
Adsorption Capability of Activated Carbon Prepared from Silica
Extracted Rice Husk by Chemical Activation
Anis Tasnim Md Yusof
1
, Nasrul Aizuddin Ab Fatah
1
, Dasmawati Mohamad
2
and Nora Aziz
2
1
Chemistry Section, School of Distance Education, Universiti Sains Malaysia, 11800 USM, Penang, Malaysia.
2
School of Dental Sciences, UniversitiSains Malaysia, 16150 KubangKerian, Kelantan, Malaysia.
Keywords: rice husk, activated carbon, silica, chemical activation, adsorption
Abstract: This research is done to optimize the rice husk residue usage with the production of activated carbon by
using the chemical activation method consecutively after the silica extraction. The silica extracted rice husk
was impregnated with different concentration of KOH solution and then burned to activation at the
predetermined activation temperature to obtain the activated carbon. The optimum condition to produce the
activated carbon is impregnation with 30 % concentration of KOH solution at 750
o
C activation
temperature. The maximum methylene blue uptake of the activated carbon produced are tested to be 322.36
mg g
-1
. The adsorption isotherm model study was done using Langmuir isotherm. The Langmuir isotherm
adsorption capacity, Q
e
was calculated to be 416.67 mg g
-1
while the rate of adsorption, b value was 4.8 mg
-1
with R
2
0.9995. The results obtained from this study show that the silica extracted rice husk is a suitable
precursor for preparing an activated carbon and the activated carbon have good absorption capacity.
1 INTRODUCTION
Rice is the world’s second most important crop in
the world after wheat and is a primary source of
food for more than half of the world’s population
mainly for Asian. More than 90 % of the world’s
rice is grown in Asia making it as both the largest
producer and consumer of rice (Rajamoorthy et al,
2015).
Among major rice producing countries in Asia
are China, India, Indonesia, Bangladesh, Vietnam
and Thailand with rice production rate around 145
million to 16.4 million metric tonne per year. Still, it
is estimated that about 70 % increase in rice
production yearly is needed to cater the demand of
Asian population growth in the future. Malaysia,
being a part of Asia has also been a country where
rice is the staple food of Malaysian. The food
consumption pattern of Malaysian adult population
shows that rice is at top of the list of 10 most
consumed food daily where majority of Malaysian
consume rice twice a day and on average, two and a
half plates of rice per day (Noor Shuhadah et al,
2012). The importance of rice as Malaysian staple
food has made The Ministry of Agriculture and
Agro-based Industry (MOA) implemented
DasarAgromakanan Negara (DAN) to ensure the
stability of the country’s rice stock by increasing the
production of Malaysia local rice (Norimah et al,
2008). However, the increase in rice production also
means the increase of waste income due to the
milling process of rice. Rice grains are generally
coated by a protective covering layer known as rice
husk. The husk is indigestible to human as it is made
of hard materials including silica and lignin in order
to protect the seed during the growing season. The
rice husk are removed from the grain during the
milling process to create the brown rice which then
milled further to produce the white rice. There is
roughly 0.28 kg of rice husk by-product produced
for each kg of milled white rice. The worldwide
annual rice husk output is about 80 million tonnes
and according to statistic by Malaysian Ministry of
Agriculture, 408,000 metric tonnes of rice husk are
produced in Malaysia each year. In the past, rice
husk was considered and treated as useless
agricultural waste by farmers hence it was usually
burned. This leads to the release of carbon dioxide
(CO
2
) gas to the atmosphere which is not only
harmful to the environment but also gives negative
impact to human health as it can affect the
respiratory function (Noor Syuhadah et al, 2012).
Thus, many major rice producing countries
Md Yusof, A., Ab Fatah, N., Mohamad, D. and Aziz, N.
Adsorption Capability of Activated Carbon Prepared from Silica Extracted Rice Husk by Chemical Activation.
DOI: 10.5220/0008881600110018
In Proceedings of the 7th International Conference on Multidisciplinary Research (ICMR 2018) - , pages 11-18
ISBN: 978-989-758-437-4
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
11

including Malaysia have made finding on the usage
of rice husk and ways to utilize it commercially as
their research priority. Findings have led to a few
implementations of rice husk as fuel in power plant,
due to its high calorific power (Noushad et al, 2012),
as insulation or building materials since it is highly
resistant to 3 penetration of fungal and moisture
decomposition, as fertilizer, capacitors (Le Van et al,
2014), pollutant and odour removal, gas separation
and catalysis (Liu et al. 2011). The high silica
content of silica in rice husk has attracted interest on
how to use it commercially as a viable raw material
for the production of silicates and silica. The rice
husk ash especially obtained from the combustion
step of rice husk contains silica of over 60 %. Apart
from silica, other major component of rice husk and
rice husk ash are about 10-40 % carbon and minor
other mineral compositions (An et al, 2011)
Production of activated carbon from rice husk has
also attracted the interest of various parties.
