
The Effect of Heating, Boiling and Acidification Techniques on the
Total and Exchangeable Contents of K, Ca, and Mg in Volcanic Ash
of Sinabung of North Sumatra
Basyaruddin
1
, Khusrizal
1
and A. Malik
2
1
Faculty of Agriculture, Universitas Islam Sumatera Utara, Medan, Indonesia
2
Faculty of Agriculture, Universitas Malikussaleh, Aceh Indonesia
Keywords: Volcanic Ash, Heating, Boiling, Acidification.
Abstract: This research is aimed at studying the effect of heating, boiling, and acidification techniques of Sinabung
Volcanic Ash (AVS) material on the total and exchangeable concentrartion of K, Ca, and Mg, to obtain a
technique that can increase the exchangeable content of K, Ca, and Mg, and to produce products of
agrotechnolgy containing exchangeable K, Ca, and Mg in high concentration. This experiment uses a non
factorial completely randomized design. The treatments tested were 3 types of techniques consisting of:
oven-heating technique of 100oC (PO), 100oC (PA) water-boiling technique, and 0.01N HCl acidification
technique (US). Each treatment technique was carried out for 6 hours. The variables observed included the
total content of K, Ca, and Mg (extractant ingredients H2SO4 (p) and HCl (p), and the exchangeable content
of K, Ca, and Mg (NHOAc, pH 7.0), the ratio of K, Ca, and Mg to be exchangeable for the total amount,
and the relative ratio between elements K, Ca, and Mg. Measurement of K, Ca and Mg elements uses the
Atomic Absorption Spectrophotometer (AAS) tool. The results showed that the effect of heating technique
treatment with oven 100
o
C (PO), boiling water 100
o
C (PA), and acidification techniques with 0.01 N HCl
were significantly different in increasing total and exchangeable contents of K, Ca, and Mg, releasing rate
of exchangeable contents of K,Ca, and Mg. Acidification technique is the best technique in increasing all
the variables observed and manufacturing products of agrotechnology, followed by heating and boiling
techniques. Three products were found and marked by codings of AVS-A0,01-6, AVS-O100-6, dan AVS-
W100-6.
1 INTRODUCTION
Sinabung Mountain is one of the mountains located
in Karo District of North Sumatra, Indonesia at
coordinate position of 03
o
10’ NL and 98
o
23’ EL
and highest peak of 2.460 m from the sea level.
Historically, Sinabung Mountain has never erupted
since 1600. However it is reactive and some
eruptions have occured, and the first one occured on
August 27, 2010.
Although Sinabung eruptions have distructed the
crops, resulting bad impacts on the people in its
surroundings, and high financial loss (Retnaningsih,
2013; Sudiarso, 1987), the volcanic ash produced
from the Sinabung eruption is reported to be able to
improve the quality of chemical and physical
properties of soil (Andhika, 2011; Rostaman et al.
2011). It is also reported that Volcanic Ash of
Sinabung (VAS) contains some nutrients (Sinuhaji,
2011; Barasa, 2012) and primary minerals as sources
of plant nutrients (Khusrizal et al.2018). According
to Khusrizal et.al (Khusrizal et.al. 2018), the VAS in
depth of 0-24 cm contains primary minerals such as
plagioclas (34%), hipersthene (9%), augite (3%),
and amphibole/hornblende (5%). These minerals are
sources of plant nutrients such as K, Mg, and Ca.
The total contents of each element in VAS are
found in the amount of 2,27 %, 8,12 %, and 2,28 %
respectively. All these cations are unavailable to be
absorbed by plants as they are still in mineral
structures. These cations can be available to plants if
the minerals have been weathered by the rections as
the following:
Calsium (Ca) sources from weathering of Ca-
plagiclase
Basyaruddin, ., Khusrizal, . and Malik, A.
The Effect of Heating, Boiling and Acidification Techniques on the Total and Exchangeable Contents of K, Ca, and Mg in Volcanic Ash of Sinabung of North Sumatra.
DOI: 10.5220/0008881700190025
In Proceedings of the 7th International Conference on Multidisciplinary Research (ICMR 2018) - , pages 19-25
ISBN: 978-989-758-437-4
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
19

CaAlSi
2
O
8
+H
2
CO
3
+ ½O
2
= Al
2
Si
2
O
5
(OH)
4
+ Ca
2+
+ CO
3
2-
..............(1)
Magnesium (Mg) sources from weathering of
hypersthene are:
MgFeSi
2
O
3
+ 2H
2
CO
3
= Mg
2+
+ Fe
2+
+ 2HCO
3-
+ SiO
2
+ H
2
O.......(2)
Potassium (K) sources from weathering of feldspar
or orthoclase are:
2KAlSi
3
O
8
+ 2H
+
+ 9H
2
O = HAlSi
2
+ 4H
4
SiO
4
+
2K
+
.......... (3)
The concentration of K, Ca, and Mg is
exchangeable form and/or available to the plants
depending on the reaction rate that is controlled by
some factors such as mineral species, temperatures,
soil acidity, and soil moisture. According to Bowen
Series, the rate of weatherable minerals is
deteriorated from amphibole, biotite until K-felsdpar
(Bowen, 1928). Under the condition of high
temperature and low pH, it could support promoting
the rate of dilution process resulted from the
minerals destroyed releasing exchangeable cations.
