
Bcl2 Gene Expression Profile on Administration of Novel
Active Compound from Soursop Leaves (SF-1603) as a New
Molecular Target in Liver Cancer Therapy
Maya Tejasari
*1
, Siti Aminah Abdurachman
3
, Dwi Prasetyo
4
, Herri S. Sastramihardja
2
1.Histology Department, Faculty of Medicine, Bandung Islamic University, Indonesia
2.Professor of Clinical Farmacology, Faculty of Medicine, Bandung Islamic University, Indonesia
3.Professor of Internal Medicine Department, Faculty of Medicine, Padjadjaran University,Indonesia
4.Professor of Pediatric Department, Faculty of Medicine, Padjadjaran University, Indonesia
Keywords: Apoptosis, Bcl-2, Liver Cancer, Soursop (Annona Muricata), Targetted Therapy
Abstract: Most cases of liver cancer present in advanced stages so the prognosis remains poor. Apoptotic
dysregulation of liver cancer cells by BCL-2 gene expression is linked to tumor progression and resistance
to treatment. Soursop plant compound is believed will be able to induce apoptosis by interfering Bcl2 gene
expression. The objectives of the study was to explore the role of novel active compound isolated from
soursop leaves againts Bcl2 gene expression in order to find new molecular target for liver cancer therapy.
This study was in vitro experimental to assess active compound (SF-1603) effects on Bcl2 mRNA
expression. Treatment groups were treated with SF-1603 dosage of 0,5xIC
50
, IC
50
and 2xIC
50
. Observations
were assessed in hours 0, 24, 48, and 72. The results showed that Bcl-2 gene optimum expressions were
achieved with 2xIC
50
dose at hour 24. There were strong correlation between Bcl-2 gene expression with
apoptosis level (r=0,558). This evidence indicates that administration of SF-1603 promote expression of
Bcl-2 gene to produce a peak signal that activate the apoptosis. It was concluded that SF-1603 affect Bcl2
gene expression as liver cancer therapy molecular target on HepG2 cell line culture.
1 INTRODUCTION
As an effort to reduce mortality, various studies to
find effective treatments for liver cancer are still
being developed. Curative approaches such as
surgery and transplantation can only be done on a
limited basis due to various causes such as lack of
donors and considering its effects on liver function.
Therapy recommendations based on the
classification of the Barcelona Clinic Liver Cancer
(BCLC) for liver cancer with advanced stages are a
systemic chemotherapy. Curative approaches such
as resection and transplantation can only be limited
to patients with early stage liver cancer.
Chemotherapy is still the best choice for patients
with advanced liver cancer, however until now the
effectiveness of chemotherapy in liver cancer
patients is often considered relatively ineffective.
This therapeutic option for patients with liver cancer
is still very limited which is also one of the reasons
for the prognosis of liver cancer remain poor. (Ho et
al., 2009, Huynh et al., 2010, Lencioni et al., 2008,
Robotin et al., 2009, Park et al., 2006, Ghassan and
Abou-alfa, 2004, Wirth et al., 2005, Sherman et al.,
Bruix and Sherman, 2005)
Systemic chemotherapy has been shown
repeatedly to provide no benefit in improving
survival, regardless of whether it is given as a single
agent or as a combination chemotherapy part.
Systemic chemotherapy using existing
chemotherapy agents is generally stated to be
relatively ineffective for liver cancer. Liver cancer is
conciders resistant to chemotherapy because of the
high mutation load and also the mechanism of drug
resistance. The mechanism of resistance that arises
relates to the administration of low-dose
chemotherapy because it considers liver dysfunction
and is also carried out to reduce toxicity (Park et al.,
2006, Wirth et al., 2005, Ghassan and Abou-alfa,
2004)
Although many chemotherapeutic agents have
been tested, the role of systemic chemotherapy for
liver cancer remains unclear. New therapeutic
Tejasari, M., Abdurachman, S., Prasetyo, D. and Sastramihardja, H.
Bcl2 Gene Expression Profile on Administration of Novel Active Compound from Soursop Leaves (SF-1603) as a New Molecular Target in Liver Cancer Therapy.
DOI: 10.5220/0008790800570064
In Proceedings of the 2nd Syiah Kuala International Conference on Medicine and Health Sciences (SKIC-MHS 2018), pages 57-64
ISBN: 978-989-758-438-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
57

strategies with more specific targets are needed to
improve treatment effectiveness. (Ghassan and
Abou-alfa, 2004, Park et al., 2006) There are no
prospective controlled studies showing that systemic
chemotherapy prolongs the survival of liver cancer
patients compared with supportive care. (Park et al.,
2006, Ghassan and Abou-alfa, 2004, Wirth et al.,
2005)
Antihormonal therapy with tamoxifen or
octreotide does not provide better patient survival.
Therapeutic strategies based on molecular targets
through pathway intervention signal transmission
and apoptotic regulation, offering new hope for
more effective treatment options. Potential targets
for systemic chemotherapy strategies in liver cancer
include mechanisms of oxidative and inflammatory
stress, growth factors, cell cycle checkpoints,
oncogene viruses, telomere shortening,
carcinogenesis, stem cells, angiogenesis and
antiapoptosis. (Wirth et al. , 2005, Park et al., 2006,
Ghassan and Abou-alfa, 2004) Dysregulation of
apoptosis influence the process of carcinogenesis,
progression of the tumor and tumor resistance to
radio-chemotherapy, therefore the development of
anticancer agents by inducing apoptosis is very
potential to be developed. Understanding in genetics
and treatment has improve a lot, but liver cancer
remains a deadly disease causing high mortality.
