and increases
HDL-Cholesterol significantly (p <0.01).
This study observed the blood serum protein
profiles of hyperlipidemic rats which were given the
methanol extract of Parijoto. This research is
expected to reveal the anti-hyperlipidemia effects of
parijoto extract on proteomic levels. Differences in
protein profiles that occur during pathological
conditions can indicate the presence of biomarker
proteins (Naz et al., 2009).
2 MATERIALS AND METHOD
Material and method in this paper will in this
section.
2.1 Materials
Parijoto (M.speciosa) fruits were obtained from
Muria Mountain, Kudus, Central Java, Indonesia;
male Wistar rats (R. norvegicus) aged 2 months and
weighing 110 g to 150 g were obtained from the
experimental animal laboratory, Faculty of
Pharmacy, Airlangga University.
The content of the high-lipid diet included reused
cooking oil and duck yolk. The other materials used
were Comfeed
®
(Japfa) as a basal feed for the rats;
methanol; Acrylamide; TrisHCl; sterile distilled
water; 10% SDS; 10% APS and Temed.
2.2 Method
Step to reach the method will explain about
preparation, treatment and collection.
2.2.1 Preparation of Experimental Animals
The rats (R. norvegicus) were acclimated for a
week, where feed and drink were given ad libitum.
After one week acclimatization, the rats were
weighed and divided into five groups. Each of the
groups had four individual replicates.
Group I: Control without hyperlipidemia treatment
Group II: Control with Hyperlipidemia treatment
Group III:Hyperlipidemia which was given 500
mg·kg
˗1
of parijoto extract
Group IV:Hyperlipidemia which was given 1000 mg·
kg
˗1
of parijoto extract
Group V:Hyperlipidemia which was given 1500 mg·
kg
˗1
of parijoto extract.
2.2.2 Hyperlipidemia Treatment
Rats (R. norvegicus) were conditioned to be
hyperlipidemic following a procedure from Sa’adah
(Sa’adah et al., 2017). The experimental rats were
orally fed a mixture of duck yolk and reused cooking
oil (ratio 2:1) at the amount of 1% of body weight
(BW) for 30 days. The hyperlipidemia treatments
were given to all the groups except the control group
(group I).The body weight of the rats were weighed
on a weekly basis.
2.2.3 The Methanolic Extract of Parijoto
Treatment
The treatment of the methanolic extract of parijoto
with a concentration of 500 mg·kg
˗1
, 1000 mg·kg
˗1
,
and 1500 mg·kg
˗1
was given for 30 days after the rats
were in hyperlipidemic condition. The control and
hyperlipidemic rats (Groups I and II) were only given
basal feed and drink by ad libitum for 30 days. The
body weight of the rats were weighed weekly.
2.2.4 Blood Serum Collection
Blood was taken from the treated rats after the
treatment of the methanolic extract of parijoto. The
blood serum was centrifugally separated from the
blood cells at 3000 rpm for 10 minutes. The serum
blood was collected in a microtube.
BEST ICON 2018 - Built Environment, Science and Technology International Conference 2018
124

2.2.5 Proteins Profile of Rats
The protein profile of the rats’ (R. norvegicus) serum
was determined by the Sodium Dodecyl Sulphate-
Polyacrylamide Gel Electrophoresis (SDS-PAGE)
method, which consisted of several stages:
preparation of the polyacrylamide gel, assembling of
the chamber and the glass plate, injection of sample
(serum) in the comb, the process of running SDS -
PAGE, staining, and destaining of gels.
2.2.6 Standard Curves and Analysis of
Protein Bands
The molecular weight of the protein sample was
calculated by using a standard curve (y = ax + b). A
standard curve was constructed by measuring
distance marker bands of well. The marker used was
the PageRuler™ Prestained Protein Ladder
®
(gel
concentration of 10%) with a molecular weight of 10
kDa to 170 kDa. The bands distance was used as the
ordinate (x axis) and abscissa (y axis) was the
logarithm of marker molecular weight.