Soil moistures are responsible to control hydration
and reduction–oxidation process. By this process,
iron (Fe) in mineral structure, and pyroxene can be
reduced or oxidized so that the mineral is destroyed
and some cations are released (Schott et al., 1981).
All the processes are able to increase the
concentration of exchangeable cations.
In order to improve the concentration of
exchangeable contents of K, Ca, and Mg, the
volcanic ashes can be treated by promoting the
decomposition of mineral such as heating, boiling,
acidification, and fermentation. All of these
technique tereatments are recommended to be
conducted by Khusrizal et al (Khusrizal et al. 2018).
This research aims to study the effect of heating,
boiling, and acidification techniques on total and
exchangeable form contents of K, Ca, and Mg, to
find technique that is able to increase the
exchangeable forms of K, Ca, and Mg and to
produce the argotechnology product containing
exchangeable forms of K, Ca, and Mg in high
amount.
In addition, these products can potentially be
applied to substitute or reduce the fertilizer material
from other sources, such as KCl and/or dolomite
(CaMgCO
3
). Finally, it can reduce the financial
budget or increases benefits in farming.
2 RESEARCH METHOD
The experiment was conducted in Laboratory of
Faculty of Agriculture, Islamic University of North
Sumatra. The materials of VAS were colleted in
depth of 0-10 cm from Karo District, Taman Taran
Sub District, Sigarang-garang village. The location
is in coordinate position of 3
0
11’27.1“N 98
0
24’52.1” E. Morphologically, VAS is characterized
by gray color, and used to distinguish between VAS
and soil materials in its surrounding. The VAS
collected was sieved by using the screener of 40
mesh in size in order to minimize the texture and
homogenity of VAS materials.
The experiment was arranged in Non Factorial
Randomized Complete Design. Three techniques as
treatments were tested consiting of heating 100
o
C
by oven (PO), boiling 100
o
C (PA), and acidification
by HCl 0,01N (AS) techniques. Heating technique
of 100
o
C by oven was run in accordance with the
procedure in which 2 kg of VAS material was
heated in 100
o
C in oven for 6 hours. Afterwards,
the VAS material was cooled under closed room
temperature, and direct blow of wind is to be
avoided. After VAS material was cool, it was put in
a bucket of 2 kg in capacity. This product was
marked with a code as AVS-O100-6.
Boiling technique was conducted by using the
procedure in which 2 kg of VAS material was boiled
in 1 liter of water in 100
o
C for 6 hours, and stirred
until turning into mud. Then, The VAS material was
cooled under closed room temperature, and the
direct blow of wind is to be avoided. The cooled
VAS material was put into a bucket of 2 kg in
capacity.
This product is signed with a code as
AVS-W100-6.
Acidification technique was done by the
procedure in which 2 kg of VAS material was mixed
with 1 liter of 0,01N HCl and stirred for 6 hours in
mixer. VAS material was dried in closed room
temperature for 6 hours, and then put into the bucket
of 2 kg in capacity. This was marked with a code as
AVS-A0,01-6
The variables observed consisted of the total
concentration of K,Ca, and Mg (extracted by
concentrate of H
2
SO
4(c)
dan HCl
(c)
), exchangeable
content of K, Ca, and Mg (NH
4
OAc, pH 7,0),
exchangeable ratio of K,Ca, and Mg to totality of
K,Ca, dan Mg, and each product of AVS-O100-6,
AVS-W100-6 and AVS-A0,01-6 and VAS was
standard. Both the total or exchangeable contents of
K, Ca, and Mg were determined by using Atomic
Absorption Spectrophotometer (AAS).
