
The Effects of Phaleria macrocarpa (Scheff.) Boerl Extract on Tumor
Necrosis Factor-Alpha (TNF-α) Level in Preeclampsia-induced
Human Umbilical Vein Endothelial Cell (HUVEC) Culture
Leo Simanjuntak
1,2*
, M. Fidel Ganis Siregar
3
, Johannes C. Mose
4
and Sarma N. Lumbanraja
3
1
Doctoral Program, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia
2
Department of Obstetrics and Gynecology, Faculty of Medicine, Nommensen HKBP University,
Medan, Indonesia
3
Department of Obstetrics and Gynecology, Faculty of Medicine, Universitas Sumatera Utara,
Medan, Indonesia
4
Department of Obstetrics and Gynecology, Faculty of Medicine, Universitas Padjadjaran,
Bandung, Indonesia
Keywords : Phaleria Macrocarpa, Preeclampsia, HUVEC, TNF-Α
Abstract : Preeclampsia is a major cause in both maternal and perinatal mortality and morbidity. The
etiopathogenesis of preeclampsia is still not fully elucidated but it is believed to be a multifactor.
Endothelial dysfunction plays a big role in the pathophysiology of preeclampsia. Tumor Necrosis Factor-α
(TNF-α) is considered as one of the potentially specific markers for preeclampsia. In vitro model research is
considered the best and most effective way to understand the disease pathophysiology. HUVEC (Human
Umbilical Vein Endothelial Cell) culture is an in vitro model widely used to study the pathogenesis of
preeclampsia. Phaleria macrocarpa (Scheff.) Boerl also known as Mahkota Dewa is widely used as an anti-
inflammation and antioxidant because of alkaloids, saponins, flavonoids and polyphenols properties. This
study aimed to determine the effects of Phaleria macrocarpa Extract on Tumor Necrosis Factor – Alpha
(TNF- α) Level In preeclampsia-induced Human Umbilical Vein Endothelial Cell (HUVEC). Our results
showed the Phaleria macrocarpa’s extract reduce TNF-α level siginifcantly at concentration of 7.813
μg/mL. Phaleria macrocarpa’s extract at concentration of 62.5 μg/mL reduce TNF-α level to normal level.
Thus, Phaleria macrocarpa’s extract might be used as agent to overcome endothelial dysfunction in
preeclampsia.
1. INTRODUCTION
Preeclampsia is one of the leading causes of
maternal morbidity and mortality worldwide. It is
estimated that maternal deaths worldwide are around
500,000 annually and about 10% - 15% are due to
preeclampsia and eclampsia (Maynard et al. 2008).
In 2006 WHO reported that 16% of maternal deaths
in developed countries due to hypertension in
pregnancy, higher than due to bleeding of 13%,
abortion of 8% and sepsis of 2% (Cunningham et al.
2014).
Preeclampsia and eclampsia also adversely affect
the fetus and the neonates. It is estimated that 15%
of preterm births due to preeclampsia, where labor
has to be performed to prevent the progression of
preeclampsia (Roberts & Gammill 2005).
Although there have been many studies but the
etiopathogenesis of preeclampsia is still not fully
elucidated but it is believed to be a multifactors.
Therefore preeclampsia remains a 'disease of
theories'. The difficulties increase because the
syndrome of preeclampsia usually occurs in the third
trimester when the underlying disorder has occurred
10
Simanjuntak, L., Siregar, M., Mose, J. and Lumbanraja, S.
The Effects of Phaleria macrocarpa (Scheff.) Boerl Extract on Tumor Necrosis Factor-Alpha (TNF-) Level in Preeclampsia-induced Human Umbilical Vein Endothelial Cell (HUVEC) Culture.
DOI: 10.5220/0008789900100016
In Proceedings of the 2nd Syiah Kuala International Conference on Medicine and Health Sciences (SKIC-MHS 2018), pages 10-16
ISBN: 978-989-758-438-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

in the early stages of placentation, thus difficult to
understand its progression (Cross 1996).
