Isolation and Characterization of Chitosan from Coconut Crab Skin
Origin of Halmahera Island with Ftir
Zulkifli Zam Zam, Sudir Umar, Tamrin Robo, Fadlan Muin
Faculty of Teacher Training and Education, Khairun University
Jln. Bandara Babullah, Akhedua, North Ternate, Ternate
Keywords: Coconut crabs, Isolation, Characterization, Chitosan, FTIR
Abstract: This study aims to determine the isolation and characterization of chitosan from the skin of coconut crabs.
Isolation of chitosan from coconut crabs was carried out through four stages, namely deproteination (4%
NaOH, 80
o
C), demineralization (HCl 1.25 N, 70
o
C), depigmentation (4% NaOCl, 75
o
C), deasitilation (50%
NaOH, 80
o
C). The degree of deasitilation of chitosan from walnut crabs is 82.52%. Chitosan FTIR
spectrum analysis results of several main examples of wave numbers 3371.57 cm
-1
which show symmetrical
stretching vibrations due to overlapping OH and amines (NH), stretching vibrations of 1597.06 cm
-1
caused
by propagation of C=O and stretch vibrations 1651.07 cm
-1
which shows secondary amide. Characterization
of FTIR spectroscopy which showed the extraction of coconut crabs was chitosan.
1 INTRODUCTION
Halmahera Island has abundant natural resources in
the fisheries sector, one of which is coconut crabs.
The results of observations conducted in the markets
showed that the sale of coconut crabs carried out
was limited to the sale of the meat while the shell
crabs shell was discarded and left alone until it roted
without any utilization. If left unchecked it will
cause environmental pollution and damage to
environmental aesthetics. Crustacea (coconut crab)
skin waste consists of three main components,
namely protein (25% - 44%), calcium carbonate
(45% -50%), and chitin (15% 20%) (Fohcher et al,
1992). The chitin content in shrimp skin waste is
around 20-50% dry weight. Chitin polymers are
composed of monomers; 2-acetamide-2-deoxy-D-
Glucose (N-acetyl glucosamine) (Horton et al,
2002). The bond between chitin monomers is the
glycoside bond in the β- (1-4) position. The structure
of chitin molecules is a long straight chain. Chitin is
the largest natural polymer in the world after
cellulose (Yanming et al, 2001).
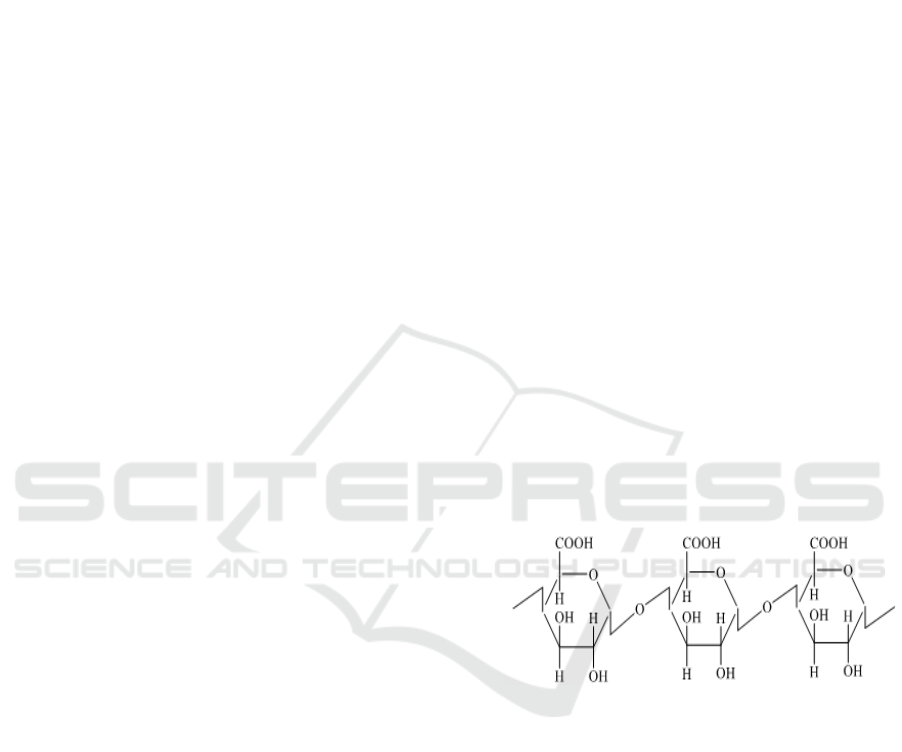
Chitosan [poly-2-amino-2-deoxy-β- (1-4) D-
glucopiranose)] is a poly-aminosaccharide
compound synthesized by partially removing 2-
acetyl groups from chitin [poly (2 acetamido-2-
deoxy-β-(1-4)-Dglukopiranosa)], linear biopolymers
with 2000-5000 monomer units, bound together by
β- (1-4) glycosidic bonds. Chitosan (C
6
H
11
NO
4
)n is
a yellowish white amorphous solid, polyelectrolyte
(Chen et all, 2007). Generally soluble in organic
acids, the pH is around 4–6.5, insoluble at lower or
higher pH. Solubility is influenced by molecular
weight and degree of deacetylation (Mima et al.,
1983).