Further studies are needed to identify agents that
have more effective activity against liver cancer.
Cancer occurs because of fundamental changes
in cell physiology, one of the common
characteristics of which is avoiding apoptosis,
therefore the target of developing cancer therapy
agents is directed to induction of apoptosi. (Cha and
DeMatteo, 2005, Ho et al., 2009) Neoplastic cell
accumulation can occur not only because of
activation of oncogenes that promote tumor growth
or inactivation of tumor suppressor genes that
suppress growth, but also because of dysregulation
in genes that control apoptosis. As cell growth is
controlled by growth and suppressor genes, cell
survival is also controlled by genes that promote and
inhibit apotosis (Kumar et al., 2003, Ho et al., 2009)
Apoptosis is a form of programmed cell death
that depends on the results of intracellular gene
expression. The apoptosis process is divided into
two phases, initiation phase and execution phase.
Apoptosis initiation occurs because of signals
transmission from different pathways, namely the
extrinsic pathway (death receptor pathway) and
intrinsic pathway (mitochondrial pathway). These
two pathways eventually activate the caspase
enzyme and relate to each other in several stages.
(Kumar et al., 2003, Xu et al., 2010, Liu et al., 2011)
Apoptosis is induced by a cascade of sequences
of molecular events initiated through many
pathways that eventually activate caspase enzymes
(Kumar et al., 2003, Wirth et al., 2005, Chiu et al.,
2003) Apoptosis processes can be divided for two
phases, namely (Kumar et al., 2003, Martin)
initiation phase, when the caspase enzyme is
catalyzed to become active and the execution phase,
when the caspase enzyme causes cell death.
Apoptosis initiation occurs because of signals from
different pathways, namely the extrinsic pathway
(death receptor pathway) and intrinsic pathway
(mitochondrial pathway). Both of these pathways
eventually activate caspase enzymes and relate to
each other in several stages. (Kumar et al., 2003)
Intrinsic pathway involves one of the pro and
antiapoptotic molecules including Bcl-2.
Intrinsic signaling pathways are initiated by
increased mitochondrial permeability and release of
proapoptotic molecules into the cytoplasm, without
the involvement of death receptors. Hormones,
growth factors and other survival signals stimulate
antiapoptotic production from the Bcl-2 protein
group. Two proteins which are the main
antiapoptosis moleculs are Bcl-2 and Bcl-x. Both of
these antiapoptotic proteins are normal in the
mitochondrial membrane and cytoplasm of the cell.
(Kumar et al., 2003, Ho et al., 2009, Xu et al., 2010,
Robotin et al.,2009) There is an increase in
expression of Bcl2 and IAP in liver cancer, which is
an antiapoptotic protein resulting in apoptosis
inhibition via intrinsic pathways. (Chang Y.,2011,
Bassiouny A et al.,2008, Yildiz L et al.,2008)
The principal of intrinsic pathway is the
existence of a balance between pro-apoptotic
molecules such as Bax, Bak, and Bim with
antiapoptotic molecules such as Bcl2 and Bcl-xl,
which control mitochondrial permeability and
release of factors that induce cell death in normal
mitochondria. Mitochondria play an important role
in this pathway by releasing cytochrome c, which
eventually forms a complex with apoptosis-inducing
factor 1 (APAF-1), procaspase- 9 and ATP. Inside
this complex, procaspase-9 is activated to caspase-9,
which then triggers caspase-3 as executor caspase.
(Kumar et al., 2003, Ho et al., 2009, Xu et al., 2010)
In our study, induction of cell apoptosis was
investigated through one of the intrinsic pathways
which would be proven biochemically with changes
in the Bcl2 expression.
Bcl-2 is a protein family that is a CED-9
homologue found in mammals. Bcl-2 was first
SKIC-MHS 2018 - The 2nd Syiah Kuala International Conference on Medicine and Health Sciences
58

discovered in B-cell lymphoma as a proto oncogen.
Bcl-2 gene overexpression is a protective
mechanism to deal with various stimuli that cause
cell death. Bcl-2 gene is responsible for synthesizing
protein bcl-2. The Bcl-2 protein family consists of
antiapoptosis groups, namely bcl-2, bcl-xl, bcl-w,
Mcl-1, Nr13, and A1 / Bfl1, as well as proapoptosis
groups namely Bax, Bak, Bok, Diva, Bcl-xs, Bik,
Bim, Hrk, Nip3, Nix, Bad, and Bid. These proteins
are characterized by the Bcl-2 homology (BH)
domain in their structures, namely BH1, BH2, BH3,
and BH4 (Lu et al., 2011, Cagle and Allen, 2009,
Marschitz et al., 2000)
The proapoptosis group has two subfamily,
multidomain groups (Bax, Bak, Bok, Diva, and Bcl-
xs) and groups that only have BH3 domains (Bik,
Bim, Hrk, Nip3, Nix). This relative ratio between
pro protein and antiapoptosis determines cell
sensitivity to various apoptotic stimuli. Proapoptosis
proteins that have been studied well are Bax and
Bid. Exposure to various apoptotic stimuli causes
translocation of Bax from the cytosol to the
mitochondrial membrane. Bax oligomeration in the
mitochondrial membrane together with other
proapoptotic proteins, Bak eventually releases
cytochrom-c from within the mitochondria to the
cytosol. Other proapoptotic proteins, especially
those that only have BH3 domains, play a role in the
Bax-Bak oligomeration process in the mitochondrial
membrane. Bcl-2 antiapoptotic protein plays a role
in inhibiting Bax-Bak oligomeration in the
mitochondrial membrane and eventually inhibits the
release of cytochromic cytosol (Marschitz et al.,
2000, Lu et al., 2011, Cagle and Allen, 2009)
Hepatocarcinogenesis is a slow process, genomic
changes that progressively alter hepatocellular
phenotypes and produce cellular intermediates that
develop into hepatocellular carcinoma (Thorgeirsson
and Grisham, 2002, Cha and DeMatteo, 2005) In
liver cancer pathogenesis, apoptotic dysregulation
occurs in the form of low Fas expression which
inhibits caspase-8 and caspase-3 activation which
ultimately inhibits the apoptosis process through the
extrinsic pathway, and an increase in Bcl2 and IAP
expression which inhibits cascapse 9 activation and
caspase 3 which ultimately inhibits the apoptosis
process through intrinsic pathways. (Kumar et al.,
2003, Ho et al., 2009, Xu et al., 2010, Bassiouny A
et al.,2008, Cagle and Allen, 2009,Yildiz L et
al.,2008) Based on this thought, a study was
conducted to observe Bcl2 gene profile as one of the
genes that play a role in the liver cancer
pathogenesis.