Endothelial dysfunction plays an important role
in the pathophysiology of preeclampsia. Endothelial
dysfunction is defined as an altered state of
endothelial cell differentiation in response to
sublethal injury or cytokine stimulation (Hubel
1999). Under normal circumstances, endothelial
cells maintain vascular integrity, regulating blood
pressure, preventing intravascular coagulation, and
regulating vascular smooth muscle tone by
producing various substances including nitric oxide
(NO), endothelin, prostacyclin and thromboxane
(Davidge 1998; Scalera , Schlembach & Beinder
2001). Rodgers et al. (1988) suggests that
endothelial dysfunction occurs due to cytotoxic
factors in the circulation. Impaired utero-placental
perfusion in PE causes hypoxia, ischemia, placental
oxidative stress, so the placenta produces free
radicals such as superoxide anions (O
2
-
) and H
2
O
2
,
trophoblast debris, pro-inflammatory cytokines, and
antiangiogenic factors which are thought to cause
vascular endothelial dysfunction and causing
excessive maternal inflammatory responses.
Systemic maternal vascular endothelial dysfunction
is the underlying cause of clinical manifestations in
preeclampsia (Roberts et al. 1989).
Tumor Necrosis Factor-α (TNF-α) is considered
as one of the potentially specific markers for
preeclampsia and contributes to the formation of free
radicals such as peroxides (H
2
O
2
), and superoxide
(O
2
-
) (Amash et al. 2010). In endothelial cells TNF-α
causes endothelial dysfunction by increasing
oxidation of low-density lipoprotein (LDL)
(Maziere, Auclair & Maizere 1994), inhibiting
eNOS enzymes causing NO levels decrease (Zhang
et al. 2009) and increasing free radical production by
xanthine oxidase enzyme, then binds to endothelial
cells and produces an O
2
-
anion in endothelial cells
(Page et al., 1997).
Wang & Walsh (1996) found that TNF-α levels
in placenta preeclampsia were significantly higher
than normal pregnancies (17.32 ± 1.97 pg / mg
protein vs 11.62 ± 0.93 pg / mg protein) with ELISA
method and allegedly TNF-α increases oxidative
stress by stimulating the formation of ROS. Hayashi
et al. (2005) found that TNF-α level in preeclampsia
serum is significantly higher than in the serum of
normal pregnancies (4.68 pg/mL vs 3.31 pg/mL).
Zuspan (1978) suggested that PE treatment will
only be successful and rational if based on
understanding the disease pathophysiology. In an
attempt to determine the pathophysiology of a
disease, in vitro model research is considered the
best and most effective way (Orendi et al. 2011).
HUVEC (Human Umbilical Vein Endothelial Cell)
cell line culture and trophoblast cell line is an in
vitro model widely used to study the pathogenesis of
preeclampsia.
The preventions of preeclampsia consist of
primary, secondary, and tertiary prevention. Primary
prevention aims to prevent the onset of disease by
avoiding pregnancy with contraception because the
pathogenesis of preeclampsia remains unclear.
Secondary prevention aims to inhibit the disease
progression before the onset of clinical
manifestations. Tertiary prevention aims to prevent
the complications of a disease, in preeclampsia, the
complications such as seizures, HELLP syndrome,
and IUGR, which tertiary prevention can be
interpreted as treatment Tertiary prevention includes
regular antenatal examination, appropriate referral,
anti-hypertensive administration, anti-convulsant
administration, and appropriate timing of delivery
(Dekker & Sibai 2001)..
The use of traditional medicines in Indonesia is
part of a culture that has been going on since long
time ago. Act No. 381 of 2007 on national
traditional drug policy regulates the development of
traditional medicines in order to obtain good quality,
safe, and scientifically tested traditional medicine
(Departemen Kesehatan RI 2007).
Herbs or medicinal plants have been used
traditionally as alternative medicine since ancient
times. Phaleria macrocarpa (Scheff.) Boerl also
known as Mahkota dewa belongs to the
Thymelaceae family, that originated from Papua
province, is very popular in Indonesia used in the
treatment of various diseases such as cancer,
hemorrhoids, diabetes mellitus, allergies, liver
disease, heart disease, kidney disease, hypertension,
migraine, skin diseases and others (Hendra et al.
2011; Anggraini & Lewandowsky 2015; Alara,
Alara & Olalere 2016). Phaleria macrocarpa
(Scheff.) Boerl is widely used as an antioxidant
because of alkaloids, saponins, flavonoids and
polyphenols properties. The phenol and flavonoid
compounds in the extract of Phaleria macrocarpa
have antioxidant and anti-inflammatory activity
(Tiwari 2001; Hendra et al. 2011).