ICMR 2018 - International Conference on Multidisciplinary Research
20
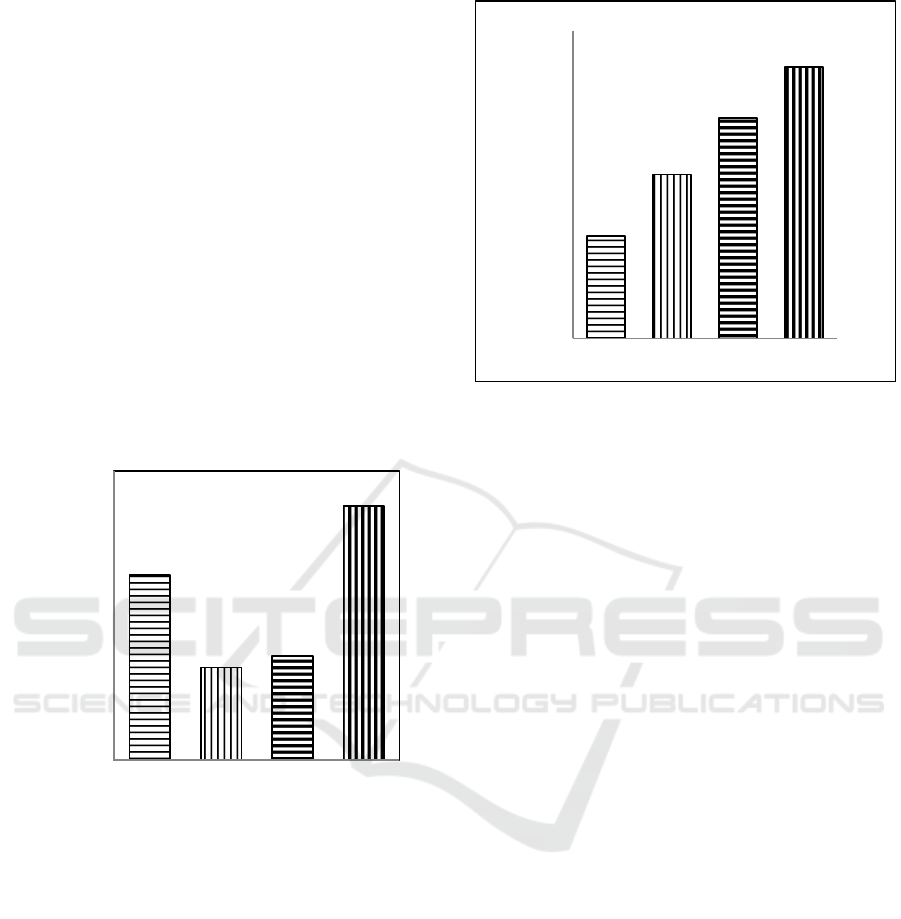
3 RESULTS AND DISCUSSION
3.1 The Effect of Heating, Boiling, and
Acidification Techniques on Total
Contents of K, Ca, and Mg in VAS
3.1.1 Potassium Total (K)
The effects of heating, boiling, and acidification
techniques on total content of K in VAS are
presented in Figure 1. Figure 1 shows that the effect
of heating and boiling techniques (oven and water)
and acidification technique are different significantly
on the total concentration of K, Ca, and Mg in VAS.
The effect of acidification technique is the highest of
that of heating and boiling techniques. The latter
techniques of heating and boiling are not different
significatly according to DMRT test.
Treatments
Figure 1: The Effects of Heating (PO), Boiling (PA) , and
Acidification (AS) Techniques on Total content of K in
VAS.
Treatments
Figure 2: The Effects of Heating (PO), Boiling (PA), and
Acidification (AS) Techniques on Total of Ca in VAS.
3.1.2 Calsium Total (Ca)
The effects of heating, boiling, and acidification
techniques on total content of Ca in VAS are
presented in Figure 2. The effect of acidification
technique is highest on the total content of Ca
(8,46%) folowed by boiling technique (8,26%), and
heating technique (8,04%), and controlled
treatments (7,80%).
3.1.3 Magnesium Total (Mg)
The effects of heating, boiling, and acidification
techniques on total of Ca in VAS are presented in
Figure 3.
