
Dengue Virus (DENV)-1 Induces High Expression of Anti- and
Pro-inflammatory Cytokines in Human Macrophages
Winda Yulia
1
, Benediktus Yohan
2
, Febrina Meutiawati
2
, Alida R. Harahap
2,3
,
R. Tedjo Sasmono
2
1
Department of Microbiology, Faculty of Medicine, Universitas Syiah Kuala, Aceh, Indonesia
2
Eijkman Institute for Molecular Biology, Jakarta, Indonesia
3
Department of Clinical Pathology, Faculty of Medicine, Universitas Indonesia, Jakarta
Keywords: Dengue Viruses, Cytokines, Monocyte-Derived Macrophages (MDMs).
Abstract: Dengue is caused by dengue virus (DENV) infection. The pathological mechanism of dengue infection is
still poorly understood. Serotype variation and host’s immune response are thought to have roles in disease
severity. This study compared the ability of four DENV serotypes (DENV-1, -2,-3,-4) isolates from
Indonesia to induce the expression of anti- and pro-inflammatory cytokines in Monocyte-Derived
Macrophages (MDMs) differentiated from a healthy human. Monocytes were isolated from healthy human
donors and differentiated into macrophages under the stimulation of Macrophage Colony Stimulating Factor
(M-CSF). Mature MDMs were infected with four different serotypes of DENV isolates from Indonesia. The
resulting expression kinetics of Interferon alpha (IFN-2) and Interleukin-10 (IL-10) as anti-inflammatory
cytokines and IL-1 as pro-inflammatory cytokine was measured at six different time points using Luminex
immunoassay. The presence of DENV Non-Structural Protein 1 (NS1) as a marker of virus replication was
also measured using ELISA. DENV-1 showed a tendency to induce the expression of IFN2 and IL-10
more rapidly and at a higher level than other serotypes albeit of lower NS1 expression. The expression of
IL-1 was down-regulated in response to all DENV serotypes infection. This study demonstrates the
differences in the ability of four DENV serotypes in regulating the expression of cytokines in MDMs with
DENV-1 showed a superior inducing capability compared to other serotypes. This data provides
information of possible serotype-specific role on disease severity.
1 INTRODUCTION
Dengue is a febrile illness caused by dengue virus
(DENV) infection.(Gubler, 1998)
DENV is a
member of the Flaviviridae family, which includes
four serotypes identified as DENV-1, DENV-2,
DENV-3, and DENV-4.(Lindenbach & Rice,
2003)(Kuno, Chang, Tsuchiya, & Karabatsos, 2014)
The mechanisms that lead to clinical manifestations
of dengue are believed to be multifactorial. There
are two main factors which have been shown to
contribute to disease severity, i.e., viral and host
factors.(Halstead, 2008)
Viral factors (viral load and variations of viral
serotypes) have been shown to play critical roles in
the emergence of symptoms.(Halstead, 2008)(Clyde,
Kyle, & Harris, 2006) Previous research has argued
that the role of immune mediators such as anti- and
pro-inflammatory cytokines may be as important as
host immune factors.(Green & Rothman, 2006) In
humans, macrophages have been determined as
DENV targets.(Chen & Wang, 2002) The targeting
of these cells by DENV may then lead to the
immunological modulation,(Sun & Kochel, 2013) as
evidenced by the expression of various cytokines.
This study aimed to compare the ability of the
four serotypes of DENV in inducing the expression
of anti- and pro-inflammatory cytokines in MDMs
by looking at the kinetic patterns of cytokine
expression in vitro.
Previously, we have described the
cytokine/chemokine expression of four DENV
serotypes in the human A549 cell line.(Yohan,
Kendarsari, Mutia, Bowolaksono, & Harahap, 2014)
Here, we study the expression profile of MDM cells,
which more reflecting the natural pathogenesis
mechanism of DENV infection in human.
44
Yulia, W., Yohan, B., Meutiawati, F., Harahap, A. and Sasmono, R.