Description:
𝑦 = 𝑎𝑥 + 𝑏
x = Bands distance from well
y =logarithm of marker molecular weight
The bands of the protein sample were analyzed by
comparing the marker.
2.2.7 Data Analysis
The data was analyzed descriptively, such as the
presence or lack of presence of protein bands,
molecular weight of protein bands, and if the protein
bands were thin or thick. The analysis of the protein
profile was performed only on consistent protein
bands, protein bands which were present in all
replications ( running replications and individuals)
and protein bands which have relatively the same
thickness.
3 RESULTS AND DISCUSSION
Sa’adah et al. (2017 & 2018) reported that rats that
were given high-lipid diets for 30 days showed
significant increases of total cholesterol, LDL-C, TG
levels and atherogenic index value (p<0.01) at
approximately 184.06 mg · dL
˗1
, 80.11 mg ·
dL
˗1
,130.25 mg ·dL
˗1
, and 5.7 respectively; the HDL-
C level also decreased significantly (p<0.01) after the
intake of the lipid-rich diet from 55.99 mg · dL
˗1
to
27.72 mg · dL
˗1
.
Lipids are associated with blood plasma proteins
and remain in a dissolved state in the blood. Primary
hyperlipidemia is caused by specific genetic
abnormalities (Nirosha et al., 2014). Previous studies
have reported that hyperlipidemia causes alteration
of gene expression in the heart and some of these
genes have been suspected to be related to
cardiovascular diseases (Puskas et al., 2004).The
differences in gene expression during pathological
conditions can indicate the presence of biomarker
proteins (Naz et al., 2009). An intake of the
methanolic extract of parijoto reduced total
cholesterol, LDL-C levels, atherogenic index values,
and increased the HDL-C level significantly (p<0.01)
(Sa’adah et al., 2017). The lipid level is associated
with blood plasma proteins; when the lipid level of
the blood serum decreases, it may alter the serum
protein profile of the hyperlipidemic rats.
3.1 Consistency of the Serum Protein
Profile of Rats
The marker used in the method was the PageRuler™
Prestained Protein Ladder
®
which contains proteins
with molecular weights of 170, 130, 100, 70, 55, 40,
35, 25, 15 and 10 kDa. Various proteins were
obtained from the results of the SDS-PAGE of blood
serum of the rats (R. norvegicus). The results of
running and individual replications showed
consistent bands, protein bands which were present
on all replicates (running and individual replications)
with relatively the same thickness (Figure 1).
The consistency of blood serum protein bands is
affected by the physiological condition of individual
rats, such as feed intake or specific immune responses
to the pathogen. The rats in all groups were given
Comfeed® (Japfa) as the basal feed and were located
in the same condition. It was presumed that the rats
had no differences physiologically. Protein band
consistency describes the protein profile differences
of treatment groups and controls (Sa’adah et al.,
2016).