To date there has been no research on the effect
of the Phaleria macrocarpa’s extract on the levels of
TNF-α in preeclampsia-induced Human Umbilical
Vein Endothelial Cell (HUVEC). The aim of this
study is to determine the tffects of Phaleria
macrocarpa (Scheff.) Boerl Extract on Tumor
Necrosis Factor – Alpha (TNF- α) Level In
The Effects of Phaleria macrocarpa (Scheff.) Boerl Extract on Tumor Necrosis Factor-Alpha (TNF-) Level in Preeclampsia-induced Human
Umbilical Vein Endothelial Cell (HUVEC) Culture
11
preeclampsia-induced Human Umbilical Vein
Endothelial Cell (HUVEC).
2. MATERIALS AND METHOD
Serum samples used were obtained from women
at >20 - 42 weeks of gestational age, which were
diagnosed preeclampsia and normal pregnancy at
Dr. Hasan Sadikin General Hospital. Research
subjects have fulfilled inclusion and exclusion
criteria.
2.1. Cell Culture
HUVEC cell line was obtained from American
Type Collection Culture with ATCC CRL-1730
code number. HUVEC cell line was growth into
tissue culture flask (25 cm
2
) containing RPMI 1640
media, 20% (v/v) FBS qualified (fetal bovine serum)
supplementation, 10% endothelial supplement, 1%
Penicillin G - Streptomycin solution stabilized, and
1% antimycotic Fungizone Amphotericin B and 1%
gentamicin. The cells were then incubated at 37
O
C
and 5% CO
2
(v/v). Culture medium is replaced every
2 - 3 days. Then cells are passaged every seven days
until reach 80-90% confluence.
2.2. Phaleria macrocarpa’s Extract
Phaleria macrocarpa (Scheff.) Boerl was
obtained from the Research Institute for Industrial
Plants at Manoko, Lembang, West Java, Indonesia.
The plant species was identified by the laboratory of
Plant Taxonomy staff at Herbarium Bogoriense,
Bogor, Indonesia.
2.3. Measurement of TNF- α Level
As many as 6x10
5
cells/mL induced with normal
and preeclampsia serum, were placed into 60-well
plate, then incubated at 37
O
C and 5% CO
2
(v/v).
Each well then was washed with 37
O
C PBS 3-4
times. Furthermore, various concentrations of
Phaleria macrocarpa’s extract ((0,977; 1,953; 3,906;
7,813; 15,625; 31,25; 62,5; 125; and 250 μg/mL)
were added into each well, then incubated for 24 and
72 hours 37
O
C and 5% CO
2
(v/v). Each well then
was washed with 37
O
C once for five minutes.
Transfer the cells into centrifugation tube using 1.5
mL pipette. Centrifuged at 1.500 rpm for 10 minutes
at 4°C. Use the supernatant as a sample for the
ELISA method measurement, then the rest of the
sample can be stored at -80 °C.
2.4. Data Analysis
Data distribution was analyzed with Shapiro-
Wilk normality test. Data was analyzed with
repeated ANOVA (analysis of variance) test and
followed by Bonferroni test as post hoc comparison
test.