Natural polyphenols are a large group of
compounds derived from plants, which are
chemically characterized by two or more phenol
units (Dai and Mumper, 2010, Fraga and Oteiza,
2011) Some chemists know the term polyphenols as
White-Bate-Smith Swain-Haslam wihch is described
in several characteristics. (Quideau et al., 2011) This
definition does not include low molecular weight
structures, which have been shown to have potential
benefits for human health. There are thousand
compounds derived from plants with higher
biological potential, which only have one or more
aromatic rings and at least two hydroxyl groups,
categorized as polyphenols. (Sies, 2010)
Natural polyphenols are secondary metabolites
derived from plants that are produced as defense
agents against various types of stress, such as
ultraviolet radiation, pathogenic aggression, low soil
fertility, changes in ambient temperature and
drought. (Dai and Mumper, 2010, Manach et al.,
2004) Based on its chemical structure, there are four
main classes of polyphenols: phenolic acids,
flavonoids, stilbenes, and lignans (Manach et al.,
2004, Bravo, 1998) Knowledge and implications of
these compounds on human health including their
effects on cancer (Kampa et al., 2007, Guo et al.,
2009, Korkina et al., 2009) nervous system
protection, (Zhao, 2009, Gutierez-Merino et al.,
2011) cardiovascular system dysfunction and
damage, (Sies, 2010, Grassi et al., 2009) metabolic
syndrome, (Agouni et al., 2009, Cherniack, 2011)
diabetes, (Milne et al., 2007) aging, (Queen and
Tollefsbol, 2010, Accomando et al., 2010) and
various pathologies (Tejasari M et al., 2014) and
condotion related to inflammation. (Accomando et
al., 2010) Polyphenols have been used for thousands
of years as traditional medicine in eastern countries.
However, the inclusion of these compounds in
western medicine is still a pending issue, perhaps
because of lack information and still not widely
known scientifically. (Rodriguez et al., 2013,
Dashwood, 2007)
Annona muricata Linn is a plant generally
known as soursop or graviola which contain a large
group of phytochemicals that naturally have
anticancer activities with high selectivity between
cancer cells and normal cells. (Fraga and Oteiza,
2011, Song et al., 2014, Martin, 2006, Young et al.,
2005 , Sayers, 2011) There are much studies show
that the active ingredients of soursop leaves have
strong anticancer activity on various types of cancer
cell lines. (He et al., 2010, Xiao et al., 2011, Coloma
et al., 2002a). Other studies also report that active
from the leaves of scales proved to be able to induce
Bcl2 Gene Expression Profile on Administration of Novel Active Compound from Soursop Leaves (SF-1603) as a New Molecular Target in
Liver Cancer Therapy
59

apoptosis, but research on the ability of soursop
leaves to induce apoptosis in liver cancer cells and
its mechanism of action has not been widely carried
out.(Coloma et al., 2002b)
This study aims to test the effect of
administration of pure compounds (SF-1603)
isolated from soursop leave, to analyze its ability to
induce apoptosis in liver cancer cells, and explore its
mechanism of action in inducing apoptosis by
analyze Bcl2 mRNA expression in liver cancer cell
line to determine potential pathways as a new
molecular target of liver cancer therapy.
2 MATERIAL AND METHODS
Materials used in this study was the pure compound
isolated from the soursop leaves code SF-1603,
HepG2 cell line (HB-8065TM) from the American
Type Culture Collection (ATCC), Dulbecco's
Modified Eagle Medium (DMEM ) containing 10%
fetal bovine serum (FBS), penicillin, streptomycin
and trypsin for cell culture.
2.1 HepG2 Cell Line Culture
HepG2 cell line used in this study were less than 15
passages. Cell were seeded into well in Medium
(DMEM / F12) containing 10% Fetal Bovine Serum,
previously release cells using trypsin 0.05% - EDTA
0,53mM, then added to the growth medium into a
cell suspension. The cells were counted using a
hemocytometer and planted with a cell density of
25,000 cells/mL and obseved at 0, 24, 48 and 72
hour at 37oC with 5% CO2 atmosphere. There were
control group and intervention group given SF-1603
with concentration of 0,5xIC50, IC50 and 2xIC50.
Determination of IC50 concentration was done using
3-4-5-dimetylthiazol-2yl-2,5-difenil tetrazolium
bromide (MTT) method.