2,26 b
2,18 a
2,19 a
2,32 c
2,1
2,15
2,2
2,25
2,3
2,35
AO PO PA AS
Concentration of Total K (%)
7,80cd
8,04c
8,26 b
8,46 a
7,40
7,60
7,80
8,00
8,20
8,40
8,60
AO PO PA AS
Concentration of Ca-total ,%
The Effect of Heating, Boiling and Acidification Techniques on the Total and Exchangeable Contents of K, Ca, and Mg in Volcanic Ash of
Sinabung of North Sumatra
21
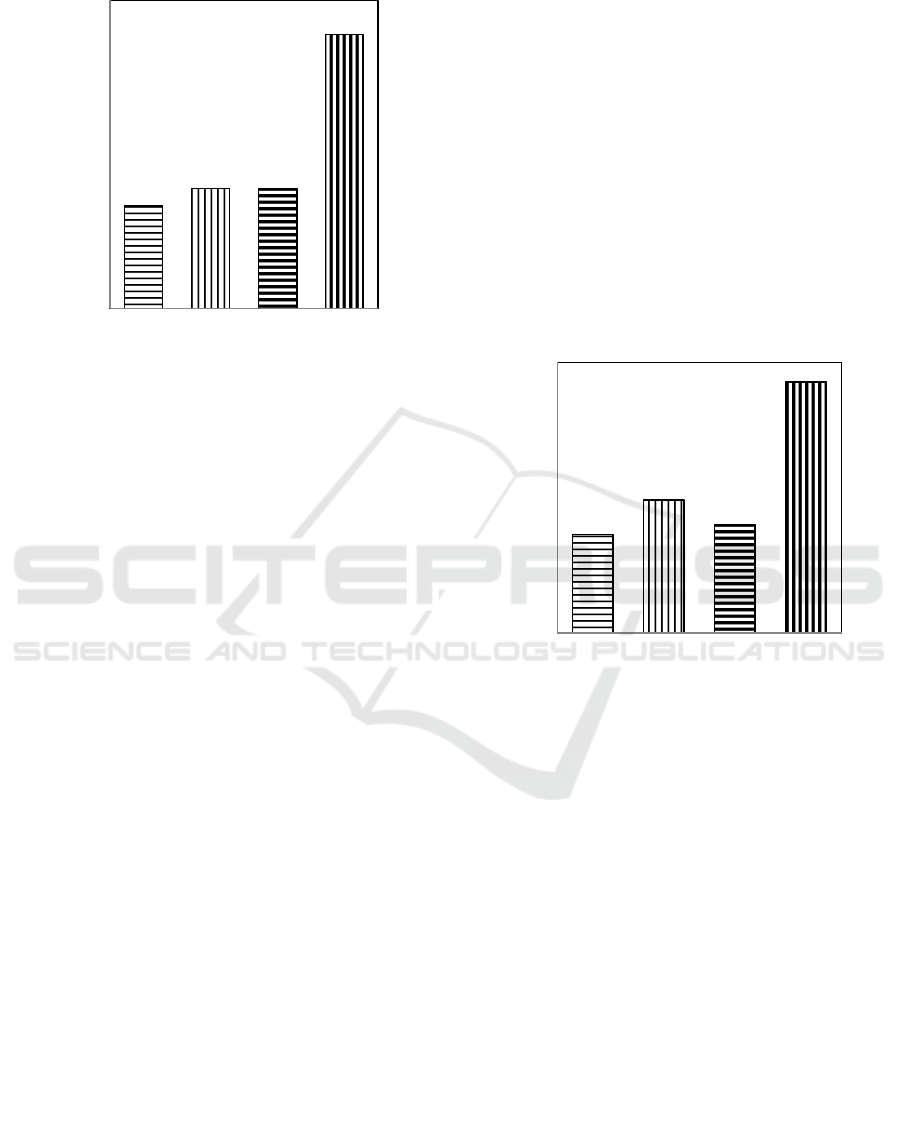
Treatments
Figure 3: The Effects of Heating (PO), Boiling (PA), and
Acidification (AS) Techniques on Total of Ca in VAS.
Figure 3 shows that the effects of the techniques
are significantly different on concentration of total
of Mg. Acidification techniques produce highest
concentration of total of Mg followed by boiling
(2,27%), heating technique (2,27%), and VAS as
standard tereatment (2,26%). Both the techniques,
boiling and heating are not significanly different.
Acidification technique produces highest
concentration of total of K, Ca, and Mg, and it can
be caused by some alternative reasons such as: (1)
acidification reaction involved is responsible in
weathering of minerals as representive in weathering
reaction of Ca-plagioklas (equation 1), hipersten
(equation 2), and feldspar/orthoklas (equation 3) in
naturally weak acid (H
2
CO
3
) (Scott et al 1998); (2)
in acidification technique there are double
acidification treatments, both of which are by HCl
0.01N and in extraction using the mixed hard acids
(H
2
SO
4
and HCl). This action can possibly destroy
the minerals in big amount so that Ca should be
released; and (4) under natural condition, the
weathering of minerals involves carbonate acid.
(H
2
CO
3
) is weak acid; while in application of
acidification technique, the hard acid (HCl) is used.
Environment under control by hard acid can
support to accelarate the reaction rate to improve
weathering of mineral intensively and dialution of
K, Ca, and Mg could be exchangeable increasing the
concentration.
3.2 The Effect of Heating, Boiling, and
Acidification Techniques on
Exchangeable Contents of K, Ca,
and Mg in VAS
3.2.1 Exchangeable Content of K
The effects of heating, boiling, and acidification
techniques on exchangeable content of K in VAS
are significantly different as presented in Figure 4.