3. RESULTS
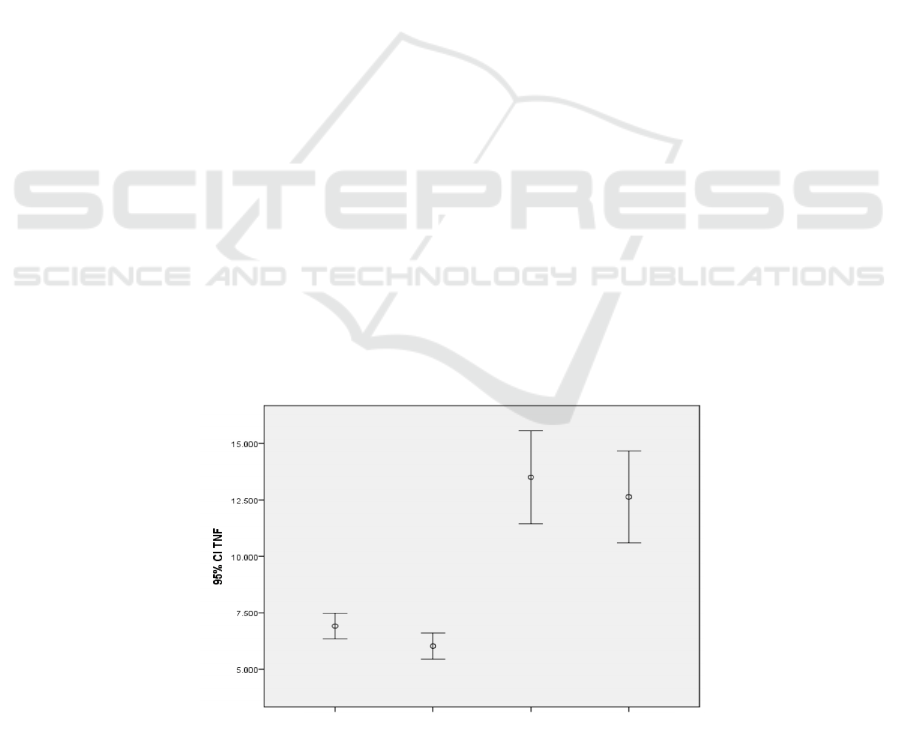
Figure 1. TNF-α levels in normal and preeclampsia-induced HUVEC based on incubation time.
NORMAL 24H NORMAL 72H PE 24H PE 72H
SAMPLE
SKIC-MHS 2018 - The 2nd Syiah Kuala International Conference on Medicine and Health Sciences
12
As shown in figure 1 TNF-α levels in
preeclampsia HUVEC culture model is higher than
normal pregnancy HUVEC culture model. The
TNF-α levels at 72 hours incubation time was lower
than the 24 hours incubation time in both normal and
preeclampsia models.
Variables tested in this study were normally
distributed both in normal and preeclampsia model
treated with Phaleria macrocarpa’s extract in various
concentrations incubated for 24 and 72 hours.
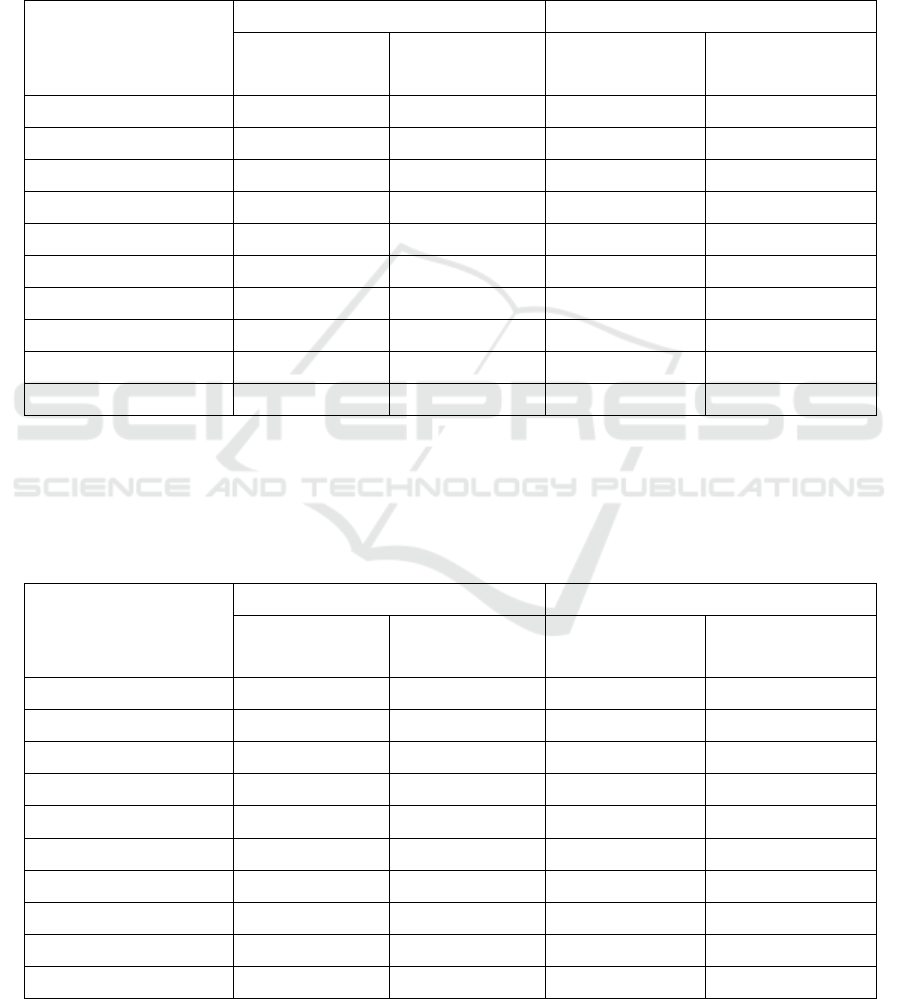
Table 1. TNF-α levels (pg/mL) in preeclampsia and normal serum-induced HUVEC culture model treated with Phaleria
macrocarpa’s extract in various concentrations incubated for 24 and 72 hours.