Figure 1: Molecular Structure of Chitosan.
Things related to the environment are by
utilizing coconut crabs that form chitin and then
transformed into chitosan which can be applied in
various fields (Hargono et al., 2008). Since chitosan
has high economic value, it is very important to
conduct research to process skin into chitosan.
2 MATERIALS AND METHOD
2.1 Material
The materials used are coconut crab skin, acetic acid
(CH
3
COOH), hydrochloric acid (HCl), sodium
298
Zam, Z., Umar, S., Robo, T. and Muin, F.
Isolation and Characterization of Chitosan from Coconut Crab Skin Origin of Halmahera Island with Ftir.
DOI: 10.5220/0008901202980302
In Proceedings of the 1st International Conference on Teaching and Learning (ICTL 2018), pages 298-302
ISBN: 978-989-758-439-8
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

hydroxide (NaOH), technical sodium hypochlorite
(NaOCl), filter paper and aquades.
2.2 Tools
Petri dishes, pH meters, analytics, a set of reflux
tools, glass plates, magnetic stirrers, shakers, ovens,
mes machines, magnetic support devices, shakers,
ovens, mes machines, supporting tools in the form of
glassware, plastic and FTIR.
2.3 Experimental
2.3.1 Stages of Sample Preparation
Wash the skin of the coconut crabs with running
water to remove the impurities that are attached,
then dry them in an oven at 80˚C for 24 hours. After
that the dried coconut crab skin is mashed to 100
mesh, then processed to get chitosan.
2.3.2 Deproteination
Add 4% NaOH with a ratio of 1: 6 (b/v) to the skin
of finely ground coconut crabs, then heated at 80˚C
for 30 minutes. Then cool the resulting solution and
filter it so that it gets a solid, after that the solid is
dried at 80˚C for 24 hours (Roberts, 1992).
2.3.3 Demineralization
Mixing coconut crabs skin with HCl 1.25 N with a
ratio of 1:20 (b/v), then heated at a temperature of 70
˚C for 1 hour. The solution formed is then filtered so
that it gets solids. The solid is washed with water to
neutral pH, then dried at 80˚C for 24 hours. The
product produced is chitin
2.3.4 Depigmentation
Depigmentation stages using 4% NaClO to remove
impurities that may have been produced in the
previous process
2.3.5 Deacetylation of Chitin to Chitosan
Mix chitin powder with 50% NaOH solution with a
ratio of 1:10 (w / v) then heat it for 6 hours at a
temperature of 80˚C. Washing solids obtained with
distilled water to neutral pH after drying with an
oven at 80˚C for 24 hours. The product formed from
this process is chitosan. The chitosan obtained was
then analyzed by FTIR to determine the Degree of
Deacetylation (DD). Deacetylation degree
calculations using equations:
3 RESULTS AND DISCUSSION
3.1 Isolation of Chitosan Coconut Crabs
Coconut crabs skin is washed first to remove the dirt
that sticks. After washing, the coconut crabs skin is
dried in the sun to remove the water content. Then
the coconut crabs skin is mashed with a blender
(powder), aiming to simplify the deproteination,
demineralization, depigmentation and deaetylation
treatment processes.