2.2 Measurement of mRNA Expression
using Quantitative Real-time
Polymerase Chain Reaction
This study using real-time PCR for quantitative
analysis. The working principle of real time PCR is
similar to conventional RT-PCR with the
fundamental difference that is: (i) Analysis of
amplicons using fluorescent reporter and not using
conventional gel electrophoresis, (ii) amplicon can
be analyzed from each cycle, and not only when the
end point. This study uses SsoFast
TM
EvaGreen
SUPERMIX containing a mixture of ready-made for
the qPCR reaction except the primer and template, ie
2x reaction buffer with dNTPs, sso7d-fusion
polymerase, MgCl2, EvaGreen dye and stabilizers.
2.3 Statistical Analysis
All quantitative data are representative of at least
three independent experiments. Values are expressed
as mean±SD. Statistical analyses were conducted
using independen test, ANOVA test and simple
linear regression. The statistical package IBM SPSS
Statistics 21 for Windows was used in the analysis.
2.4 Implications of Ethical Aspects
This study has obtained ethical approval from
Medical Research Ethics Committee Medical
Faculty Padjadjaran University No.988 /
UN6.C2.1.2 / KEPK / PN.
3 RESULTS AND DISCUSSION
The group with 0.5xIC
50
administration dose showed
the of Bcl2 mRNA expression in the treatment group
was much different from the control group. In
general, in the control group there were not many
changes, there was only a slight increase in the
expression from the 48th to 72nd hours, whereas in
the treatment group there were quite dynamic
changes. During the first 24 hours there was only a
slight decrease in Bcl2 mRNA expression in the
treatment group, whereas the control group did not
experience changes in expression level. From the
24th to the 42nd hours there was a sharp increase in
the expression level of reatment groups until it
reached the highest level, while the control group
only showed very little increase. From the 48th hour
to the 72nd hour there was a sharp decrease in the
level of expression in the treatment group to the
lowest point while the control group showed a slight
increase in expression.
In the group with the administration of IC
50
dose,
it was seen that the expression of Bcl2 mRNA in the
treatment group was significantly different from the
control group. There were not many changes in the
control group, there was only a slight increase in
expression from 48 hours to 72 hours, whereas in the
treatment group there was a quite dynamic change.
In the first 24 hours there was only a slight decrease
in Bcl2 mRNA expression in the treatment group
while the control group did not experience changes
in expression level. From the 24th to the 42nd hours
SKIC-MHS 2018 - The 2nd Syiah Kuala International Conference on Medicine and Health Sciences
60
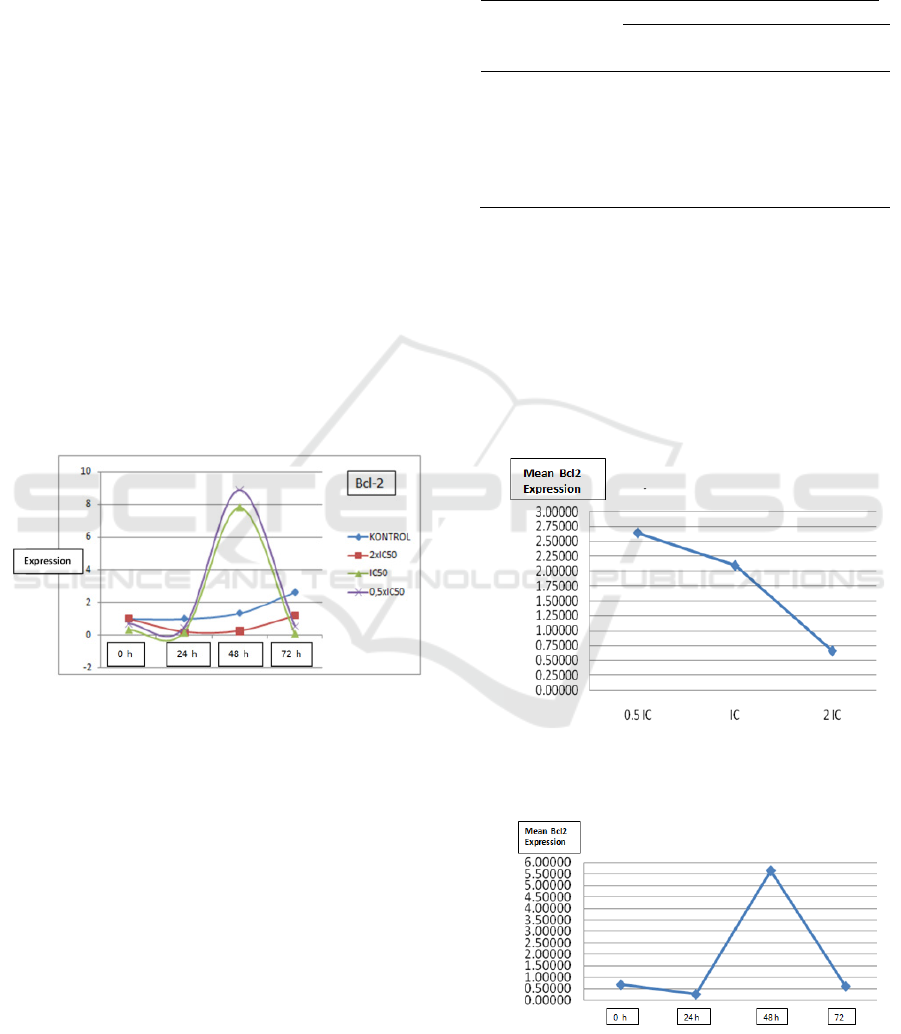
there was a sharp increase in the expression level
until it reached the highest score, while the control
group only showed very little increase. From the
48th hour to the 72nd hour there was a sharp
decrease in the level of expression in the treatment
group to the lowest point while the control group
showed a slight increase in expression.