Activated carbon is a versatile adsorbent due to its
good adsorption property from its high surface area,
fast adsorption kinetic and large adsorption capacity
(Cheenmatchaya et al. 2014). It can be produced
from variety of raw materials such as fruit shell,
straw, residual waste and from agricultural by-
products (An et al. 2011). In earlier researches,
many researchers’ focuses were on the production of
either an activated carbon or a silica from rice husk
ash. The usage of rice husk ash is preferred because
of its higher content of silica and carbon compared
to rice husk. The procedure was usually complex
hence the carbon source and silicone source could
not be sufficiently utilized. Only a few researches
found the production of silica and activated carbon
done consecutively (An et al. 2011).
Activated carbon also known as activated
charcoal is the oldest yet versatile adsorbent that is
derived from charcoal. It is an excellent adsorption
property as it can adsorb various substances from
gas and liquid streams. For adsorption of gases and
vapours, a granular types of activated carbon is
usually used while for 5 purification of liquid, a
powdered activated carbon is more desired.
Activated carbon has been reportedly used as
adsorbent for a wide varieties of inorganic and
organic pollutants dissolved in aqueous media or
from gaseous environment (Gupta et al. 2008), such
as for different types of dyes (Ahiduzzaman et al.
2016) and heavy metals (Bishnoi et al. 2004), as
electrode materials for batteries and capacitors (Le
Van et al. 2014), as odour removal, gas separation
and catalysis (Chen et al. 2011). The effectiveness of
activated carbon to react as adsorbent is because of
its highly developed pore structure and large internal
specific surfaces area (Le Van et al. 2014).
Activated carbon is deem as a material of major
industrial and has been produced extensively on a
commercial scale. The commonly used materials to
prepare commercial activated carbon are coal (El
Qada et al. 2006), wood (Sahu et al. 2010),
petroleum (Niasar et al. 2018), pitch (Gao et al.
2017), etc. However, this non-renewable starting
materials are relatively expensive and sometimes
even low in availability hence this has increased the
production cost of activated carbon with limited
usage. This has led to a growing research interest
mainly by developing nations in the production of
activated carbon from a natural, renewable and low
cost materials especially for application regarding
waste water treatment (Tan et al. 2010), remediation
and decontamination process (Chen et al. 2011).
Focus is given especially in the production of
activated carbon from waste materials such as
agricultural by-products as it will not only solve the
problem of waste disposal but also convert a
potential waste to a valuable product (Thomas et al.
2017). Among the agricultural by-product studied by
researchers for the production of activated carbon
are almond shells (Thitame et al. 2015) , date pits
(Girgis et al. 2002), coconut shells (Huang et al.
2015), sugarcane waste and rice husk (Kalderis et al.
2008). Activated carbon made from this agricultural
by-product are found to be effective in the removal
of pollutants from water (Thomas et al. 2017).
However, the properties of the activated carbon 6
produced is governed not only by the raw materials
used, but also by the method of the activation used
About 571 million tonnes of rice resulting in
approximately 140 million tonnes or rice husk are
produced annually in the world (Kalderis et al.
2008), 96 % of which are generated in developing
country. Rice husk major constituents are cellulose,
hemicellulose, lignin and mineral components
though the content of each depends on the rice
variety, climate conditions and geographic
localization of the culture (Chen et al. 2011). On
early days, rice husk is considered as a low energy
resource, thus is always discarded or burned on the
field which are unfavourable to environment (Noor
Syuhadah et al. 2012). The production of activated
carbon from rice husk does not only produce an
activated carbon with good adsorption properties but
will also alleviate the problem of disposal and
management of this waste by-product. Production of
activated carbon from rice husk generally can be
achieved through 2 routes, physical activation also
known as thermal activation and chemical activation
ICMR 2018 - International Conference on Multidisciplinary Research
12

(An et al. 2011, Chen et al. 2011 and Thomas et al.
2017). In physical activation, rice husk is firstly
carbonized into rice husk char at high temperatures
(between 600-900
o
C) followed by activation at an
elevated temperature (between 600-1100
o
C) in the
presence of a suitable oxidizing gases such as CO
2
,
steam, air or their mixture (Chen et al. 2011 and
Thomas et al. 2017).