Antihiperlipidemic Activity of the Methanolic Extract of Parijoto (Medinilla speciosa) on the Protein Profile of Hyperlipidemic Rats
125
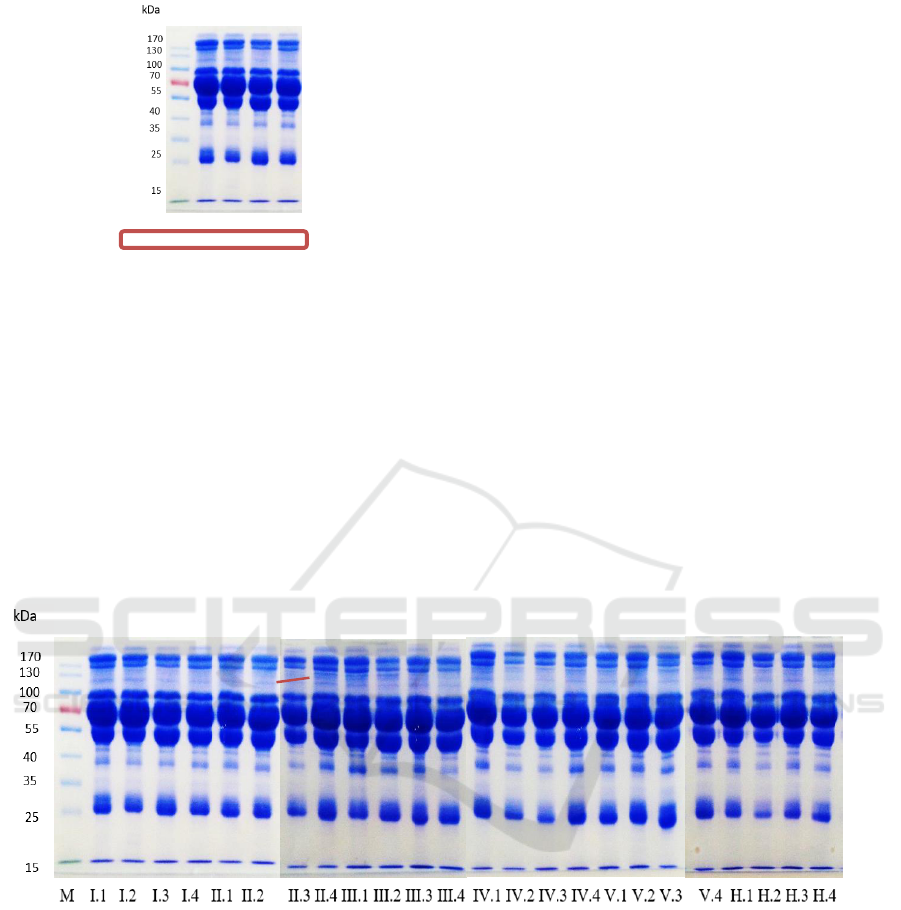
M I.1 I.2 I.3 I.4
Figure 1: Blood serum protein profile of control rats using
the SDS-PAGE method
3.2 Analysis of Serum Protein Profile of
Rats
The proteins that appeared in each group were
proteins with the molecular weights of 160; 144; 131;
124; 117; 110; 93; 76; 59; 52; 49; 42; 33; 25 and 14
kDa, however protein 117 kDa was not present in
group I (control) (Figure 2). Protein 117 kDa was
presumed as the sterol regulatory element binding
protein-1c (His-SREBP-1c), the transcription factor
that transduces the insulin signal (FoufelleandFerre,
2007).
The sterol regulatory element-binding protein-1c
(SREBP-1c) plays a major role in hepatic lipogenic
gene expression (Botolin and Jump, 2003). SREBP-
1c is one of the major isoforms of SREBP expressed
in mammalian liver (Khesht and Hassanabadi, 2012).
Overexpression of active SREBP-1c in the liver is
accompanied by increases in lipogenic enzymes
levels (Hansmannel et al., 2006). Dif et al. (2006) also
reported that the SREBPs are transcription factors
which have been shown to regulate gene expression
of several enzymes implicated in cholesterol, lipid
and glucose metabolism. The SREBP-1c promoter
was activated by insulin and can also be induced by
the activation of the nuclear receptors LXRs that have
been implicated in the control of lipid and cholesterol
metabolism (Schultz et al., 2000).
A large number of studies have demonstrated that
SREBP-1c is tightly regulated by nutritional and
hormonal status, especially at the transcriptional
level, in various tissues. Feeding a high carbohydrate
diet increases SREBP-1c mRNA and protein,
whereas they are markedly decreased upon fasting
(Gosmain et al., 2005).Therefore, the protein 117 kDa
appeared in the groups of hyperlipidemic rat groups
and does not appear in the control rat group.