The group with the administration of 2xIC50
doses showed that overall the expression of Bcl2
mRNA in the treatment group was lower than the
expression of the control group. In the first 24 hours
there was a decrease in Bcl2 mRNA expression in
the treatment group, whereas in the control group
there was no change in expression level. From the
24th to the 72nd hours there was an increase in the
expression of Bcl2 mRNA in the treatment and
control groups, with the expression level of the
treatment group being around 3 times lower than the
control group. The lowest Bcl2 mRNA expression
occurred at the 24th hour and the highest was at 72
hours, both in the treatment and control groups.
The expression profile of Bcl2 mRNA in HepG2
cell line culture after administration of pure
compounds SF-1603 can be seen in Figure 1.
Figure 1: Bcl-2 expression profile based on observation
time in all groups, on HepG2 cell line culture after
administration of pure compound SF-1603.
The normality test was performed using the
Kolmogorov-Smirnov Test and the test results with
α = 5% showed that at 95% confidence level, all
data were normally distributed. (p> 0.05) To find out
the differences in of Bcl-2 mRNA expression after
administration of pure SF-1603 compound at each
dose and time of observation, we used dependent
test and analysis of variance (ANOVA) method by
making decisions using the F distribution table.
Based on statistical calculations using ANOVA
method with α = 5% obtained F count value
compared with Ftable value as shown in Table 1
which showed that the F
count
> F
table (0.05).
It can be
concluded that at 95% confidence level there were
significant differences in Bcl-2 expression between
each group in all doses and time of observation (p
<0.05).
Table 1: Test of different expressions of Bcl-2 in HepG2
cell Line culture between groups
Group mRNA Bcl-2 expression
F count F table:
(
0,05
)
Control group
0.5xIC
50
group
IC
50
group
2xIC
50
g
roup
21.3872
8741
2.81647
ANOVA (Analisis of varians)
To determine the dose and time that produces the
optimum Bcl-2 mRNA expression for apoptosis
initiation, we used an average comparison test.
Figure 2&3 showed the administration of SF-1603
soursop leaf pure compound on HepG2 cell line
culture resulted in optimum Bcl-2 gene expression
for apoptosis initiation at a dose of 2xIC50 at 24
hours.
Figure 2: Effect of Doses on Bcl-2 Gene Expression on
All Observations time, After Giving Soursop Leaf Pure
Compounds to HepG2 Cell Line Culture.
Figure 3: Effect of Time Factors on Bcl-2 Genes in All
Doses, After Giving Soursop Leaves Compound to HepG2
Cell Line Culture.
Bcl2 Gene Expression Profile on Administration of Novel Active Compound from Soursop Leaves (SF-1603) as a New Molecular Target in
Liver Cancer Therapy
61

Various chemical compositions have been
isolated from various parts of soursop plants such as
leaves, roots, bark, flesh and seeds. Some
phytochemicals have been reported to be isolated
and characterized from various parts of soursop
plants and one of them isolated from soursop leaves
has strong anticancer activity and is able to induce
apoptosis in liver cancer cells. (Tejasari et al., 2018)
Suppression of Bcl-2 gene expression will
initiate the process of apoptosis through intrinsic
pathways. This apoptotic pathway occurs due to
increased mitochondrial permeability and release of
proapoptotic molecules into the cytoplasm, without
the involvement of death receptors. Several growth
factors and other survival signals stimulate
antiapoptosis production from the Bcl-2 protein
group (Martin, Wirth et al., 2005, Kumar et al.,
2003, Albert and Johnson, 2002)
Lots of proteins that belong to this group which
all play a role in regulating the apoptosis process.
One of the proteins which is the main antiapoptosis
is Bcl-2 protein. This antiapoptotic protein is present
in the mitochondrial membrane and cytoplasm of
cells. When the cell receives a survival signal or
experiences stress, Bcl-2 will disappear from the
mitochondrial membrane and be replaced by
proapoptotic proteins such as Bak, Bax, and Bim
(Kumar et al., 2003, Albert and Johnson, 2002).
When Bcl-2 and / or Bcl-x level decreases,
permeability of the mitochondrial membrane will
increase so that some proteins are released which
can activate the caspase cascade. One of these
proteins is cytochrome c, which is known to play a
role in mitochondrial respiration. In cytosol,
cytochrome c binds to a protein called Apaf-1
(apoptosis activating factor-1, homologous with
Ced-4), and this complex activates caspase-9. Bcl-2
and Bcl-x may also directly inhibit Apaf-1 activation
so that in the absence of Bcl-2 and Bcl-x, Apaf-1
activation can occur (Albert and Johnson, 2002,
Kumar et al., 2003). is the caspase cascade initiation
process. It can be concluded that the essence of this
intrinsic pathway is the balance between pro-
apoptotic molecules and protective molecules that
control mitochondrial permeability and the release
of factors that induce cell death that are normally in
mitochondria. (Kumar et al., 2003).
The study conducted in 2005was reported that in
liver cancer pthogenesis, there was an increase in the
Bcl2 expression which is an antiapoptotic protein
and strengthened by a study in 2008 which stated
that Bcl2 activation played a role in the progression
towards liver cancer. (Bassiouny et al., 2008,
Coloma et al., 2002, Chang and Xu, 2000, Yildiz et
al., 2008, Wu et al., 1995, Liu and 2009, 2009)
Inhibition of apoptosis through the intrinsic
pathway involving the Bcl-2 gene is one of the
pathways involved in the pathogenesis of liver
cancer. The ability of SF1603 soursop leaf pure
compound to suppress the expression of Bcl-2 can
be seen in figure 1 which showed the expression
level of Bcl-2 was lower than the control group in all
treatment groups. This is reinforced by the graph in
figure 2&3 which showed the optimum point of
expression of Bcl-2 by calculating the dose and time
factors. Statistical test with ANOVA in Table 1
strengthens the evidence with conclusions on 95%
confidence levels there are significant differences in
Bcl-2 expression between each group in all doses
and time of observation (p <0.05), in HepG2 cell
line culture after the administration of soursop leave
pure compounds SF-1603.