Figure 4 shows that the concentration of
exchangeable content of K is treated by acidification
technique of the highest of (2,01 me%), and then
the heating is decreased to (1,77 me%) and (1,72
me%) and AVS is standard (1,70 me%)
respectively.
Treatments
Figure 4: The Effect of Heating (PO), Boiling (PA) , and
Acidification (AS) Techniques on Exchangeable content
of K in VAS.
3.2.2 Exchangeable Content of Ca
The effects of heating, boiling, and acidification
techniques on exchangeable content of Ca in VAS
are presented in Figure 5. The Figure 5 shows that
the exchangeable concentration of Ca in VAS that
are treated by heating, boiling, and acidification
techniques produce 21,7 me%, 21,46 me%, and 21,8
me% respectively. All the techniques are not
significantly different, but they are significantly
different from the standard treatment (20,45 me%).
2,26b
2,27b 2,27b
2,36 a
2,20
2,22
2,24
2,26
2,28
2,30
2,32
2,34
2,36
2,38
AO PO PA AS
Concentration of Mg Total (%)
1,70b
1,77b
1,72b
2,01a
1,50
1,55
1,60
1,65
1,70
1,75
1,80
1,85
1,90
1,95
2,00
2,05
AO PO PA AS
Exchangeable of K (me %)
ICMR 2018 - International Conference on Multidisciplinary Research
22
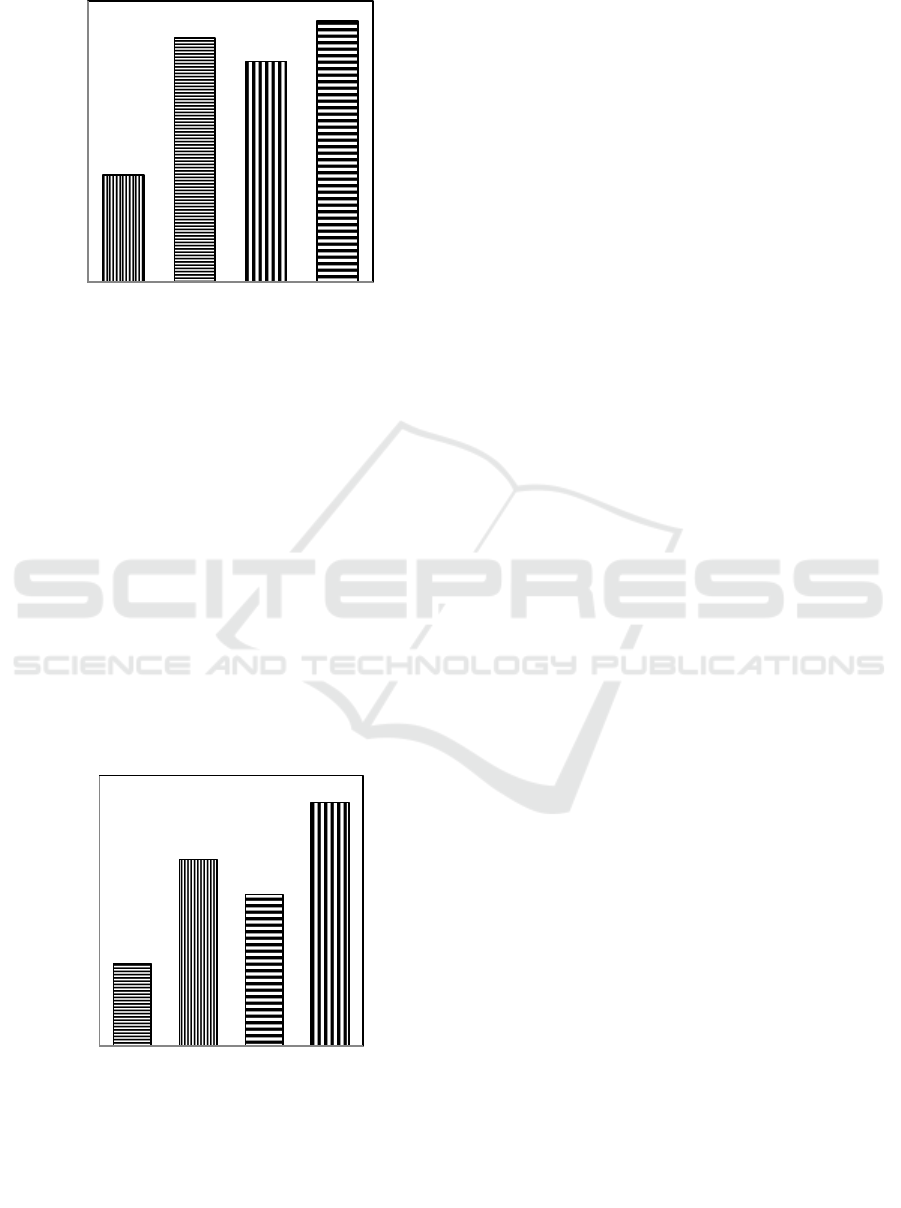
Treatments
Figure 5: The Effects of Heating (PO), Boiling (PA), and
Acidification (AS) Techniques on Exchangeable Content
of Ca in VAS.