3.2 Deproteination
The deproteination process aims to break the bonds
between protein and chitin by adding NaOH. The
skin of a 50 gram coconut crabs is dissolved with
500 ml of 4% NaOH then heated at 80
o
C for 2 hours
while continuing to stir. After being heated the
solution is cooled for 30 minutes then filtered with a
filter while washing with distilled water until the pH
is neutral. Then the coconut crabs skin was dried in
the oven for 24 hours and weighed. Deproteinization
of coconut crabs skin was 31.86 grams so that the
protein content contained in shrimp skin ranged
from 36.28%. In this process, NaOH functions to
break the intermolecular bond between chitin and
protein, then the protein will bind to Na
+
proteinat
which dissolves in water.
3.3 Demineralization
Demineralization to remove inorganic salts or
minerals contained in the skin of coconut crabs. The
main minerals contained in the skin of coconut crabs
are CaCO
3
and Ca
3
(PO
4
)
2
. Marganov, 2003,
minerals contained in the skin and shells of
crustaceans (shrimp, crabs, etc.) are more easily
separated than proteins because they are only
physically bound. The mineral separation process is
indicated by the formation of CO
2
gas in the form of
air bubbles when the HCl solution is added in the
sample, so that the addition of HCl into the sample
is done in stages so that the sample does not
overflow (Hendry, 2008). Reactions that occur:
Isolation and Characterization of Chitosan from Coconut Crab Skin Origin of Halmahera Island with Ftir
299
3.4 Depigmentation
Depigmentation stages using sodium hipolorit
(NaClO) to remove impurities that may be present in
the previous process, and produce a yield of 91.04%.
The product of this stage is called chitin and a
further process is needed to obtain chitosan which is
deacetylation.
3.5 Deacetylation
Deacetylation is the process of removing acetyl
groups (-COCH
3
) from chitin using an alkaline
solution to change to an amine group (-NH
2
) (Sirait,
2002). Chitin has a long crystalline structure with
strong hydrogen bonds between nitrogen atoms and
carboxylic groups in adjacent chains (Muzzarelli,
1986). Termination of the bond between the acetyl
group and the nitrogen group so that it turns into an
amine group (-NH
2
) needs to use NaOH with a
concentration of 50% at 80
o
C for 4 hours. The use of
alkali solutions with high concentrations and high
temperatures during the deacetylation process can
affect the degree of deacetylation produced (Kim et
al., 2004).
Depigmentation stages using sodium
hypochlorite (NaClO) to remove impurities that may
be present in the previous process, and produce a
yield of 91.04%. The product of this stage is called
chitin and a further process is needed to obtain
chitosan which is deacetylation.