In chemical activation, rice husk is mixed with a
chemical agent such as potassium hydroxide (KOH)
(An et al. 2011), sodium hydroxide (NaOH) (Le Van
et al. 2014), phosphoric acid (H
3
PO
4
)
(Cheenmatchaya et al. 2014), zinc chloride (ZnCl
2
)
(Chen et al. 2011). Then the carbonization and
activation are performed simultaneously at activated
temperature (between 400-900
o
C). During
activation, disorganized carbon is removed by
exposing the crystallites to the action of activating
agent which leads to the development of porous
structure. The efficiency of the activated carbon is
also strongly influenced by a relatively 7 small
amount of chemically bonded heteroatoms (mainly
oxygen (O) and hydrogen (H) (Chen et al. 2011 and
Thomas et al. 2017).
Chemical activation is usually a much more
preferred method to produced activated carbon as it
provides two important advantages in comparison to
physical activation and the process can be performed
at a lower temperature and the global yield of
chemical activation tends to be greater because
burning off charcoal is not required (Mohanty et al.
2006). However, an admixed method of physical and
chemical process can also be applied (Le Van et al.
2014).
2 EXPERIMENTAL
2.1 Acid Pre-treatment of Rice Husk
300.0 g of the rice husk sample was washed
thoroughly 2 times with normal water and 5 times
with distilled water respectively. The rice husk was
then dried in a universal oven (Memmert, UFE 600,
Germany) at 110
o
C for 24 h. 40.0 g of the dried rice
husk was weighted into a 1000 mL beaker and
treated with 1 M solution of HCl at 75
o
C for 90
minutes in a water bath (Julabo, tw20, Germany).
The suspension was filtered to extract the solid
residue of rice husk which was then dried again in a
universal oven (Memmert, UFE 600, Germany) at
110
o
C for 24 h.
2.2 Extraction of Silica
40.0 g of the acid pre-treated rice husk was
immersed in 600 mL of 10 % of NaOH solution in a
beaker and heated at 90
o
C for 60 minutes in a water
bath (Julabo, tw20, Germany). The suspension was
let cool for 2 h and then filtered to extract the
sodium silicate solution from the rice husk.
2.3 Preparation of Activated Carbon
The silica extracted rice husk was washed with
distilled water and dried in an universal oven
(Memmert, UFE 600, Germany) at 110
o
C for 24 h.
10 g of the silica extracted rice husk was then
impregnated with 100 mL of 10 %, 20 % and 30 %
of KOH solution in a beaker and heated at 90
o
C for
60 minutes in a water bath (Julabo, tw20, Germany).
After that, the mixtures were let cool to room
temperature before being filtered to remove the
excess KOH solution. The impregnated rice husk
was then transferred into a 100 mL porcelain
crucible and dried overnight at 80
o
C in a universal
oven (Memmert, UFE 600, Germany). The dried
impregnated rice husk was then burned in a burn out
furnace (UginDentaire, Programix 100, Freance) at
heating rate of 10
o
C/min from room temperature to
the final activated temperature of 750
o
C, and 800
o
C
and the final temperature was maintained for 60
minutes. The samples were then let cool to room
temperature and then washed with distilled water
repeatedly by using vacuum filtering setup to
remove the activating agent. The product obtained
was then dried in the universal oven (Memmert,
UFE 600, Germany) at 110
o
C for 24 h. Finally, the
activated carbon obtained was grinded and stored in
adesiccators.
2.4 Adsorption Study of Activated
Carbon
Firstly, the activated carbon obtained was evaluated
with Methylene Blue (MB) dye adsorption test. The
test was done to find out the best combination of the
activation factors used (temperatures and rice husk:
KOH % concentration ratio) to obtain the best
product of activated carbon from the silica extracted
rice husk. A commercial activated carbon was also
used as control.
A standard calibration graph was plotted by
finding the absorbance value for a series of
Methylene Blue (MB) solution concentration from
0.5, 1.0, 1.5, 2.0 and 3.0 mg L
-1
that were prepared
in 5 different volumetric flasks. The maximum
Adsorption Capability of Activated Carbon Prepared from Silica Extracted Rice Husk by Chemical Activation
13
absorbance of the solution at 664 nm of wavelength
(λ) was measured by using a spectrophotometer
(Thermo scientific, Genesys 20, USA). Distilled
water was used as blank.