Phaleria macrocarpa’s
extract concentration
(μg/mL)
24 H INCUBATION TIME 72 H INCUBATION TIME
NP* (Mean ± SD) PE (Mean ± SD) NP* (Mean ± SD) PE (Mean ± SD)
Control
8.718 ± 0.043 18.709 ± 0.007 7.858 ± 0.029 17.848 ± 0.035
0.977
8.218 ± 0.003 18.273 ± 0.051 7.445 ± 0.015 17.395 ± 0.007
1.953
7.886 ± 0.005 17.888 ± 0.001 6.987 ± 0.006 16.778 ± 0.015
3.906
7.382 ± 0.071 15.295 ± 0.087 6.335 ± 0.02 14.666 ± 0.312
7.813
6.814 ± 0.007 14.533 ± 0.000 5.950 ± 0.057 13.763 ± 0.000
15.625
6.009 ± 0.003 12.778 ± 0.000 5.103 ± 0.007 11.492 ± 0.708
31.25
6.000 ± 0.000 10.089 ± 0.000 5.077 ± 0.014 9.325 ± 0.000
62.5
5.994 ± 0.006 8.578 ± 0.000 5.009 ± 0.000 7.668 ± 0.001
125
5.455 ± 0.001 7.654 ± 0.000 4.662 ± 0.000 6.834 ± 0.001
250
5.003 ± 0.000 6.089 ± 0.000 4.101 ± 0.001 5.404 ± 0.000
NP : Normal Pregnancy
Table 1. shows TNF-α levels mean in difference
preeclampsia and normal serum-induced HUVEC
culture model treated with Phaleria macrocarpa’s
extract in various concentrations incubated for 24
and 72 hours.
Table 2. TNF-α levels (pg/mL) mean comparison before and after various concentrations of Phaleria macrocarpa’s extract
treatment at 24 hours and 72 hours incubation time in preeclampsia HUVEC culture model.