3.2.3 Exchangeable Content of Mg
The effects of heating, boiling, and acidification
techniques on exchangeable content of Mg in VAS
are presented in Figure 6. Figure 6 shows that
heating and boiling techniques are not different, but
signifiacantly different with acidifiacation
techniques in producing the concentration of
exchangeable content of Mg. Acidification
technique produces the highest exchangeable
concentration of Mg, and decreases in heating (8,41
me%), boiling (8,34 me%), and AVS is in standard
treatment (8,18 me%).
Treatments
Figure 6: The Effect of Heating (PO), Boiling (PA) , and
Acidification (AS) Techniques on Exchangeable Content
of Ca in VAS.
Treatment by using acidification technique
produces significantly the highest exchangeable
concentration of K and Mg compared to Ca. This
fact can be caused by some possible reasons such as:
(1) acidification is able to distruct directly the
mineral structure by reaction with elements in
meneral structure and release K,Ca, and Mg to be
exchangeble form or available to plant. (2)
meanwhile, both heating and boiling techniques only
occur in the addition of heat energy, and indirectly
react with elements in mineral structure with lower
result in releasing K,Ca, and Mg in exchangeable
form; (3) duration of heating and boiling for 6 hours
is not enough to optimalize to destruct or dilute the
primer minerals and release the exchangeable forms
of K,Ca, and Mg. But, both of the techniques have
the ability to produce the exchangeable forms of K,
Ca, and Mg higher than standard treatment or
control of VAS. On the other hand, all of the
techniques have potential ability to increase or
release the exchangeable concentration of K, Ca,
and Mg or available to plant.
3.3 Rates of K,Ca, and Mg Release
Release of K,Ca, and Mg is an exchangeable form
due to destruction of mineral structure resulted from
the treatment of heating, boiling, and acidification
techniques. The exchangeable contents of K, Ca,
and Mg are available form that can be absorbed by
plant roots. Rate of K,Ca,and Mg release can be
predicted base on the exchangeable ratio to total
form of K, Ca, and Mg. The ratio value is presented
in Figure 7, 8, and 9. Figur 7 and 9 show that the
rate of release of the exchangeable contents of K and
Mg are significantly different. The highest
concentrations of K and Mg are found in treatment
of acidification technique and decreased in boiling
technique and heating respectively (Figure 7).