A series of Methylene Blue (MB) solution
concentration 50, 100, 150, 250 and 300 mg L
-1
was
prepared in 5 different volumetric flasks. 0.01 g of
the each activated carbon sample obtained was then
weighted (Sartorius, BSA224S CW analytical
balance, Germany) and mixed with 15 mL of each
MB solution prepared in a beaker. The mixture was
stirred and kept for 24 h at room temperature. Next,
the dye solution was transferred into a falcon mask
and centrifuged (Hettich, Universal 32 R, Germany)
for 20 min at 1500 rpm to settle down the carbon
particle at the bottom of the tube. Clean solution
obtained was then filtered using a pore size 0.45 µm
filter paper to remove the remaining carbon particle
from the solution. The maximum absorbance of the
solution at 664 nm of wavelength was then
measured by using a spectrophotometer (Thermo
scientific, Genesys 20, U.S.A). Distilled water was
used as blank.
The coefficient of extinction was calculated by
plotting a calibration chart of absorbance with
respect to the MB concentration. The concentration
of MB after adsorption was then determined by
using equation:
C
e
=
A
E
(1)
where C
e
is the concentration of MB solution after
adsorption, mg L
-1
; A is the absorbance ; E is the
coefficient of extinction, L mg
-1
.
The amount of MB absorbed was than calculated
by using the following equations:
q
e
= ቆ
ሺ
C
o
- C
e
ሻ
V
m
ቇ (2)
where q
e
is the uptake of dye adsorbent, mg g
-1
; C
o
is
the initial concentration of dye, mg L
-1
; C
e
is the
final concentration of dye, mg L
-1
; V is the volume
of dye solution, L; m is the weight of activated
carbon, g.
3 RESULT AND DISCUSSION
The effect of different activated conditions variable
on physical and chemical characteristics of the
activated carbon products will be discussed in this
section. The influencing factors on the methylene
blue adsorption capacity of activated carbon
products are investigated and compared with the
commercially bought activated carbon.
3.1 Methylene Blue Dye Adsorption
Test
Table 1: The uptake value of methylene blue by activated
carbon, q
e
for activation temperature 750
o
C.
Initial
MB
Conc
(
m
g
L
-1
)
q
e
(mg g
-1
)
AC750
-10
AC750
-20
AC750
-30
CAC
50.0
73.121 73.313 74.981
74.814
100.0
139.78 142.95 149.97
147.53
150.0
177.91 212.31 224.59
183.43
200.0
220.73 268.52 298.01
202.77
300.0
251.90 331.51 418.88
219.96
Table 2: The uptake value of methylene blue by activated
carbon, q
e
for activation temperature 800
o
C.
Initial
MB
Conc
(mg L
-1
)
q
e
(
m
g
g
-1
)
AC800
-10
AC800
-20
AC800
-30
CAC
50.0
71.946 72.768 74.257 74.814
100.0
138.25 140.95 147.86 147.53
150.0
176.97 181.43 219.65 183.43
200.0
210.40 216.97 262.42 202.77
300.0
245.44 276.67 322.35 219.96
Initial
MB
Conc
(mgL-1)
q
e
(
m
g
g
-1
)
AC750
-10
AC750
-20
AC750
-30
CAC
50.0
73.121 73.313 74.981
74.814
100.0
139.78 142.95 149.97
147.53
150.0
177.91 212.31 224.59
183.43
200.0
220.73 268.52 298.01
202.77
300.0
251.90 331.51 418.88
219.96
and table 2 contain the uptake value of methylene
blue by all activated carbon prepared in the 750
o
C
and 800
o
C respectively and the commercial
activated carbon bought. From the experimental data
obtained it is seen that activated carbon with good
adsorption properties can be made from a silica
extracted rice husk.
This meansthat the porosity of the activated
carbon structure can be created not only just by
ICMR 2018 - International Conference on Multidisciplinary Research
14
leaching out silica and removing the chemical
activating agents in the carbonized samples by
washing. In the case of low or absence of silica
content in the solid residue of rice husk, the
activating agent KOH will reacted with carbon to
produce an activated carbon (An et al 2011).
The used of KOH as an activating agent in the
preparation activated carbon has been known to
produce activated carbon with good pore
development, greater specific surface area though at
the cause of a typically low percent yield. The
intercalation of metallic potassium ions into the
carbon network during the development of pores of
the activated carbon accelerates the carbon loss.
During the activation process, the following
reactions take place (Hui et al, 2015).
C+2KOH → 2K+ H
2
+CO
2
(3)
C+2KOH → 2K+ H
2
+CO
(4)
CO
2
+2KOH → K
2
CO
3
+ H
2
O
(5)
The potassium carbonate decomposed during
activation and CO
2
gas was released. The reaction
between the activating agent and the carbon
precursor lead to the decomposition of the volatile
organic compounds and in return formed the porous
surface of the activated carbon samples (Muniandy
et al. 2014).