3.6 Degrees of Deacitilation and
Characterization of Chitosan with
FTIR
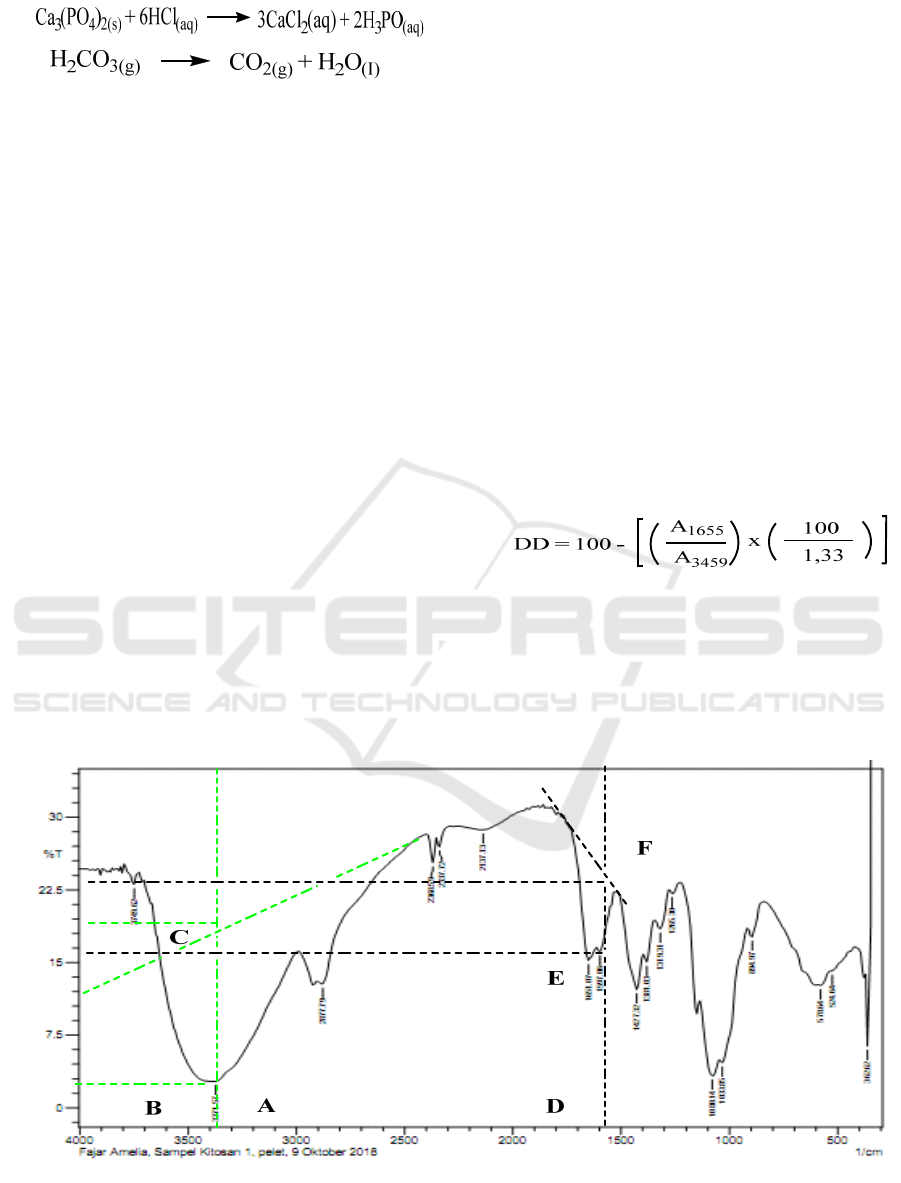
3.6.1 Degree of Deacitylation of Chitosan
The FTIR spectrum analysis of chitosan from the
skin of coconut crabs was carried out in the
functional group and fingerprint region with a
frequency of 4000 cm
-1
-400 cm
-1
. The degree of
deacetylation of chitosan is determined by the base
line method based on the FTIR spectrum, the
formula used:
Where, A
1655
shows absorption in the amide
band, A
3459
shows absorption in the hydroxyl band,
and factor 1.33 shows the value of the ratio
A
1655
/A
3459.
Figure 2: Calculation of the Degree of Deacetylation of Chitosan from the Skin of Coconut Crabs.