Meanwhile, rate of Ca release is also significantly
different and highest concentration is found in
treatment of boiling technique, but there is no
difference to all other treatments (AO, PO, and AS)
(Figure 8).
20,45 a
21,7 b
21,46 b
21,8 b
19,50
20,00
20,50
21,00
21,50
22,00
AO PO PA AS
Exchangeable of Ca (me%)
8,18b
8,41b
8,34b
8,54a
8,00
8,10
8,20
8,30
8,40
8,50
8,60
AO PO PA AS
Exchangeable of Mg (me/100g)
Treatmen
The Effect of Heating, Boiling and Acidification Techniques on the Total and Exchangeable Contents of K, Ca, and Mg in Volcanic Ash of
Sinabung of North Sumatra
23
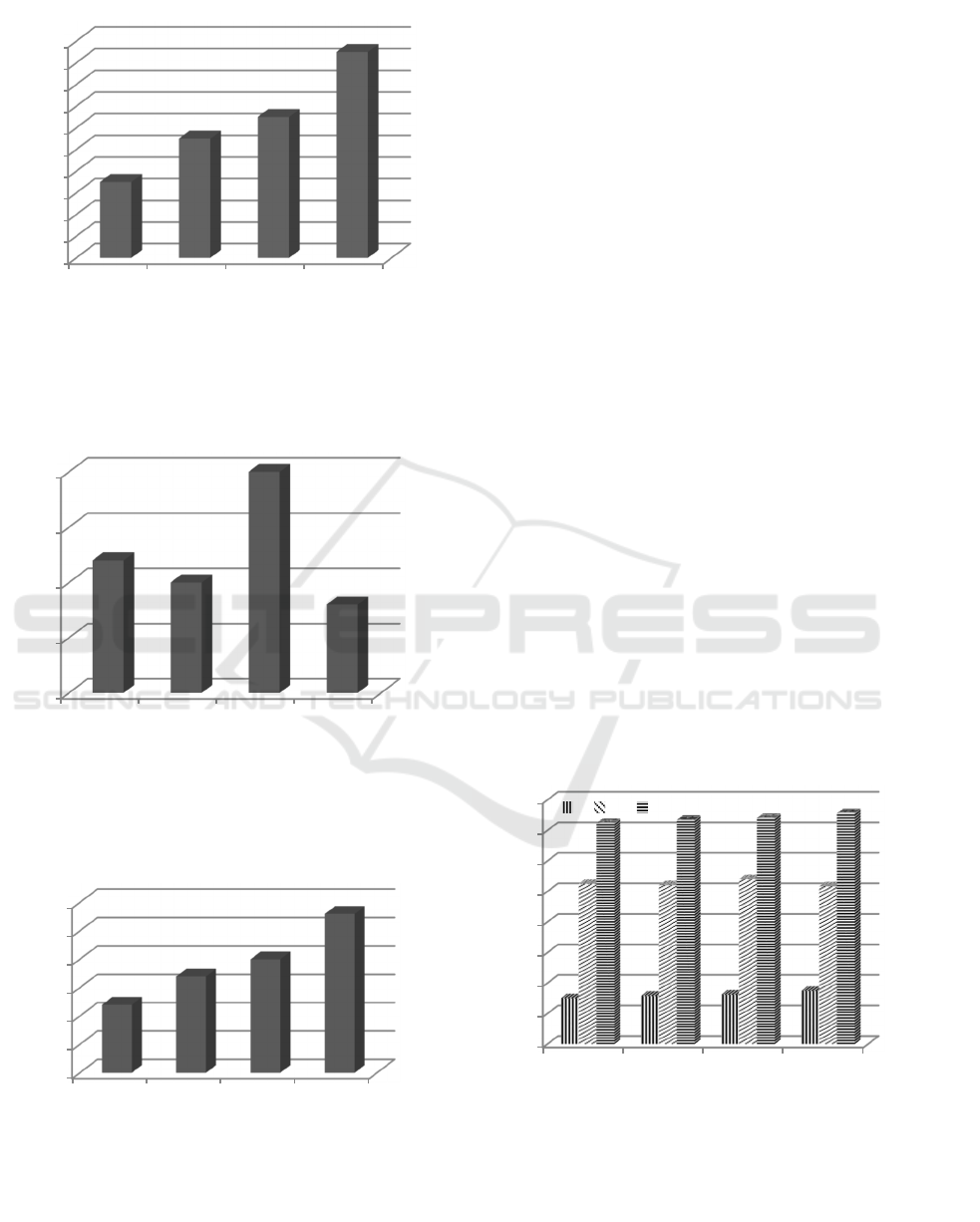
Treatments
Figure 7: The Effect of Heating (PO), Boiling (PA), and
Acidification (AS) Techniques on Ratio of K-exch./K-
total in VAS.
Treatments
Figure 8: The Effect of Heating (PO), Boiling (PA) , and
Acidification (AS) Techniques on Ratio of Ca-exch./Ca-
total in VAS.
Treatments
Figure 9: The Effect of Heating (PO), Boiling (PA) , and
Acidification (AS) Techniques on Ratio of Mg-exch./Mg-
total in VAS.
3.4 Relative Rate of Release for
Exchangeable Contents of K,Ca,
and Mg
Relative rate of release for exchangeable contents of
K,Ca, and Mg can be explained based on ratio of
the exchangeable concentration to total
concentration of K,Ca, and Mg. Based on the ratio,
relative rate of release of K,Ca, and Mg shows
significant difference in all treatments as presented
in Figure 10. The figure also shows that the relative
rate of release between elements of K, Ca, and Mg
in all of treatments are different significantly.
Relative rate of release for Mg is highest and
decreased for Ca and K (Mg>Ca> K) and in
standard treatment respectively.
The highest release for Mg is possibly correlated
with source of Mg originating from hipersthene that
contains Fe in its structure. Hypersthene belongs to
mineral of ferromagnesian group. It is relatively
weathering minerals due to exchanges of Fe
3+
(ferri)
to Fe
2+
(fero) resulted from the influence of
reduction-oxidation condition and low stage in pH
(VAS pH, more or less 4.0). Under this condition
this can facilitate or support the weathering of
hipersthene efectively and release Mg to be
exchangeable form faster than those of K and Ca.
Both of the elements, K and Ca, are sourced from
felspar/orthoclase and plagioclase. These minerals
do not have Fe in their structure resulted from the
weathering that may occur in relative lower stage so
that the release rate of the element becomes
exchangable form, also relatively low.