Phaleria macrocarpa’s
extract concentration
(μg/mL)
24 H INCUBATION TIME 72 H INCUBATION TIME
PE (Mean ± SD) P value* PE (Mean ± SD) P value*
Control 18.709 ± 0.007 17.848 ± 0.035
0.977 18.273 ± 0.051 1.000 17.395 ± 0.007 1.000
1.953 17.888 ± 0.001 0.227 16.778 ± 0.015 0.362
3.906 15.295 ± 0.087 0.470 14.666 ± 0.312 1.000
7.813 14.533 ± 0.000 0.034 13.763 ± 0.000 0.175
15.625 12.778 ± 0.000 0.024 11.492 ± 0.708 1.000
31.25 10.089 ± 0.000 0.017 9.325 ± 0.000 0.082
62.5 8.578 ± 0.000 0.014 7.668 ± 0.001 0.070
125 7.654 ± 0.000 0.013 6.834 ± 0.001 0.065
250 6.089 ± 0.000 0.012 5.404 ± 0.000 0.058
* : statistically significant if p < 0.05
The Effects of Phaleria macrocarpa (Scheff.) Boerl Extract on Tumor Necrosis Factor-Alpha (TNF-) Level in Preeclampsia-induced Human
Umbilical Vein Endothelial Cell (HUVEC) Culture
13
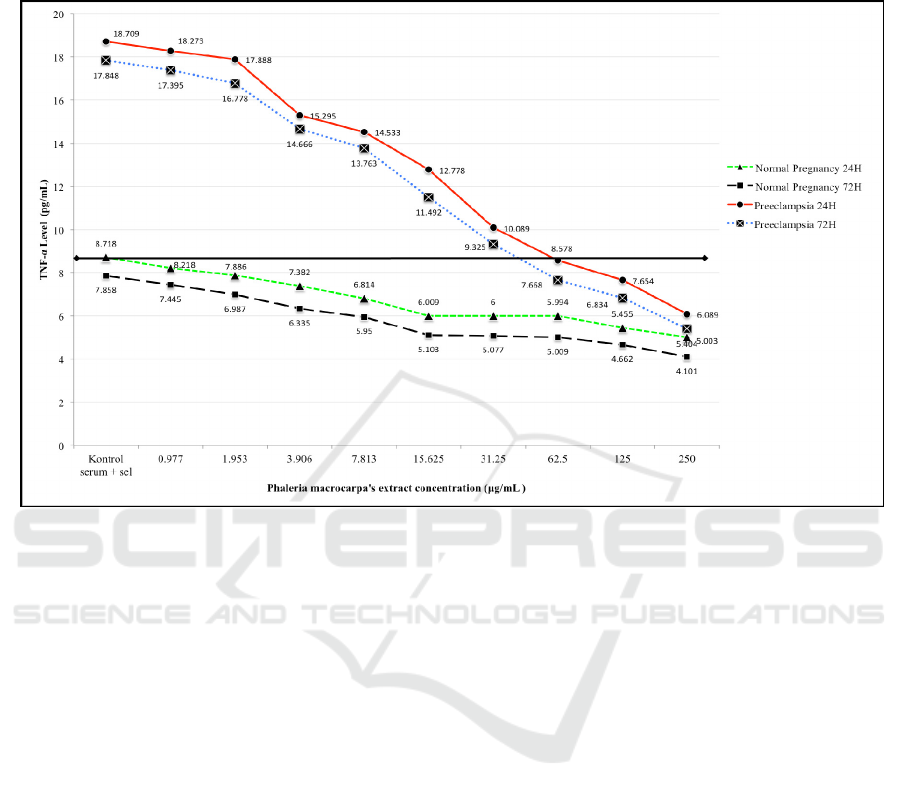
Table 2 shows TNF-α level decreased in
preeclampsia serum-induced HUVEC ATCC CRL
1730 following increased Phaleria macrocarpa’s
extract concentration. TNF-α level significantly
decreased after exposure of Phaleria macrocarpa’s
extract on concentration 7.813 μg/mL. (p<0,05).
Figure 2. TNF-α levels in relation with Phaleria macrocarpa’s extract concentration
Figure 2 shows that Phaleria macrocarpa’s
extract at concentration of 62.5 μg/mL reduce
TNF-α level in preeclampsia model to normal
pregnancy level.
4. DISCUSSION
This were the first study to evaluate the effects of
Phaleria macrocarpa (Scheff.) Boerl extract on
Tumor Necrosis Factor – Alpha (TNF- α) level in
Preeclampsia-Induced Human Umbilical Vein
Endothelial Cell (HUVEC). Preeclampsia and
eclampsia have been known since ancient times but
their pathophysiology is still not clearly understood.
Abnormal trophoblast invasion and placental
perfusion disorders are thought to be the underlying
cause of preeclampsia.
There is compelling evidence that endothelial
dysfunction plays a role in the pathophysiology of
preeclampsia. A consistent finding is the presence of
glomerular endotheliosis in more than 70% of
primiparous preeclampsia patients and this
glomerular endotheliosis will disappear after
delivery (Roberts et al., 1989).
To date, invitro research using HUVEC has been
done a lot recently. Previous invitro research on
HUVEC cultures by treating with anti-inflammatory
and antioxidant compounds such as curcumin and
Papua ant nest (Myrmecodia pendens) decrease
oxidative stress and inflammation characterized by
decreased levels of MDA, and TNF-α. These studies
conclude that the Papuan ant nests and curcumin
have a therapeutic effect on preeclampsia (Yeni et
al., 2017, Gunardi et al., 2016).