To measure the correlation strength of Bcl2
expression with apoptosis level, a simple correlation
test was calculated using the Pearson formula, and
presence coeficcient correlation between Bcl2
mRNA expression and the level of apoptosis is
r=0,558, which means the strength of the correlation
is strong. With the ability of SF-1603 soursop leave
pure compound in suppressing the expression of
Bcl-2, this compound can be used as a candidate for
HCC therapy agent by making the Bcl-2 gene as a
molecular target of therapy to initiate the HCC
apoptosis induction process.
4 CONCLUSIONS
The study conclude that the novel soursop leaves
active compound (SF-1603) is a powerful anticancer
that affect Bcl2 gene expression in apoptosis
induction on liver cancer cell, so it can be used as a
candidate for new therapeutic agent for liver cancer
treatment with Bcl2 as a new molecular target
ACKNOWLEDGEMENTS
This research was made possible thanks to the full
support of the Faculty of Medicine, Bandung Islamic
University and Faculty of Medicine Padjadjaran
University, as well as with the cooperation of the
Chemistry Research Laboratory Graduate School of
Science, Padjadjaran University and Biotechnology
Laboratory Rajawali Hospital Bandung..
SKIC-MHS 2018 - The 2nd Syiah Kuala International Conference on Medicine and Health Sciences
62

REFERENCES
Accomando, S., Pellitteri, V. & Corsello, G. 2010. Natural
Polyphenols As Anti Inflammatory Agents. Fornt
Biosci, 2, 318-31.
Agouni, A., Lagrus-Lak-Hal, A., Tesse, A., Mulder, P. &
Al, E. 2009. Red Wine Polyphenols Prevent Metabolic
And Cardiovascular Alterations Associated With
Obesity In Zucker Fatty Rats (Fa/Fa). Plos One, 4,
E5557.
Albert & Johnson 2002. Molecular Biology Of The Cell,
New York., Garland Science Taylor And Francis
Group Company.
Bassiouny, A., Bassiouni, N., Nosseir, M., Zoheiry, M., El-
Ahwany, G., F, F. S. & Al., E. 2008. Circulating And
Hepatic Fas Expression In Hcv-Induced Chronic Live
Disease And Hepatocellular Carcinoma. Medscape J
Med. , 10, 130.
Bravo, L. 1998. Polyphenols: Chemistry, Dietary Source,
Metabolism And Nutritional Significance. Nutr Rev,
56, 317-33.
Bruix, J. & Sherman, M. 2005. . Management Of
Hepatocellular Carcinoma. Hepatology, 42, 1208-36.
Cagle, P. & Allen, T. C. 2009. Apoptosis. Basic Concept
Of Molecular Pathology. Springer Dordrecht
Heidelberg.
Cha, C. & Dematteo, R. 2005. Molecular Mechanisms In
Hepatocellular Carcinoma Development.
Practise&Research Clinical Gastroenterology, 19, 25-
37.
Chang, Y. & Xu, Y. 2000. Expression Of Bcl-2 Inhibited
Fas-Mediated Apoptosis In Human Hepatocellular
Carcinoma Bel-7404 Cells. 10.
Cherniack, E. 2011. Polyphenols: Planning The Seeds Of
Treatment For The Metabolic Syndrome Nutrition, 27,
617-23.
Chiu, H., Chih, T., Hsian, Y., Tseng, C., Wu, M. & Wu, Y.
2003. Bullatacin, A Potent Antitumor Annonaceous
Acetogenins, Induces Apoptosis Through A Reduction
Of Intracellular Camp And Cgmp Levels In Human
Hepatoma 2.2.15 Cells. . Biochemical Pharmacology.,
65, 319-327.
Coloma, A., Guadan-O, A., Ine’sa, C., Martinez-Diazb, R.
& Cortesc, D. 2002a. Selective Action Of Acetogenin
Mitochondrial Complex I Inhibitors. Z Naturforsch,
57c, 1028-1034
Dai, J. & Mumper, R. 2010. Plant Phenolics: Extraction,
Analysis And Their Antioxidant And Anticancer
Properties. Molecules, 15, 7313-52.
Dashwood, R. 2007. Frontiers In Polyphenols And Cancer
Prevention. J Nutr, 37, 267s-269s.
Fraga, C. G. & Oteiza, P. I. 2011. Dietary Flavonoids :
Role Of (-)-Epicatechin And Related Procyanidins In
Cell Signaling. Free Radical Biology & Medicine, 51,
813-23.
Ghassan, K. & Abou-Alfa, M. 2004a. Current And Novel
Therapeutics For Hepatocellular Carcinoma.
American Society Of Clinical Oncology.
Ghassan, K. & Abou-Alfa, M. 2004b. . Current And Novel
Therapeutics For Hepatocellular Carcinoma
Grassi, D., Desideri, G., Groce, G., Tiberti, S., Anggio, A.
& Al, E. 2009. Flavonoids, Vascular Function And
Cardiovascular Protection. Curr Pharm Des, 15,
1072-84.
Guo, W., Kong, E. & Meydani, M. 2009. Dietary
Polyphenols, Inflammation And Cancer. Nutr Cancer,
61, 807-10.
Gutierez-Merino, C., Lopez-Sanchez, C., Lagoa, R.,
Samhan-Arias, A., Bueno, C. & Al, E. 2011.