3.1.1 The Effect of Initial Concentration of
Methylene Blue on Uptake Value of
Methylene Blue by Activated Carbon
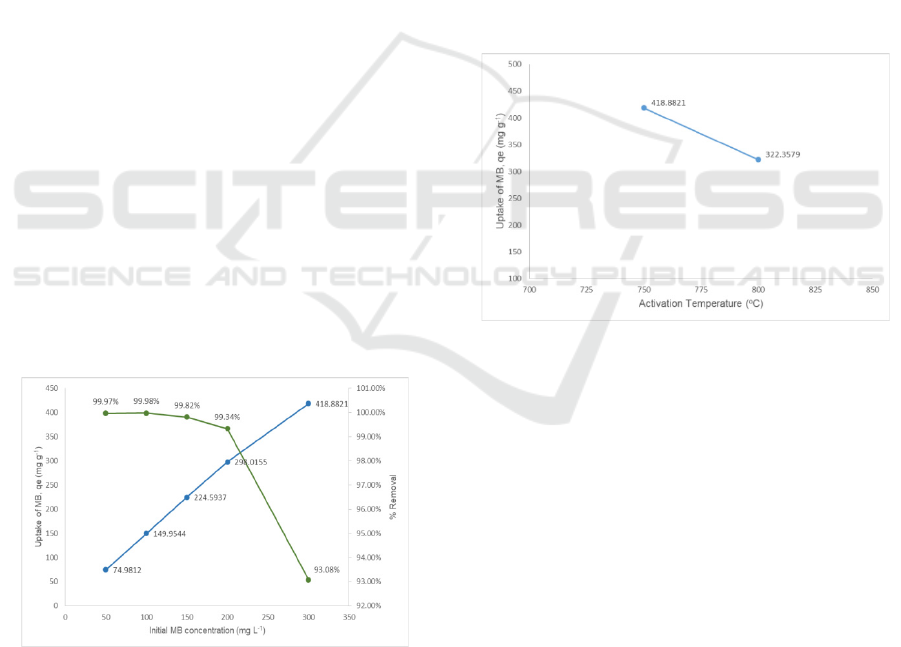
Figure 1: The effect of initial concentration of methylene
blue on uptake value of methylene blue by activated
carbon.
Figure 1 shows the effect of initial concentration of
methylene blue on the uptake value and % removal
of methylene blue by activated carbon prepared at
activation temperature of 750
o
C and 30 %
concentration of KOH solution. The figure shows
that though the uptake of methylene blue by
activated carbon used increased from the low to high
concentration of methylene blue, the % removal of
methylene blue were actually decreased.
At low concentration of methylene blue,
sufficient adsorption sites of activated carbon are
available for the adsorption of methylene blue. At
higher concentration, relatively less available sites of
activated carbon caused the reduction in the % of
adsorption. Hence, increasing the initial methylene
blue concentration decreased the % removal of
methylene blue from the solution due to the
saturation of adsorbent or activated carbon with
methylene blue solution.
3.1.2 The Effect of Activation Temperature
of Activated Carbon on the Uptake
Value of Methylene Blue
Figure 2: The effect of activated temperature of activated
carbon on methylene blue uptake.
Figure 2 shows the uptake value of methylene blue
with initial concentration of 300 mg L
-1
by activated
carbon produced with 30 % concentration of KOH
solution. The figure shows that the uptake value of
the methylene blue by activated carbon produced at
activation temperature of 750
o
C is higher compared
to activated carbon produced at activation
temperature of 800
o
C. This shows that activation
temperature influenced the adsorption capacity of an
activated carbon. This is because the activation
temperature used is among the parameters that
highly influenced the formation of pore and its
structure of an activated carbon.
The decrease of uptake value of methylene value
from the activated carbon produced using activation
temperature of 800
o
C in comparison to 700
o
C
suggest that 700
o
C is the optimum activation
temperature for the preparation of the activated
Adsorption Capability of Activated Carbon Prepared from Silica Extracted Rice Husk by Chemical Activation
15
carbon. Hence, the methylene blue adsorption
capability of the activated carbon is actually
increasing with the increase of activation
temperature to a certain temperature and then
decrease again if heat is still supplied (Rhaman et al.
2015)
The formation of pore is simultaneous with the
destruction of pore. This suggest that at activating
temperature of 750
o
C, the active reaction is at its
maximum and increase the adsorption ability of the
activated carbon. At this temperature, the active
reaction led to a much more number of pore forming
hence increased the specific surface area of the
activated carbon. But, at activating temperature of
800
o
C, the destruction of pore is higher than the
formation of pore hence resulting in a reduction of
the specific surface area of the activated carbon
(Guo et al. 2002).