Dengue Virus (DENV)-1 Induces High Expression of Anti- and Pro-inflammatory Cytokines in Human Macrophages.
DOI: 10.5220/0008790500440048
In Proceedings of the 2nd Syiah Kuala International Conference on Medicine and Health Sciences (SKIC-MHS 2018), pages 44-48
ISBN: 978-989-758-438-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2 METHODS
2.1 Monocyte Isolation and MDM
Differentiation.
Monocytes were isolated from venous blood of
healthy human volunteers. Ethical clearance was
obtained from the Eijkman Institute Research Ethics
committee.
Peripheral blood mononuclear cell (PBMCs)
isolation was performed using Ficoll gradient
centrifugation (Ficoll-Paque PLUS, GE Healthcare).
Monocytes fraction from PBMC were enriched by
overnight cell adherence into the surface of cell
culture flasks in RPMI-1640 medium (Gibco-
Thermo Scientific) supplemented with 10% FBS
(Gibco), 2 mM L-glutamine (Gibco), 100 U/ml
Penicillin and 100 μg/ml streptomycin (Gibco).
The enriched monocytes were further
differentiated into macrophages by stimulation with
10 ng/ml Macrophage Colony Stimulating Factor
(M-CSF) (Sigma) for eight days. Differentiation of
monocytes into macrophages was monitored based
on morphological observation and the expression of
c-fms gene as macrophage marker by RT-PCR. β-
actin gene was also amplified as RNA loading
control. The primer pairs used were as follows:
c-fms forward, 5-ACACTAAGCTCGCAATCCC-3,
and
revese 5’-GTATCGAAGGGTGAGCTCAAA-3’; β-
actin forward,
5’-CATCTCTTGCTCGAAGTCCA-3’, and
reverse,5’-ATCATGTTTGAGACCTTCAACA-
3’.(Jia et al., 2010)
2.2 DENV Infections
Four DENV clinical strains representing all four
serotypes were isolated from Indonesian dengue
patients’ sera. Viruses were cultured in Vero cells.
Viral titers were measured in plaque forming
units/ml (PFU/ml), using a modified plaque assay
method.(Lambeth, White, Johnston, & Silva, 2005)
MDMs were infected by four serotypes of DENV
using 0.1 multiplicity of infection (moi). Controls
included non-infected and lipopolysaccharide (LPS)-
stimulated cells. For cytokines and NS1 antigen
measurement, the cell culture supernatant was
collected in 12-hour intervals for a total of 72 hours
and immediately stored at -80°C. DENV NS1
antigen expression was measured in cell culture
supernatants by using Panbio Dengue Early NS1
ELISA kit (Alere, USA).
2.3 Cytokines Assay
The level of IFN-2, IL-10 and IL-1β were
measured using Milliplex MAP Human Cytokine kit
(Merck Millipore, MPXHCYTO-60K, Germany).
Multiplex fluorescent microbead immunoassay
containing fluorescent microspheres, conjugated
with specific monoclonal antibodies for the target
protein were used to detect and quantify the
cytokines from 25 µl of culture supernatant,
simultaneously, as described elsewhere.(Yohan et
al., 2014) Results were obtained in Median
Fluorescent Intensity (MFI) and further analyzed
using MasterPlex QT software to measure the
cytokines concentration in
pg/ml.(www.ReaderFit.com)
3 RESULTS
3.1 Isolation and Differentiation of
MDMs
In the early isolation of PBMC, the monocytes were
round upon microscopic inspection. Monocytes were
observed as small and uniformly distributed on the
tissue culture flask. Morphological changes were
observed during the eight days of differentiation and
can be seen in FIGURE 1. Cell development was
observed to have accelerated rapidly from day five
onward. Morphological observation of MDMs on
day eight indicated that the cells were fully
differentiated into macrophages, demonstrating cell
adherent, fibroblast-like morphology, and the
appearance of pseudopodia.(Abbas, Lichtman, &
Pillai, 2012)(Sasmono & Hume, 2004) Detection of
the c-fms gene, a marker of macrophage
differentiation(Sasmono & Hume, 2004), was
prominent at day 8 using RT-PCR. This gene was
not detected in fibroblasts control cells.