Neuroprotective Actions Of Flavonoids. Curr Med
Chem, 18, 1195-1212.
He, H., Wu, X., Yu, B., Liu, K., Zhou, G., Qian, G. & Al, E.
2010. The Effect Of Desacetyluvaricin On The
Expression Of Tlr4 And P53 Protein In Hepg 2.2.15.
Journal Of Hepatitis, 11, 364-367.
Ho, H., Pok, S., Streit, S., Ruhe, J. E., Hart, S., Lim, K. &
Al., E. 2009. Fibroblast Growth Factor Receptor 4
Regulates Proliferation, Anti-Apoptosis And Alpha-
Fetoprotein Secretion During Hepatocellular
Carcinoma Progression And Represents A Potential
Arget For Therapeutic Intervention. . J.Jhep, 50, 118-
127.
Huynh, H., Ngo, V., Koong, H., Poon, D., Choo, S., Toh,
H. & Al., E. 2010a. Azd6244 Enhances The Anti-
Tumor Activity Of Sorafenib In Ectopic And
Orthotopis Models Of Hcc. J.Jhep, 52, 79-87.
Huynh, H., Ngo, V., Koong, H., Poon, D., S, S. C., Toh, H.
& Al, E. 2010b. Azd6244 Enhances The Anti-Tumor
Activity Of Sorafenib In Ectopic And Orthotopis
Models Of Hcc. Jjhep, 52, 79-87.
Kampa, M., Nifli, A., Notas, G. & Castanas, E. 2007.
Polyphenols And Cancer Cell Growth. Rev Physiol
Biochem Pharmacol, 159, 79-113.
Korkina, L., Luca, C. D., Kostyuk, V. & Pastore, S. 2009.
Plant Polyphenols And Tumors: From Mechanism To
Therapies, Prevention And Protection Againts Toxicity
Of Anti-Cancer Treatments. Curr Med Chem, 16,
3943-65.
Kumar, V., Cotran, R. & Robbin, S. 2003. Pathologic
Basic Of Disease, Philadelphia, Saunders-Elsevier.
Lencioni, R., Crocetti, L., Petruzzi, P., Vignali, C., Bozzi,
W., Pina, C. & Al, E. 2008. Doxorubicin-Eluting
Bead-Enhanced Radiofrequency Ablation Of
Hepatocellular Carcinoma:A Pilot Clinical Study.
J.Jhep, 49, 217-222.
Liu, C. & 2009, Y. Z. 2009. Effect Of Annonaceous
Acetogenin On Proliferation And Apoptosis Of Raji
Cell. Chinese Journal Of Information On Traditional
Medicine. .
Liu, Q., Chen, J., Liu, L., Zhang, J., Wang, D., Ma, L. &
Al, E. 2011. The X Protein Of Hepatitis B Virus
Inhibits Apoptosis In Hepatoma Cell Through
Enchancing The Methionine Adenosyltransferase 2a
Gene Expression And Reducing S-Adenosylmethionine
Production. The Journal Of Biological Chemistry,
286, 17168-17180.
Lu, Y., Zhang, Y., Jia, Z., Wu, W. & Lu, Z. 2011.
Hepatocellular Carcinoma Hepg2 Cell Apoptosis And
Caspase-8 And Bcl-2 Expression Induced By
Injectable Seed Extract Of Coix Lacryma-Jobi.
Hepatobilliary Panreat Dis Int., 10.
Bcl2 Gene Expression Profile on Administration of Novel Active Compound from Soursop Leaves (SF-1603) as a New Molecular Target in
Liver Cancer Therapy
63

Manach, C., Scallbert, A., Morand, C., Remesy, S. &
Jimenez, L. 2004. Polyphenols: Food Sources And
Bioavailability. Am J Clin Nutr, 79, 727-47.
Marschitz, I., Tinhofer, I., Hittmair, A., Egle, A., Kos, M.
& Greil, R. 2000. Analysis Of Bcl-2 Protein
Expression In Chronic Lymphocytic Leukemi. Am J
Clin Pathol 113, 219-229.
Martin, K. Targeting Apoptosis With Dietary Bioactive
Agents. Experimental Biology And Medicine, 231,
117-129
Martin, K. R. 2006. Targeting Apoptosis With Dietary
Bioactive Agents. Experimental Biology And
Medicine, 231.
Milne, J., Lambet, P., Schenk, S., Carney, D., Smith, J. &
Al, E. 2007. Small Molecule Activators Of Sirt1 As
Therapeutics For The Treatment Of Type 2 Diabetes.
Nature 450, 712-16.
Park, S., Lee, Y., Han, S., Kwon, S., Kwon, O., Kim, S. &
Al, E. 2006a. . Systemic Chemotherapy With
Doxorubicin, Cisplatin And Capecitabine For
Metastatic Hepatocellular Carcinoma. Bmc Cancer, 6,
10.1186/471-2407-6-3.
Park, S., Lee, Y., Han, S., Kwon, S., Kwon, O., Kim, S. &
Al., E. 2006b. Systemic Chemotherapy With
Doxorubicin, Cisplatin And Capecitabine For
Metastatic Hepatocellular Carcinoma. Bmc Cancer, 6,
10.1186/1471-2407-6-3.
Queen, B. & Tollefsbol, T. 2010. Polyphenols And Aging.
Curr Aging Sci, 3, 34-42.
Quideau, S., Deffieux, D., Douat-Casassus, C. &
Pouysegu, L. 2011. Plant Pholyphenols: Chemical
Properties, Biological Activities And Synthesis. Angew
Chem Int Ed Engl.