Treatments
Figure 10: The Effect of Heating (PO), Boiling (PA) , and
Acidification (AS) Techniques on Ratio of K-exch/K-
total,Ca-exch/Ca-total, dan Mg-exch/Mg-total AVS.
0,68
0,70
0,72
0,74
0,76
0,78
0,80
0,82
0,84
0,86
0,88
AO PO PA AS
2,50
2,55
2,60
2,65
2,70
AO PO PA AS
3,50
3,55
3,60
3,65
3,70
3,75
3,80
AO PO PA AS
0,00
0,50
1,00
1,50
2,00
2,50
3,00
3,50
4,00
AO PO PA AS
K Ca Mg
ICMR 2018 - International Conference on Multidisciplinary Research
24

4 CONCLUSIONS
1. The effects of heating by oven at 100
o
C, boiling
at 100
o
C, and acidification techniques are
different significantly and could increase the
total and exchangeable concentration, and release
rate of K, Ca, and Mg
2. The effect of acidification technique is highest in
producing the total and exchangeble
concentration of and in improving the release
rate of K, Ca, and Mg and decreased for heating
and boiling techniques respectively.
3. The acidification is the best technique and can
be potentially used to manufacture VAS, useful
as a soil amendment product in future.
4. The best product based on exchangeable
concentration of K,Ca, and Mg or available form
is produced by acidification technique and
folowed by heating and boiling techniques.
5. Each product is marked by the coding as AVS-
A.01-6, AVS-O100-6, and AVS-W100-6
respectively.
5 RECOMMENDATION
Exchangeable concentration of K,Ca, and Mg are
still relatively low compared to total concentration.
All products produced above can potentially be used
in agrotechnology, but need to be tested in order to
impove all the existing techniques.
REFFERENCES
Andhika, M. M. 2011. Dampak Debu Vulkanik Gunung
Sinabung Terhadap Perubahan Sifat Fisika Dan
Kandungan Logam Berat Pada Tanah Inceptisol.
Skripsi Universitas Sumatera Utara. Medan.
Barasa, R., F. 2012. Dampak DebuVulkanik Letusan
Gunung Sinabung Terhadap Kadar Cu, Pb dan B
Tanah di Kabupaten Karo. Skripsi. Universitas
Sumatera Utara. Medan.
Bowen, N. 1928. The Evolution on the Igneous Rock.
Princeton University Press, New Jersey.
Fiantis. D. 2006. Laju Pelapukan Kimia Debu Vulkanis
Gunung Talang dan Pengaruhnya Terhadap Proses
Pembentukan Mineral Liat Non Kristalin. Universitas
Andalas, Padang.
Khusrizal, Basyaruddin, R.D.H. Rambe, I. Setiawan,
2018. Study of Mineralogy Composition, Total, and
Exchangable Content of K, Ca, and Mg of Volcanic
Ash from Sinabung Mountain Eruption in North
Sumatera, Indonesia" In Proceedings of MICoMS
2017. Published online: 11 Jul 2018; 199-
207.Permanent link to this document:
https://doi.org/10.1108/978-1-78756-793-1-00029
Lubis, A. H. 2011. Dampak Debu Vulkanik Letusan
Gunung Sinabung terhadap Ketersediaan dan Serapan
Hara P oleh Tanaman Jagung Serta terhadap Respirasi
Mikroorganisme pada Tanah Dystrandepts. Skripsi
Universita Sumatera Utara. Medan.
Retnaningsih, H. 2013. Letusan Gunung Sinabung dan
Penanganan Bencana di Indonesia.Info Singkat
Kesejahteraan Sosial Vol. V, No. 18/II/P3DI.
Rostaman, T., A. Kasno dan L. Anggaria.2011.Perbaikan
Sifat Tanah dengan Dosis AbuVulkanik pada Tanah
Oxisols. Bdan Litbang Pertanian. Bogor
Schott, J., Berner, R.A. and Sjoberg, E.L. 1981.
Geochemical et chosmochimica Acta, Vol. 45, pp.
2123-2135.
Sinuhaji, N. F. 2011. Analisis Logam Berat dan Unsur
Hara Debu Vulkanik Gunung Sinabung Kabupaten
Karo - Sumatera Utara. Skripsi USU. MEDAN.
Surdiyarso, A. S. 1987. Dampak Negatif Abu Vulkanik
Terhadap Lingkungan dan Kesehatan. Pusat
Pengkajian, Pengolahan Data dan Informasi, Medan
The Effect of Heating, Boiling and Acidification Techniques on the Total and Exchangeable Contents of K, Ca, and Mg in Volcanic Ash of
Sinabung of North Sumatra
25