Dengue Virus (DENV)-1 Induces High Expression of Anti- and Pro-inflammatory Cytokines in Human Macrophages
45
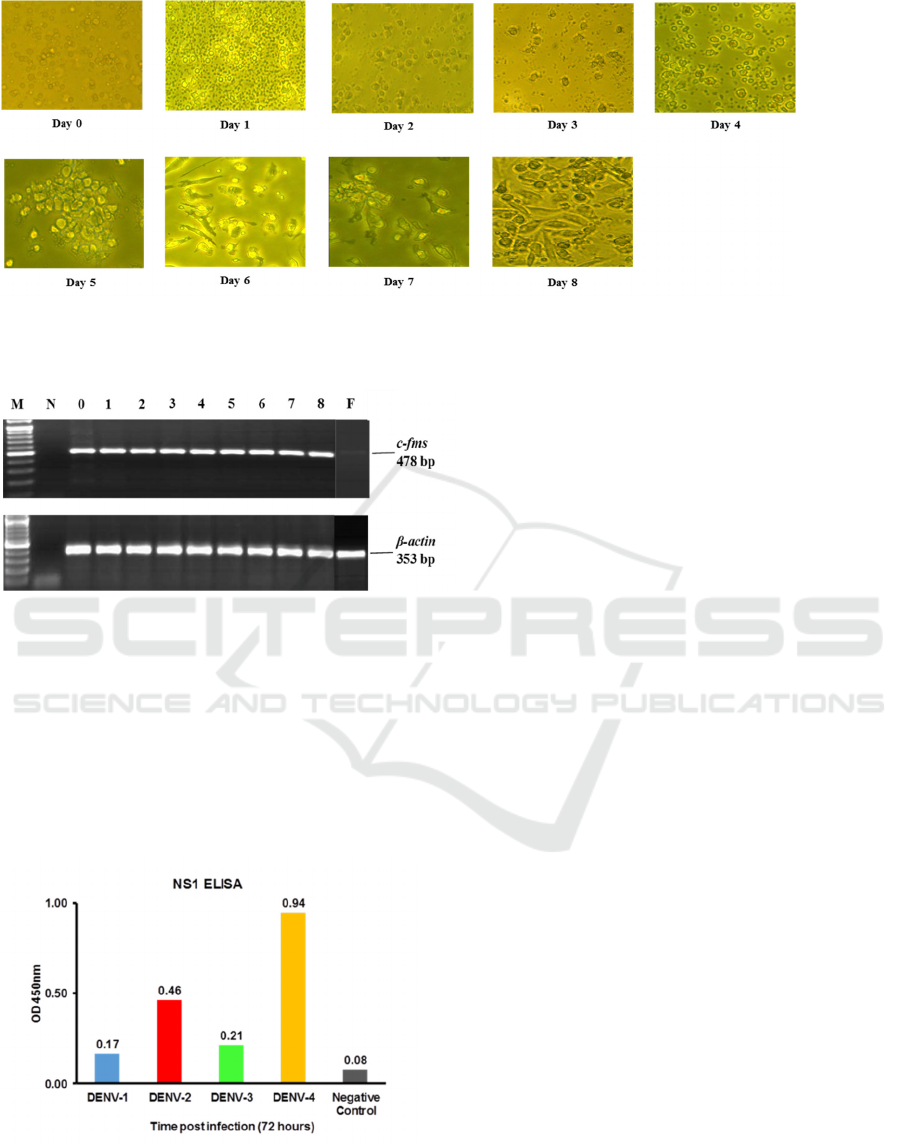
Figure
1. Morphology MDMs isolation and differentiation on day 0 to day 8. Cell growth appears slow
from day 0 to 4 of isolation, and rapid growth seen up to day 8. 20 x magnification.