ICTL 2018 - The 1st International Conference on Teaching and Learning
300
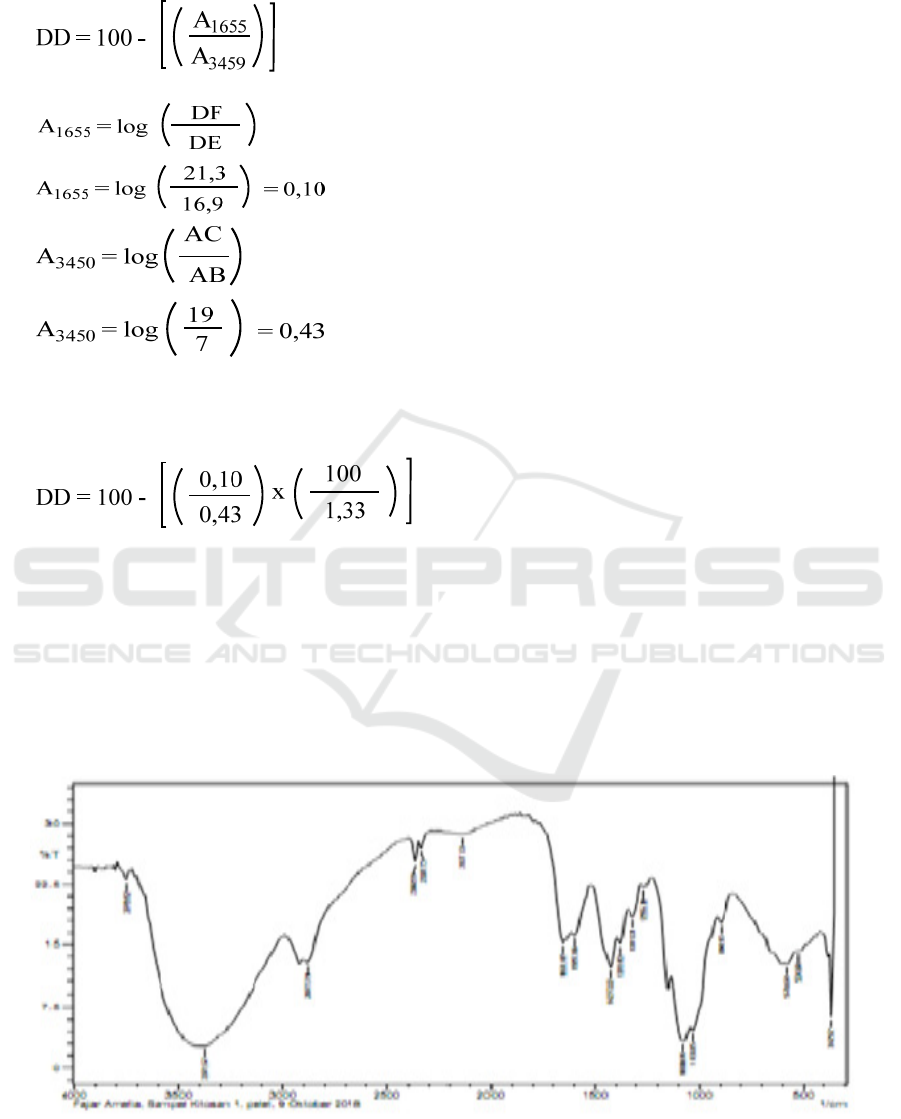
Determination of the degree of deacetylation of
coconut crabs:
Where,
So that, the value of the degree of deacetylation
obtained:
DD = 100 - 17,48
DD = 82,52%
Degree of deasitilation of coconut crabs skin is
82.52%, indicating the provision of an amine
group (NH
2
) as an active group. The degree of
deacetylation is carried out to determine the
formation of chitosan from chitin. The degree of
deacetylation of chitosan in this study was 82.52%,
which was determined based on the FTIR
spectrophotometer method with the Base Line
method as proposed by Baxter et al (Khan et al.,
2002). The degree of deacetylation is still in
accordance with the value of deacetylation degree
according to Protan Laboratory which states that
the degree of deacetylation of chitin to chitosan
usually ranges from 70-100%.
3.6.2 Characterization of Coconut Crabs
Skin with FTIR Spectrophotometer
In figure 3 shows the FTIR spectrum of the skin of
coconut crabs in the area 400 - 4000 cm
-1
. Chitosan
produced from coconut crabs was characterized by
FTIR spectroscopy. The chitosan IR spectrum is
presented in Figure 3, to identify the functional
groups. Characteristic absorption of chitosan is at
wave number 3371.57 cm
-1
which indicates the
presence of hydrogen bonds from the -OH group
which overlap with the -NH range (Guibal, 2004).
Uptake at 2877.79 cm
-1
indicates the existence of a
range of vibrations from the -CH, while the tensile
vibration -CH appears at a stirring number of
1381.03 cm
-1
. -NH gulp vibration appears at wave
number 1597.08 cm
-1
. C-O obstacle vibration is
one of the characteristic absorption of
polysaccharides. Appears at wave number 1080.14
cm
-1
. Based on the picture, it is also seen that the
absorption in the area of 1651.07 cm
-1
is getting
weaker and this indicates that diacetylation is
perfectly close (Marguerite, 2006).