Robotin, M., Kansil, M., Howard, K., George, J., Tipper,
S., Dores, G. & Al, E. 2009a. Antiviral Therapy For
Hepatitis B-Related Cancer Prevention Is More Cost-
Effective Than Cancer Screening. Jjhep, 50, 990-998.
Robotin, M., Kansil, M., Howard, K., George, J., Tipper,
S., Dores, G. & Al., E. 2009b. Antiviral Therapy For
Hepatitis B-Related Cancer Prevention Is More Cost-
Effective Than Cancer Screening. J.Jhep, 50, 990-998.
Rodriguez, M. L., Estrela, J. M. & Ortega, A. L. 2013.
Natural Polyphenols And Apoptosis Induction In
Cancer Therapy. J Carcinogene Mutagene, S6.
Sayers, T. 2011. Targetting The Extrinsic Apoptosis
Signaling Patways For Cancer Therapy. Cancer
Immunol Immunother, 60, 1173-80.
Sherman, M., Burak, K., Maraun, J., Metrakos, P., Myers,
R., Guindi, M. & Al., E. Multidiciplinary Canadian
Concensus Recommendations For The Management
And Treatment Of Hepatocellular Carcinoma. Current
Oncology;, 18.
Sies, H. 2010. Pholyphenols And Health: Update And
Perspectives. Arch Biochem Biophysics, 501, 2-5.
Song, Y., Zhang, A., Yan, G., Han, Y. & Wang, X. 2014.
Pant-Derived Natural Products As Leads To Anti-
Cancer Drugs. J.Med.Plant Herb.Ther.Res, 6.
Tejasari, M., Sastramihardja, H., Abdurachman, S. &
Prasetyo, D. 2018. Anticancer Activity Of Novel
Soursop Leaves Active Compound (Sf1603) Through
Apoptotic Induction In Liver Cancer Malaysian
Journal Of Fundamental And Applied Sciences, 14,
226-234.
Tejasari M, S. H., Abdurrachman Sa, Prasetyo D 2018.
Anticancer Activity Of Novel Soursop Leaves Active
Compound (Sf-1603) Through Apoptotic Induction In
Liver Cancer. Malaysian Journal Of Fundamental
And Applied Sciences 14, 226-234.
Tejasari M, S. N., Sastramihardja H, Djanuarsih I 2014.
The Role Of Soy In Preventing Apoptosis In Liver
Injury. International Journal Of Research In
Pharmaceutical And Nano Science,Issn: 2319 – 9563,
3, 373-379.
Thorgeirsson, S. & Grisham, J. 2002. Molecular
Pathogenesis Of Human Hepatocellular Carcinoma.
Nature Genetics, 31.
Wirth, T., Kuhnel, F., Fleischmann-Mundt, B., Woller, N.,
Djojosubroto, M., Rudolph, K. & Al, E. 2005a. Wirth
T, Kuhnel F, Fleischmann-Mundt B, Woller N,
Djojosubroto M, Rudolph K, Et Al. Telomerase-
Dependent Virotherapy Overcomes Resistance Of
Hepatocellular Carcinomas Against Chemotherapy
And Tumor Necrosis Factor-Related Apoptosis-
Inducing Ligand By Elimination Of Mcl-1 Cancer
Resaacrjournal 65, 7393.
Wirth, T., Kuhnel, F., Fleischmann-Mundt, B., Woller, N.,
Djojosubroto, M., Rudolph, K., Manns, M., Zender, L.
& Al., E. 2005b. Telomerase-Dependent Virotherapy
Overcomes Resistance Of Hepatocellular Carcinomas
Against Chemotherapy And Tumor Necrosis Factor-
Related Apoptosis-Inducing Ligand By Elimination Of
Mcl-1. Cancer Res.Aacrjornal. , 65, 7393.
Wu, F., Zeng, L., Gu, Z., Zhao, G., Zhang, Y., Schwedler,
J., Mclaughin, J. & Sastrodihardjo, S. 1995. New
Bioactive Monotetrahydrofuran Annonaceous
Acetogenins, Annonamuricin C And Muricatocin C,
From Leaves Of Annona Muricata. J Nat Prod 58,
909-15.
Xiao, Q., Qiu, Y., Zhou, G., Mao, C., Li, Z., Yao, Z. & Al,
E. 2011. Potent Antitumor Mimetics Of Annonaceous
Acetogenins Embedded With An Aromatic Moiety In
The Left Hydrocarbon Chain Part. J Med Chem, 54,
525-533.
Xu, L., Qian, G., Tang, L., Su, J. & Wang, J. 2010. Genetic
Variations Of Hepatitis B Virus And Serum Aflatoxin-
Lysine Adduct In High Risk Hepatocellular
Carcinoma In Southern Guangxi, China. J.Jhep., 53,
671-676.
Yildiz, L., Baris, S., Aydin, O., Kefeli, M. & Kandemir, B.
2008. Bcl-2 Positivity In B And C Hepatitis And
Hepatocellular Carcinoma. Hepatogastroeneterology
55, 2207-10.
Young, L., Hantz, H. & Martin, K. 2005. Resveratrol
Modulates Gene Expression Associated With
Apoptosis, Proliferation, And Cell Cycle In Cells With
Mutated Human C-Ha-Ras, But Does Not Alter C-Ha-
Ras Mrna Or Protein Expression. J.Nutr Biochem, 16,
663-74.
Zhao, B. 2009. Natural Antioxidant Protect Neuron In
Alzheimers Disesase And Parkonsons Disease.
Neurochem Res, 34, 630-38.
SKIC-MHS 2018 - The 2nd Syiah Kuala International Conference on Medicine and Health Sciences
64