Figure 2: Blood serum protein profile of rats with the SDS-PAGE method. Protein 117 kDa appeared in the hyperlipidemic
rat groups (thin protein bands) and did not appear in the control rat group
The number of individual repetition (n) = 4
Group I : Control without hyperlipidemia treatment
Group II : Hyperlipidemia control
Group III : Hyperlipidemia which were given 500 mg/kg of methanolic extract of Parijoto
Group IV : Hyperlipidemia which was given 1000 mg/kg of methanolic extract of Parijoto
Group V : Hyperlipidemia which was given 1500 mg/kg of methanolic extract of Parijoto
Group H : After hyperlipidemia treatment for 30 days
The hyperlipidemic rat groups were orally given a
mixture of duck yolk and reused cooking oil for 30
days. Duck yolk is a food that contains high fat
(35.80 % to 37.25 %), cholesterol (38.15 mg · g
˗1
) and
triacylglycerols (591 mg · g
˗1
) (Ganesan et al., 2014).
In addition, duck yolk has a composition of 31.85 %
117
BEST ICON 2018 - Built Environment, Science and Technology International Conference 2018
126

saturated fatty acid (SFA), 52.49 % monounsaturated
fatty acid (MUFA) and
15.66 % polyunsaturated fatty acid (PUFA) (Polat et
al., 2013).
Due to the high-lipid diet and frequent feeding,
the TG levels may be elevated all day long (Sahade et
al., 2013). Rats that were given a high-lipid diet for
30 days experienced an increase in total cholesterol,
LDL-C, TG levels and atherogenic index value, while
also showing significant decreases in the HDL-C
level (p<0.01) (Sa’adah et al., 2017). The increased
serum levels in the lipid-rich lipoproteins (LDL-C
and VLDL-C) indicate that more cholesterol and
triglyceride were transported from the liver to the
extra-hepatic tissues to be taken up by those tissues
(Adekunle et al., 2013). This process involved the
enzymes that implicate in cholesterol, lipid and
glucose metabolism. Therefore, the high-lipid diet
was presumed to increase the SREBP-1c mRNA and
protein; hence this protein appeared in the
hyperlipidemic rat groups, despite the thin protein
bands.
However, the SREBP-1c protein is actually found
in all individuals, because this protein plays a major
role in regulating the gene expression of several
enzymes implicated in cholesterol, lipid and glucose
metabolism (Dif et al., 2006).
4 CONCLUSION
The proteins that appeared in each group were
proteins with the molecular weight of 160; 144; 131;
124; 117; 110; 93; 76; 59; 52; 49; 42; 33; 25 and 14
kDa, however the protein 117 kDa was not present in
group I (control). Protein 117 kDa was presumed as
the sterol regulatory element binding protein-1c (His-
SREBP-1c), the transcription factor that transduces
the insulin signal. A high-lipid diet was presumed to
increase the SREBP-1c mRNA and protein, so this
protein appeared in the hyperlipidemic rat groups,
despite the thin protein bands.
ACKNOWLEDGEMENTS
The authors would like to express their gratitude
to Institut Teknologi Sepuluh Nopember (ITS)
Surabaya for the financial support. This research was
a grant for novice lecturers and was funded by ITS;
while the Zoology and Animal Engineering
Laboratory, Biology Department, ITS Surabaya; the
Experimental Animal Laboratory, Faculty of
Pharmacy, Airlangga University; and the
Biochemistry Laboratory, Pusat Antar Universitas
(PAU) Universitas Gadjah Mada (UGM) provided
materials and technical assistance.
REFERENCES
A. S. Adekunle, A. L. Adedeji, E. O. Oyewo, et al., Asian
Journal of Natural & Applied Sciences 2(1), 82–93
(2013).http://www.ajsc.leena-
luna.co.jp/AJSCPDFs/Vol.2(1)/AJSC2013(2.1−11).
pdf
Botolin D and Jump. DB. 2003. Selective proteolytic
processing of rat hepatic sterol regulatory element
binding protein-1 (SREBP-1) and SREBP-2 during
postnatal development.J Biol Chem. 2003 Feb
28;278(9):6959-
62.https://www.ncbi.nlm.nih.gov/pubmed/12488438
E. S. Polat, O. B. Citil, and M. Garip. 2013. Fatty acid
composition of yolk of nine poultry species kept in
their natural environment. Animal Science Papers
and Reports 31(4), 363–368 (2013).