Figure 3\: Chitosan FTIR Spectrum from Coconut Crabs Skin.
Isolation and Characterization of Chitosan from Coconut Crab Skin Origin of Halmahera Island with Ftir
301

4 CONCLUSION
This study has succeeded in isolating chitosan
compounds from the skin of coconut crabs through
the process of deproteinization reaction with
NaOH, demineralization with HCl, depigmentation
with NaOCl and deacetylation with NaOH. The
degree of deacetylation from the isolation of
chitosan from coconut crabs skin was 82.52%. The
results of infrared spectroscopy characterization
showed that the extracted compound was chitosan.
ACKNOWLEDGEMENTS
The authors acknowledge the research grant
provided by the Khairun University under the
Short Term Grant Scheme.
REFERENCES
Chen, C. H., Wang, F. Y., Mao, C. F., Yang, C. H. 2007.
Studies of chitosan. preparation and characterization
of chitosan/poly (vinyl alcohol) blend films. Journal
of Applied Polymer Science, 105, 1086–1092.
Gao, Y. M, Oshima, S. Motomizu, 2000. Adsorption
Behaiver Of Metal Ions on Cross-Linked Chitosan
and Determination of Oxoabions after Pretreatment
with a Chitosan Column. Analytical Science, Vol. 16.
Guibal, Eric 2004. Interactions of metal ions with
chitosan-based sorbents: a review. Separation and
Purification Technology 38 (2004) 43–74.
Fohcher, B., Naggi, A., Tarri, G., Cosami, A.,
Terbojevich, M. 1992. Structural differences
between chitin polymorhs and their precipitates from
solution evidences from CP-MAS 13 C-NMR, FTIR
and FTRaman Spectroscopy. Carbohydrate Polymer
17 (2) : 97-102.
Hendry, J. 2008, Enzymatic Deproteination Technique
(Portonus pelagious) by Using Pseudomonas
aeruginosa Bacteria for Making Chitin Polymers and
Deacetylation, Accessed 10 November 2014 at
http://www.fmipa. unila.ac.id / proceedings 2008.
Horton, R.H., Moran, L.A., Ochs, R.S., Rawn. J.D. dan
Scrimgeour, K.G. 2002. Principles of biochemistry
(Third Edition). New York: Prentice-Hall,Inc.
Khan, T.A, Peh, K.K, and Chang, H.S. 2002. Reporting
Degree of Deacetylation Values of Chitosan: The
Influences of Analytical Methods. J.Pharm.Sci. 5
(3), 205-212.
Kim, Eun-young, et al. 2005. Leaching Behavior of
Nickel from Waste Multi-layer Ceramic Capacitors
Marganov. 2003. Potential of Shrimp Waste as
Absorbent of Heavy Metals (Lead, Cadmium, and
Copper) in Waters. Dissertation, IPB, Bogor.
Marguerite, Rinaudo. 2006. Chitin and Chitosan:
Properties and applications. Prog Polym. Sci. (31):
603-632.
Mima, S., Miya, M., Iwamoto, R. and Yoshikawa, S.
1983. J Appl Polym Sci. 28 (6): 1909-1917
Roberts GAF. Structure of chitin and chitosan. In:
Roberts GAF, editor. Chitin chemistry. Houndmills
Macmillan; 1992. p. 1–53.
Sirait, R. I. 2002. Utilization of chitosan from the skin of
shrimps and bark shells to reduce the levels of Ni
and Cr wastes of metal coating industries. USU S-2
Thesis.
Yanming, D., Congyi, X.U., Jianwei, W., Mian,W.,
Yusong, W.U., Yonghong, R. 2001. Determination
of degree of substitution for N-acylated chitosan
using IR spectra. Science in China 44 (2) : 216224.
ICTL 2018 - The 1st International Conference on Teaching and Learning
302