Figure 2. Expression of c-fms gene in the
MDMs. Comparison of the macrophage colony-
stimulating factor receptor (c-fms gene) and human
actin (β-actin gene) as an RNA loading control
using RT-PCR from isolation day one to day eight.
M, DNA Marker (100 bp) (Invitrogen); N,
negative control; F, Fibroblast.
Figure 3. Detection of DENV 1-4 NS1 antigen in
the MDMs. DENV NS1 antigen was measured
using commercial NS1 ELISA tested to culture
supernatant collected 72 hours post-infections.
3.2 DENV NS1 Antigen Detection
NS1 protein secretion is one marker of dengue virus
replication in the host cell. The ability of dengue
virus to replicate in infected MDMs was evidenced
by the detection of DENV NS1 antigen in all four
serotypes, observed at 72 hours post-infection. NS1
antigen of DENV-4 appeared to predominate,
whereas DENV-1 NS1 expression was the lowest
among all serotypes.
The absorbance levels were considered as
Positive using the Panbio Unit calculation. Non-
infected cell control showed no detection of NS1
(FIGURE 3).
3.3 Kinetics Profile of Cytokines
Expression in the MDMs
Our study demonstrated that IFN-2, IL-10 and IL-
1 were expressed as a result of the exposure of
MDM to the four serotypes of DENV. The kinetics
of the expression patterns of cytokines is shown in
FIGURE 4. The intensity of IFN-α2 expression on
MDMs exposed by DENV-1 was significantly
different in comparison with the other serotypes.
IFN-α2 expression is seen to rise sharply after
24 hours, then decreased sharply after 60 hours. The
pattern of cytokine expression seen in DENV-4
appears to increase at a slower rate, which then starts
to increase sharply at 60 and 72 hours post-infection.
In DENV serotype -2 and -3, the increase in IFN-α2
was overall not as high as in the two other serotypes.
The induction of IL-10 expression against
DENV-1 infection tended to be higher than the other
serotypes, despite lower levels of DENV NS1
antigen being detected. The IL-10 expression
SKIC-MHS 2018 - The 2nd Syiah Kuala International Conference on Medicine and Health Sciences
46
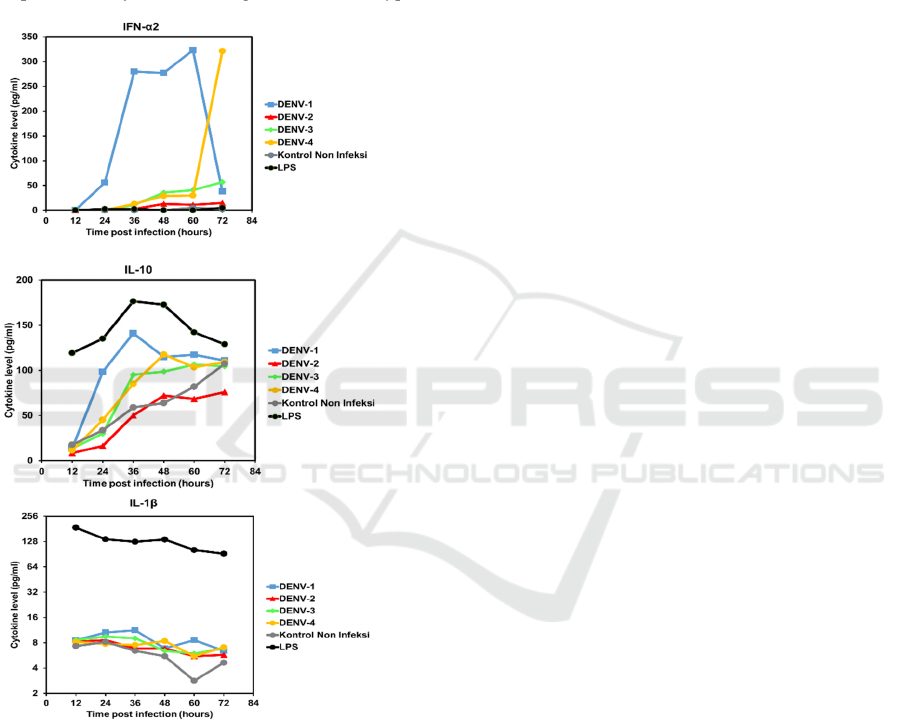
patterns showed a sharp increase at about 36
hours post-infection in all serotypes, and then
plateaued or declined.