http://agro.icm.edu.pl/agro/element/bwmeta1.elemen
t.agro-a8f6b877-4fe0-4886-b23c-ef77928fb2fe/
F. A.Khesht and A.Hassanabadi. 2012. Effects of sterol
regulatory element-bindingprotein (SREBP) in
chickens. Lipids in Health and Disease 2012, 11:20.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC330
5589/pdf/1476-511X-11-20.pdf
F.Hansmannel, S.Mordier and P. B. Iynedjian. 2006.
Insulin induction of glucokinase and fatty acid
synthase in hepatocytes:analysis of the roles of sterol-
regulatory-element-binding protein-1c andliver X
receptor. Biochem. J. (2006) 399, 275–283.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC160
9914/pdf/bj3990275.pdf
F. Martins, T. M. Noso, V. B. Porto, et al. 2010. Maté tea
inhibits in vitro pancreatic lipase activity and has
hypolipidemic effect on high-fat diet-induced obese
mice. Obesity (Silver Spring) 18 (1), 42–47.
https://www.ncbi.nlm.nih.gov/pubmed/19543216
Foufelle, F., and P. Ferre. P. 2002. New perspectives in the
regulation of hepaticglycolytic and lipogenic genes
by insulin and glucose: a role for the transcription
factor sterolregulatory element binding protein-1c.
Biochem. J. 366, 377-
391.https://www.ncbi.nlm.nih.gov/pmc/articles/PM
C1222807/pdf/12061893.pdf
Gosmain, Y., N. Dif, V. Berbe, E. Loizon, J. Rieusset, H.
Vidal, and E. Lefai. 2005. Regulation of SREBP-1
expression and transcriptional action on HKII and
FAS genes duringfasting and refeeding in rat tissues.
J. Lipid. Res. 46, 697-705.
https://www.ncbi.nlm.nih.gov/pubmed/15627654
I. Tusanti, A. Johan, and R. A. Kisdjamiatun. 2014.
Sitotoksisitas in vitro ekstraketanolikbuahparijoto
(Medinilla speciosa, reinw.ex bl.)
terhadapselkankerpayudara T47D. JurnalGizi
Antihiperlipidemic Activity of the Methanolic Extract of Parijoto (Medinilla speciosa) on the Protein Profile of Hyperlipidemic Rats
127

Indonesia 2(2), 53–58. [in Bahasa Indonesia]
https://ejournal.undip.ac.id/index.php/jgi/article/vie
w/8612
K.Nirosha, M.Divya, S.Vamsi, M. Sadiq. 2014. A review
on hyperlipidemia. International Journal of Novel
Trends in Pharmaceutical Sciences. Vol. 4, No. 5.
http://www.ijntps.org/File_Folder/0062.pdf
K. P. Kumar, A. R. N. Reddy, Y. N. Reddy, et al., Iranian
Journal of Pharmacology and Therapeutics 9(2),
73–75.
https://www.researchgate.net/publication/277849451
_Lipid_Lowering_Activity_of_Lercanidipine_in_Hy
perlipidemic_Rats/
L.G. Puskas, Z. B. Nagya, Z.Giriczb, A.Onody,
C.Csonka,K.Kitajkac, L. Hackler Jr, A.Zvaraa,
P.Ferdinandy. 2004. Cholesterol diet-induced
hyperlipidemia in£uencesgene expression pattern of
rat hearts: a DNA microarray study. FEBS Letters
562 (2004) 99-104.
https://core.ac.uk/download/pdf/82419259.pdf
N.Dif, V.Euthine, E.Gonnet, M.Laville, H. Vidal and
E.Lefai. 2006. Insulin activates human sterol-
regulatory-element-binding protein-1c(SREBP-1c)
promoter through SRE motifs. Biochem. J. (2006)
400, 179–188.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC163
5455/pdf/bj4000179.pdf
N. N. Sa’adah, A.P.D. Nurhayati, and M.Shovitri.2016. The
Anticancer Activity of the Marine
SpongeAaptossuberitoides to Protein Profile
ofFibrosarcoma Mice (Mus musculus). IPTEK, The
Journal for Technology and Science, Vol. 27, No. 3,
December 2016.
http://iptek.its.ac.id/index.php/jts/article/view/1183/
1652
N.N.Sa’adah, K.I. Purwani, and A.P.D. Nurhayati. 2017.