Tumor Necrosis Factor-α (TNF-α) is considered
as one of the potentially specific markers for
preeclampsia and contributes to the formation of free
radicals such as peroxides (H
2
O
2
), and superoxide
SKIC-MHS 2018 - The 2nd Syiah Kuala International Conference on Medicine and Health Sciences
14

(O
2
-
) (Amash et al. 2010). The study by Yoshizumi
et al. (1993) showed TNF-α decrease NOS mRNA
levels in HUVEC, indicating a decrease in NO levels
as vasodilators.
In this study results showed TNF- α levels in
preeclampsia HUVEC culture model was higher
than normal pregnancy HUVEC culture model.
TNF-α level decreased in preeclampsia and normal
serum-induced HUVEC ATCC CRL 1730 culture
following increased Phaleria macrocarpa’s extract
concentration. TNF-α level significantly decreased
after exposure of Phaleria macrocarpa’s extract on
concentration 7.813 μg/mL. Phaleria macrocarpa’s
extract at concentration of 62.5 μg/mL reduce TNF-
α level to normal level.
The result of present study suggests that Phaleria
macrocarpa’s extract contains anti-inflammatory
activity proven by decreased level of TNF-α. It was
also described that TNF-α level decreased in
preeclampsia and normal serum-induced HUVEC
ATCC CRL 1730 following increased Phaleria
macrocarpa’s extract concentration. Thus, Phaleria
macrocarpa’s extract might be used as agent to
restore endothelial dysfunction in preeclamsia.
5. CONCLUSION
The Phaleria macrocarpa’s extract reduce
TNF-α level siginificantly at concentration of 7.813
μg/mL in preeclampsia-induced HUVEC ATCC
CRL 1730 culture. Phaleria macrocarpa’s extract at
concentration of 62.5 μg/mL reduce TNF-α level to
normal level.
ACKNOWLEDGEMENT
The authors whose names are listed above certify
that they have no affiliations with or involvement in
any organizations or entity with any financial
interests in the subject matter or materials discussed
in this manuscript.
REFERENCES
Alara OR, Alara JA & Olalere OA 2016, Review on
Phaleria macrocarpa Pharmacological and
Phytochemical Properties, Drug Designing, Open
Access.
Amash, A, Holcberg, G, Sheiner, E & Huleihel, M 2010,
Magnesium Sulfate Normalizes Placental Interleukin-6
Secretion in Preeclampsia, Journal of Interferon and
Cytokine Research 30 , 9 , 683 – 690.
Anggraini T & Lewandowsky P 2015, The exotic plants of
Indonesia: Mahkota Dewa (Phaleria macrocarpa),
Sikaduduak (Melastoma malabathricum Linn) and
Mengkudu (Morinda citrifolia) as potent antioxidant
sources, Int. J. Adv. Sci. Eng. Inf. Technol, 5, 59-62.
Cross JC 1996, Trophoblast function in normal and
preeclamptic pregnancy, Fetal and Maternal Medicine
Review, 8, 57-66
Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Rouse
DJ & Spong CY 2014: Williams Obstetrics 24th. ed.
McGraw Hill Medical pp. 728 – 779.
Davidge, ST 1998, Oxidative Stress and Altered
Endothelial cell Function in Preeclampsia, Seminars in
Reproductive Endocrinology, 16, No.1.
Dekker G & Sibai BM 2001, Primary, secondary and
tertiary prevention of preeclampsia, Lancet, 357, 209-
15.
Departemen Kesehatan Republik Indonesia 2007,
Kebijakan obat tradisional nasional.
DePass LR, Ballantyne B, Fowler EH & Weil CS 1989,
Dermal Oncogenicity Studies on Two Methoxysilanes
and Two Ethoxysilanes in Male C3H Mice.
Fundamental And Applied Toxicology, 12, pp. 579-
583
Gunardi, JI, Mose, J, Satari, MH, Anwar, AD, Fauziah, PF
& Triyuli 2016, Effects of Papua Ant Nests
(Myrmecodia pendens) on Level of sFlt-2, PlGF,
MDA, and NO in Preeclampsia-induced HUVEC Cell
Line, 6, 424-435
Hayashi, M, Ueda, Y, Yamaguchi, T, Sohma, R,
Shibazaki, M, Ohkura, T et al. 2005, Tumor Necrosis
Factor – α in the Placenta is not Elevated in Pre-
eclamptic Patients Despite its Elevation in Peripheral
Blood, AJRI, 53: 113-119.