This result suggested that to the activated carbon
with optimum adsorption properties, the activation
temperature used should be around 750
o
C.
3.1.3 The Effect of KOH Percentage
Concentration of Activated Carbon on
the Uptake Value of Methylene Blue
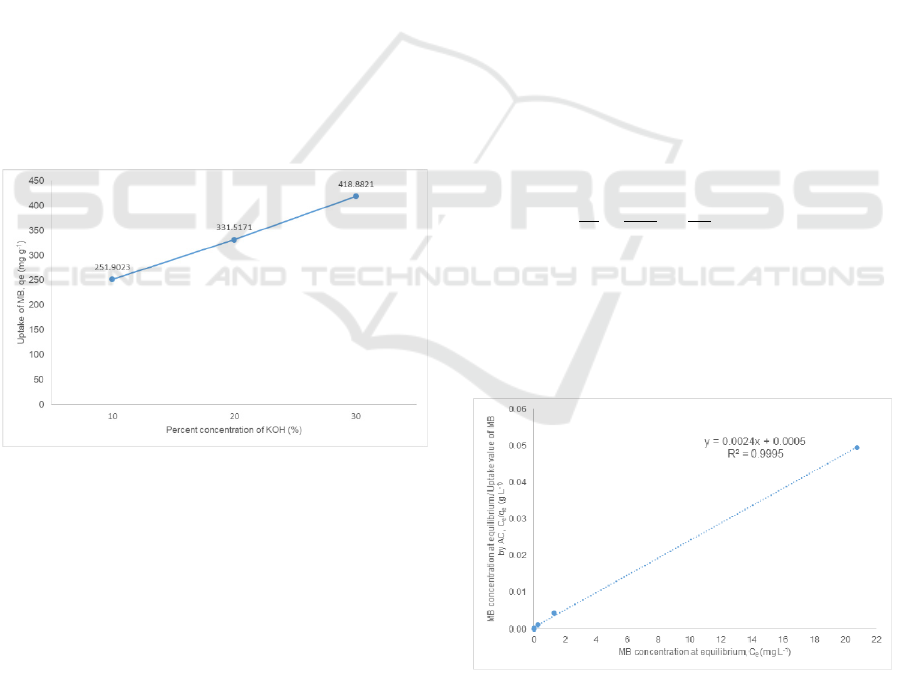
Figure 3: The effect of KOH % concentration of activated
carbon on methylene blue uptake.
Figure 3 shows the effect of KOH % concentration
of prepared activated carbon on the uptake value of
methylene blue. The uptake value of methylene blue
used are from the reaction between the activated
carbon prepared at 750
o
C with methylene blue
initial concentration of 300.0 mg L
-1
. The uptake
value of methylene blue by activated carbon can be
seen increases with the raise of KOH %
concentration. This suggest that increase in
activating agent promotes to a better production of
activated carbon.
The increase of activating agent at higher %
concentration of KOH solution promotes the contact
area between the rice husk and activating agent,
hence increasing the adsorption ability of activated
carbon. Also, since the adsorption ability of an
activated carbon is related to its pore volume it can
be said that the pore volume of activated carbon
produced also increases with the increase of the
KOH % concentration. Though further test has to be
done to prove this suggestion (An et al. 2011,
Rhaman et al. 2015 and Guo et al. 2002).
3.2 Adsorption Isotherm Model Study
The adsorption isotherm study is done to describe
the interaction between solutes and adsorbents, and
is critical in optimizing the use of adsorbents. We
used the Langmuir model for this study.
3.2.1 Langmuir Isotherm
Langmuir isotherm assumes monolayer adsorption
onto a surface containing infinite number of
adsorption site of uniform strategies of adsorption
with no transmigration of adsorbate in the plane
surface (Hameed et al. 2007). The applicability of
the isotherm equation is compared by judging the
correlation coefficient R
2
value. Langmuir equation
is written as:
C
e
q
e
=
1
Q
o
b
+ ቆ
1
Q
o
ቇCe
(6)
Where q
e
is the Where q
e
is the uptake of MB
adsorbent, mg g
-1
at equilibrium, C
e
= final
concentration of MB at equilibrium, Q
o
is the
Langmuir adsorption capacity constant, mg g
-1
and b
is the constant related to rate of adsorption, L m g
-1
.
Figure 4: Langmuir isotherm constant for methylene blue-
activated carbon.