Expression of IL-1β slightly increased and then
gradually decreased after 36 hours. All serotypes
showed decreasing patterns until 60 hours and then
continue to slightly increase after 72 hours, except
for DENV-1. In those two cytokines, there was a
tendency that DENV-1 induces relatively highest
expression of cytokines among the DENV serotypes.
Figure 4. Cytokines expression kinetics in MDMs
in response to infection of four DENVserotypes.
4 DISCUSSION
In this study, differences in the pattern of expression
of cytokines in MDMs (FIGURE 4) was
demonstrated, with DENV-1 exposed MDMs
expressing the highest levels of cytokines. The
increase in cytokine expression is inversely related
to the detection of DENV NS1 antigen (FIGURE 3).
The response to DENV-4 was observed to be
lower than most other serotypes and virus NS1
antigen was detected at highest levels than with
exposure to DENV-1 and -3. This raises the question
of whether there are intrinsic factors in DENV-1
virus titers which, although less prominent than
other serotypes, triggers an increase in cytokine
expression compared to other serotypes. Further
study is needed to determine the possible existence
of DENV-1 intrinsic factors, especially when
associated with viral genetic traits that may be
observed with the complete genome sequence of the
virus.
Interferon (IFN) is a host defense system that is
activated during the initial stages of infection.
Previous in vitro studies showed that DENV
infection in human cells could be inhibited by initial
therapy using IFN-α which inhibits translation of
viral RNA kinetics.(Diamond & Harris, 2001) The
results of this study demonstrate that the expression
pattern of IFN-α2 in MDMs infected by DENV-1
and DENV-4 may demonstrate a correlation
between the concentrations of IFN-α2 and
expression of DENV NS1 antigen ELISA (FIGURE
3 and 4). The high-level of IFN-α2 expression
during the early phase of DENV-1-infected MDM
(up to 60 hrs post-infection) resulted in the low level
of virus titer, measured as NS1 level at 72 hrs.
Inversely, the relatively low level of IFN-α2
expression in the initial stages of DENV-4 infection
yielded increased level of NS1. However, the
patterns were not clearly observed in DENV-2 and
DENV-3-infected MDM. These figures indicate a
possible role of IFN-α2 in inhibiting DENV
replication in the host and the serotype-specific
induction mechanisms.
In DENV pathogenesis, resistance against IFN
can be caused by IL-10 led immunosuppression,
followed by failure in achieving viral clearance by
the immune system and persistent infection in acute
viral infections.(Diamond & Harris, 2001) The
results of this study demonstrate that the expression
pattern of IL-10 was increasing during the
progression of DENV infection. This may be due to
the immunosuppression mechanisms intrinsic to IL-
10 as the anti-inflammatory cytokine. The increase
in IL-10 expression may be correlated to the role of
the DENV in regulating the host’s immune system.
IL-1β is a pro-inflammatory cytokine produced
by macrophages. This cytokine plays a role in the
cellular activity, including proliferation and
differentiation kinetics of cytokine expression, and
Dengue Virus (DENV)-1 Induces High Expression of Anti- and Pro-inflammatory Cytokines in Human Macrophages
47

apoptosis.(Abbas et al., 2012) In this study, most
serotypes showed a gradually decreased expression
at later stages, post-infection. The role of this
cytokine in DENV infection may need to be
explored more.