“Analysis of lipid profile and atherogenic index in
hyperlipidemic rat (Rattus norvegicusBerkenhout,
1769) that given the methanolic extract of Parijoto
(Medinilla speciosa),” in International Biology
Conference 2016: Biodiversity and Biotechnology for
Human Welfare,AIP Conference Proceedings1854,
edited by M. Murkovicet al.(American Institute of
Physics, Melville, NY, 2016),
020031.https://aip.scitation.org/doi/abs/10.1063/1.49
85422
N.N. Sa’adah, A.P.D. Nurhayati, and K.I. Purwani. 2018.
“Antihyperlipidemic and anti-obesity effects of
themethanolic extract of parijoto(Medinilla
speciosa)” in International Conference on Biological
Science2017: Inventing Prosperous Future through
Biological Research and Tropical Biodiversity
Management, AIP Conference Proceedings. 2002,
020046-1–020046-8.
https://aip.scitation.org/doi/pdf/10.1063/1.5050142
N. Verma. 2016. Introduction to Hyperlipidemia and Its
Treatment: A Review. International Journal of
Current Pharmaceutical Research. Vol 9, Issue 1,
2017.https://innovareacademics.in/journals/index.ph
p/ijcpr/article/view/16616/9004
P. Ganesan, T. Kaewmanee, S. Benjakul, et al. 2014.
Comparative Study on the Nutritional Value of Pidan
and Salted Duck Egg. Korean J. Food Sci. Anim.
Resour. 34(1), 1–6 (2014).
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC459
7835/
Pusat Data dan Informasi Kementerian Kesehatan RI.
Situasikesehatanjantung [Heart health conditions]
[Online]
http://www.depkes.go.id/resources/download/pusdat
in/infodatin/infodatin-jantung.pdf(2014), [Accessed
on 12 October 2018] [in Bahasa Indonesia]
R. Yang, Y. Shi, G. Hao, W. Li, and G. Le. 2008. Increasing
Oxidative Stress with Progressive Hyperlipidemia in
Human: Relation between Malondialdehyde and
Atherogenic Index. J. Clin. Biochem. Nutr.43 (3),
154–
158.https://www.ncbi.nlm.nih.gov/pmc/articles/PM
C2581765/pdf/jcbn-43-154.pdf
S. Naz, S. Ahmad and F. Ghafoor. 2009. Qualitative
Analysis ofSerum Proteins in Benign Prostatic
Hyperplasia Separated BySDS-PAGE. ARPN
Journal of Agricultural and BiologicalScience, vol. 4,
no. 6, pp. 24-
28.http://www.arpnjournals.com/jabs/research_paper
s/rp_2009/jabs_1109_159.pdf
Schultz, J. R., H. Tu, A. Luk, J. J. Repa, J. C. Medina, L.
Li, S. Schwendner, S. Wang, M.Thoolen, D. J.
Mangelsdorf, K. D. Lustig, and B. Shan. 2000. Role
of LXRs in control oflipogenesis. Genes. dev. 14,
2831-2838.
https://www.ncbi.nlm.nih.gov/pubmed/11090131
V. Sahade, S. Franca, and L. F. Adan. 2013. The influence
of weight excess on the postprandial lipemia in
adolescents. Lipids in Health and Disease.Vol. 12:
17.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC
3599910/
BEST ICON 2018 - Built Environment, Science and Technology International Conference 2018
128