Figure 4 was plotted using values from
adsorption of methylene blue by activated carbon
prepared by activation temperature of 750
o
C and 30
ICMR 2018 - International Conference on Multidisciplinary Research
16
% concentration of KOH. The figure indicated that
the adsorption of methylene blue followed the
Langmuir isotherm. The constant Q
o
and b were
calculated using equation and their values are as in
Table 3 Confirmation of the experimental data into
Langmuir isotherm model indicates the homogenous
nature of the rice husk carbon surface. This also
demonstrate the formation of monolayer coverage of
dye molecule at the outer surface of the activated
carbon [Hameed et. al]. The experimental data fit
well with the isotherms with R
2
values of 0.9995.
Table 3: Langmuir adsorption isotherm parameters for
methylene blue-activated carbon.
Q
o
(m
g
g
-1
) 416.67
b
(L m
g
-1
) 4.8
R
2
0.9995
The Q
o
value obtained was 416.67 mg g
-1
. Similar
result was reported by adsorption of methylene blue
onto activated carbons prepared from bamboo-based
[Hameed et. al] and by the adsorption of direct dyes
on activated carbon prepared from stawdust (Malik
et al. 2004). Table 4 shows that the activated carbon
prepared has a very large adsorption capacity
compared with some data from references.
Table 4: Comparison of the maximum mono layer
adsorption of some dyes on various adsorbents
.
Dyes Adsorbent
(Activated
Carbon)
Maximum
monolayer
adsorption
capacity
(mg g
-1
)
Reference
Methylen
e blue
Bamboo 454.20 Hameed et
al. 2007
Methylen
e blue
Coconut
shell
277.90
Adamson
1990
Methylen
e blue
Groundnu-
tshell
164.90
Adamson
1990
Methylen
e blue
Rice husk 343.50 Adamson
1990
Methylen
e blue
Jute fiber 225.64 Tsai et al.
2001
4 CONCLUSIONS
From this study, it is concluded that the produce of
activated carbon from silica extracted rice husk is
possible and doable. The produce activated carbon
from silica extracted rice husk shows promising
adsorption capacity for the methylene blue removal.
The results obtained were even better than the
commercially bought activated carbon. The
optimum experimental conditions from the study for
the produced activated carbon were from the
impregnation of the rice husk with 30 %
concentration of KOH solution and activation
temperature of 750
o
C. The maximum adsorption
capacity value, Q
o
is recorded to be 416.67 mg g
-1
.
The results obtained from this study suggest that the
silica extracted rice husk is a suitable precursor for
preparing an activated carbon.
ACKNOWLEDGEMENTS
School of Distance Education, Universiti Sains
Malaysia
School of Dental Sciences, Universiti Sains
Malaysia
REFERENCES
Khush, G., 2005. Plant Mol. Biol., 59, 1–6.
Y. Rajamoorthy, Y., Rahim, K., Munusamy, S., 2015.
Procedia Econ. Financ.,31, 861–867.
Norimah, A., K., Safiah, M., Jamal, K., Siti, H.,
Zuhaida, H., Rohida, S., Fatimah, S., Siti, N., Poh, B.,
K., Kandiah, M., Zalilah, M., S., Wan Manan, W.,M.,
Fatimah, S., Azmi, M., Y., 2008. Malays. J. Nutr., 14,
25–39.
Noor Syuhadah, S., Rohasliney, H., 2012.Heal. Environ.
J., 3, 89–95.
Noushad, M., Rahman,I., A., Husein, A., Mohamad, D.,
Ismail, A., R., 2012. Int. J. Adv. Sci. Inf. Technol., 2,
28–30.
Y. Liu, Y. Guo, W. Gao, Z. Wang, Y. Ma and Z. Wang, J.
Clean. Prod., 2012, 32, 204–209.
An, D., Guo, Y., Zou, B., Zhu, Y., Wang, Z., 2011.
Biomass Bioenergy, 35, 1227–1234.
Cheenmatchaya, A., Kungwankunakorn, S., 2014. Int. J.
Environ. Sci. Dev., 5, 171–175.
Gupta, V., K., Rastogi, A., 2008.J. Hazard. Mater.,152,
407–414.
Ahiduzzaman A., Sadrul Islam, A., K., M.,
2016.SpringerPlus, 5(1), 1248-1262.
Bishnoi, N., R., Bajaj, M., Sharma, N., Gupta, A., 2004.
Bioresour. Technol., 91, 305–307.
Adsorption Capability of Activated Carbon Prepared from Silica Extracted Rice Husk by Chemical Activation
17

Le Van K., LuongThi, T., T., 2014.Prog. Nat. Sci. Mater.