Hendra R, Ahmad S, Oskoueian E, Sukari A & Shukor
MY 2011, Antioxidant, Anti-inflammatory and
Cytotoxicity of Phaleria Macrocarpa (Boerl.) Scheff
Fruit, BMC Complementary and Alternative Medicine,
11, 110.
Hubel, CA 1999, Oxidative Stress in the Pathogenesis of
Preeclampsia, Proc Soc Exp Biol Med, 222: 222-235.
Maynard S, Epstein FH & Karumanchi SA 2008,
Preeclampsia and Angiogenic Imbalance, Annu.Rev.
Med, 59, pp. 61-78
Maziere, C, Auclair, M & Maziere, JC, 1994, Tumor
necrosis factor enhances low density lipoprotein
oxidative modification by monocyte and endothelial
cells, FEBS Lett, 338:43-46.
Orendi K, Kivity V, Sammar M, Grimpel Y, Gonen R,
Meiri H et al. 2011, Placental and trophoblastic in
The Effects of Phaleria macrocarpa (Scheff.) Boerl Extract on Tumor Necrosis Factor-Alpha (TNF-) Level in Preeclampsia-induced Human
Umbilical Vein Endothelial Cell (HUVEC) Culture
15

vitro models to study preventive and therapeutic
agents for preeclampsia, Placenta, 32, pp. 549 – 554
Page, S, Benboubetra, M, Blake, D et al. 1997, Cytokine-
induced activation of xanthine oxidase in human
mammary epithelial cells, Biochim Soc Trans, 25:
95S.
Roberts, JM, Taylor, RN, Musci, TJ, Rodgers, GM,
Hubel,CA & McLaughlin, MK 1989, Preeclampsia:
An endothelial cell disorder, Am J Obstet Gynecol,
161, 1200-4.
Roberts JM & Gammill HS 2005, Preeclampsia Recent
Insights, Hypertension 46:1243-1249.
Rodgers, GM, Taylor, RN & Roberts, JM 1988,
Preeclampsia is associated with a serum factor
cytotoxic to human endothelial cells, Am J Obstet
Gynecol, 159, 908-14.
Scalera, F, Schlembach, D & Beinder, E 2001, Production
of vasoactive substances by human umbilical vein
endothelial cells after incubation with serum from
preeclamptic patients, European Journal of Obstetrics
& Gynecology and Reproductive Biology, 99, 172-
178.
Tiwari P, Kumar B, Kaur M, Kaur G, & Kaur H 2011,
Phytochemical screening and extraction: a review,
Internationale Pharmaceutica Sciencia, 1, No. 1, pp.
98- 106
Wang, Y & Walsh, SW 1996, TNF-α concentration and
mRNA expression are increased in preeclamptic
placentac. J Reprod Immunol 32: 157 – 169.
Yeni, CM, Fauziah, PN, Maskoen, AM, Ruslami, M &
Mose, JC 2017, Effect of Curcumin in Decreasing
MDA Level in Preeclampsia-induced Human
Umbilical Vein Endothelial Cells (HUVEC)
International Journal of Pharm Tech Research, vol. 10,
No. 02, pp. 69-72.
Yoshizumi, M, Perrella, MA, Burnett, JC & Lee, ME,
1993, Tumor necrosis factor downregulates an
endothelial nitric oxide synthase mRNA by shortening
its half-life, Circ. Res, 73(1): 205-9
Zhang, H, Park, Y, Xu, J, Chen, XP, Lee, S, Yang, J,
Dellsperger, KC et al. 2009, Role of TNF-α in
vascular dysfunction, Clinical Science, 116, pp. 219-
230.
Zuspan FP 1978, Problems encountered in the treatment of
pregnancy induced hypertension. A point of view. Am
J Obstet; 131: 591
SKIC-MHS 2018 - The 2nd Syiah Kuala International Conference on Medicine and Health Sciences
16