5 CONCLUSION
In conclusion, cytokines expression in MDM
infected by various DENV serotypes showed a
marked difference in expression. These findings are
useful to assess the ability of serotypes in inducing
the host immune response by demonstrating the
variations in patterns of cytokines expression. In line
with previous research showing that cytokine IFN-
α2 has viral inhibition characteristic, our findings
suggest that IFN-α2 may contribute to DENV
inhibition. Further studies are needed to assess the
roles of infecting DENV serotype in disease
severity.
ACKNOWLEDGMENT
We thank the member of Dengue Laboratory
Eijkman Institute for support and suggestion.
REFERENCES
Abbas, A. K., Lichtman, A., & Pillai, S. (2012).
Cellular and Molecular Immunology. 7th ed.
United States of America: Elsevier.
Chen, Y., & Wang, S. (2002). Activation of
Terminally Differentiated Human Monocytes /
Macrophages by Dengue Virus : Productive
Infection , Hierarchical Production of Innate
Cytokines and Chemokines , and the Synergistic
Effect of Lipopolysaccharide †, 76(19), 9877–
9887. https://doi.org/10.1128/JVI.76.19.9877
Clyde, K., Kyle, J. L., & Harris, E. (2006). Recent
Advances in Deciphering Viral and Host
Determinants of Dengue Virus Replication and
Pathogenesis , 80(23), 11418–11431.
https://doi.org/10.1128/JVI.01257-06
Diamond, M. S., & Harris, E. (2001). Interferon
Inhibits Dengue Virus Infection by Preventing
Translation of Viral RNA through a PKR-
Independent Mechanism, 311, 297–311.
https://doi.org/10.1006/viro.2001.1114
Green, S., & Rothman, A. (2006).
Immunopathological mechanisms in dengue and
dengue hemorrhagic fever, 19, 429–436.
Gubler, D. J. (1998). Dengue and Dengue
Hemorrhagic Fever, 11(3), 480–496.
Halstead, S. B. (2008). Dengue. Imperial College
Press.
Jia, J.-B., Wang, W.-Q., Sun, H.-C., Zhu, X.-D., Liu,
L., Zhuang, P.-Y., Tang, Z.-Y. (2010). High
Expression of Macrophage Colony-Stimulating
Factor-1 Receptor in Peritumoral Liver Tissue
Is Associated with Poor Outcome in
Hepatocellular Carcinoma After Curative
Resection. The Oncologist, 15(7), 732–743.
https://doi.org/10.1634/theoncologist.2009-0170
Kuno, G., Chang, G. J., Tsuchiya, K. R., &
Karabatsos, N. (2014). Phylogeny of the Genus
Flavivirus Phylogeny of the Genus Flavivirus,
(February 1998).
Lambeth, C. R., White, L. J., Johnston, R. E., &
Silva, A. M. De. (2005). Flow Cytometry-Based
Assay for Titrating Dengue Virus, 43(7), 3267–
3272. https://doi.org/10.1128/JCM.43.7.3267
Lindenbach, B., & Rice, C. (2003). Molecular
biology of flaviviruses. Adv. Virus Res, 59, 23–
61.
Sasmono, R. T., & Hume, D. A. (2004). The Biology
of Macrophages in the Innate Immune Response
to Infection. Washington DC: ASM press.
Sun, P., & Kochel, T. J. (2013). The Battle between
Infection and Host Immune Responses of
Dengue Virus and Its Implication in Dengue
Disease Pathogenesis, 2013.
Www.ReaderFit.com. (n.d.). MasterPlex QT
readerfit for four parameters logistic (4PL) and
five parameters logistic (5PL) nonlinear
regression models with many options.
Yohan, B., Kendarsari, R. I., Mutia, K.,
Bowolaksono, A., & Harahap, A. R. (2014).
Growth characteristics and cytokine /
chemokine induction profiles of dengue viruses
in various cell lines, 36, 20–27.
https://doi.org/10.4149/av
SKIC-MHS 2018 - The 2nd Syiah Kuala International Conference on Medicine and Health Sciences
48