Int., 24, 191–198.
Chen, Y., Zhu, Y., Wang, Z., Li, Y., Wang, L., Ding, L.,
Gao, X., Ma, Y., Guo, Y., 2011. Adv. Colloid
Interface Sci., 163, 39–52.
El Qada, E., N., Allen S., J., Walker, G., M., 2006. Chem.
Eng. J., 124, 103–110.
Sahu, J., N., Acharya, J., Meikap, B., C., 2010. Bioresour.
Technol., 101, 1974–1982.
Niasar, H., S., Li, H., Das, S., Kasanneni, T., V., R., Ray,
M., B., Xu, C., C., 2018. J. Environ. Manage.,211,
63–72.
Gao, S., Ge, L., Rufford, T., E., Zhu, Z., 2017.
MicroporousMesoporous Mater., 238, 19–26.
Tan, I., A., W., Hameed, ., H., 2010. J. Appl. Sci., 10,
2565–2571.
Thomas, M., Patel, S., P., Patel, A., V., Patel, J., V.,
2017.45, 176–182.
Thitame, P., V., Shukla, S., R., 2015.Chem. Eng.
Commun.,203(6), 791-800.
Girgis, B., S., El-Hendawy, A., N., A., 2002.
MicroporousMesoporous Mater., 52, 105–117.
Huang, P., Cheng, H., Lin, S., 2015. J. Chem., 2015, 1–10.
Kalderis, D., Koutoulakis, D., Paraskeva, P.,
Diamadopoulos, E., Otal, E., Del Valle, J., O.,
Fernández-Pereira, C., 2008. Chem. Eng. J., 144, 42–
50.
Kalderis, D., Bethanis,S., Paraskeva, P., Diamadopoulos,
E., 2008. Bioresour. Technol., 99, 6809–6816.
Mohanty, K., Naidu, J., T., Meikap, B., C., Biswas, M.,
N., 2006. Ind. Eng. Chem. Res., 45, 5165–5171.
Liu, Y., Guo, Y., Zhu, Y., An, D., Gao, W., Wang, Z.,
Ma, Y., Wang, Z., 2011. J. Hazard. Mater.,186, 1314–
1319.
Hui, T., S., Zaini, M., A., A., 2015.Carbon Lett.,16, 275–
280.
Muniandy, L., Adam,F., Mohamed, A., R., Ng, E., P.,
2014. MicroporousMesoporous Mater., 2014, 197,
316–323.
Rhaman, M., T., Haque, M. A. Rouf, M., A., Siddique, M.,
A., B., Islam, M., S., 2015. 50, 263–270.
Guo, Y., Yang, S., Yu, K., Zhao, J., Wang, Z., Xu, H.,
2002. Mater. Chem. Phys., 74, 320–323.
Hameed, B., H., Din, A., T., M., M., Ahmad, A., L.,
2007.J. Hazard. Mater.,141, 819–825.
Malik, P., K., J., 2004. Hazard.Mater.,113, 81–88.
Adamson,W., A., 1990. Physical Chemistry of Surfaces,
Wiley, NewYork, 5th ed.
Tsai,W., T., Chang,C., Y., Lin, M., C., Chien, S., F., Sun,
H., F., Hsieh, M., F., 2001. Chemosphere, 45, 51–58.
Freundlich, H., M., F., 1906.Over the adsorption in
solution, J. Phys. Chem. 57, 385–470.
Haghseresht, F., Lu, G., 1998. Energy Fuels, 12, 1100–
1107.
Padiberas NasionalBerhad, World Rice Map,
<http://www.bernas.com.my/index.php/rice-
pedia/world-rice-map>, 2017
(accessed 2 April 2018).
Rice Knowledge Bank, Rice
Husk,<http://www.knowledgebank.irri.org/step-by-
step-production/postharvest/rice-by-products/rice-
husk>, 2016
(accessed 3 April 2018)
Myanmar Insider, Rice Husk - A Useful By Product For
Rice Growing Country,
<http://www.myanmarinsider.com/rice-husk-a-useful-
by-product-for-rice-growing-countries/>, 2016
(accessed 10 April 2018)
Desotec, Chemical Structure of Activated Carbon,
<https://www.desotec.com/en/carbonology/carbonolog
y-academy/chemical-structure-activated-carbon>,
2014
(accessed 20 June 2018)
Water Technology, The Basics of Activated Carbon
Adsorption, <https://www.watertechonline.com/the-
basics-of-activated-carbon-adsorption/>, 2016
(accessed 20 June 2018)
ICMR 2018 - International Conference on Multidisciplinary Research
18
