
Protective Effect of Neem (Azadirachta Indica) Leaf Extract on Liver
of Trypanosoma evansi Infected Rats (Rattus norvegicus)
Yudha Fahrimal
1
, Dwina Aliza
2
, Ayu Dwi Fitriani
1
, Erina
3
, Al Azhar
4*
1
Laboratory of Parasitology, Veterinary Faculty, Syiah Kuala University, Banda Aceh, Indonesia
2
Laboratory of Pathology, Veterinary Faculty, Syiah Kuala University, Banda Aceh, Indonesia
3
Laboratory of Microbiology, Veterinary Faculty, Syiah Kuala University, Banda Aceh, Indonesia
4
Laboratory of Biochemistry, Veterinary Faculty, Syiah Kuala University, Banda Aceh, Indonesia
Key word: Rat liver, Trypanosoma evansi, Azadirachta indica, anti-trypanosomal drugs.
Abstract: Animal trypanosomiasis is still a devastating disease of both domestic and wild animals around the
world. Control of surra is greatly depending on chemotherapy. The aim of this research was to observe
histological changes in the liver of T.evansi infected rats treated with different concentrations of neem
(Azadirachta indica) leaf extract. Samples used were livers collected from 24 male Wistar rats
randomly divided into 6 treatment groups with 4 replications each. Negative control (K
0
) was rats given
distilled water only; positive control (K1) was rats infected with T.evansi 5x10
4
; K2, K3, K4, and K5
were rats infected with T. evansi 5x10
4
and administered with neem leaf extract 50, 100, 400, and 800
mg/kg body weight (BW) per oral for 3 consecutive days, respectively. On day 4, all rats were
sacrificed by ether euthanation for liver collection. Livers obtained were histopathologically processed
using standard hematoxylin-eosin staining and microscopically observed. Histological changes in the
liver of rats in all treatment groups were as the following. Normal hepatocytes were K
0
63.65, K
1
27.25,
K
2
15.15, K
3
12.90, K
4
20.30, and K
5
24.85.Haemorrhagic hepatocytes were K
0
0.00, K
1
15.60, K
2
10.60, K
3
17.75, K
4
6.30 and K
5
9.05. Hyperemic hepatocytes were K
0
0.00, K
1
2.35, K
2
3.70, K
3
3.25,
K
4
2.25 and K
5
2.25. Infiltration of inflamation cells were K
0
0.00, K
1
2.75, K
2
3.90, K
3
14.65, K
4
2.55,
and K5 2.70. Hepatocyte degeneration was K
0
0.00, K
1
6.30, K
2
6.95, K
3
10.15, K
4
4.15, and and K
5
1.00. Necrotic hepatocytes were K
0
1.55, K
1
32.20, K
2
43.80, K
3
45.00, K
4
31.25, and K
5
34.20. Neem
leaf extract at the dose of 800 mg/kg BW was the best in preventing liver damages caused by T. evansi
infection in rats.
1. INTRODUCTION
Surra is caused by T. evansi and still becomes a
problem for animal health (Luckins, 1996). Bad
impacts caused by T. evansi in infected animals
include reduced weight, low reproduction,
immunosupression and mortality. According to
Damayanti et al. (1994), in buffalo T. evansi attacks
many organs such as brain, kidney, spleen, pulmo,
and liver. T. evansi infection causes necrosis of
hepatocytes in the Kiernan triangle, sentro-
perilobulary lipid degeneration, enlarged sinusoid,
and infiltration of polymorphonuclear cells (PMN)
around centralis vein (Lazuardi, 2008).
Control of trypanosomiasisis dependent upon
synthetic medicines such as suramin, diminazene,
isomedium, quinapyramine, and cymelarsan. Several
T. evansi strains resistant to antitrypanosomal drugs
are reported in Vietnam (Stevenson et al., 2000).
Most T. evansi isolates stored at the Tissue Culture
Collection of Veterinary Research Central Agency
of Bogor are resistant to isometamedium and
diminazen aceturate (Sukanto et al., 1988).
Therefore, it is necessary to search for new medicine
to anticipate the resistance of T. evansi isolates to
the currently available antitrypanosoma. The use of
plants extract containing phytochemicals have a
variety of beneficial biologic effects could provide
alternatives.
One of plants extensively investigated its
medicinal benefits is neem (Azadirachta indica).
This plant has been known by people as a medicinal
plant that has broad spectrum biological activities
such as antipyretic, analgesic, antifungal, mosquito
repellant, antiinflamasion, antiparasite, antiinsect,
and larvasidal as well as anticancer, antoeczema, and
antimalaria (Biswas et al., 2002; Wahyuningsih et
al., 2002).
According to Syarmalina and Laksmitawati
(2005), phytochemical contents of neem leaves
172
Fahrimal, Y., Aliza, D., Fitriani, A., Erina, . and Azhar, A.
Protective Effect of Neem (Azadirachta Indica) Leaf Extract on Liver of Trypanosoma evansi Infected Rats (Rattus norvegicus).
DOI: 10.5220/0008788401720177
In Proceedings of the 2nd Syiah Kuala International Conference on Medicine and Health Sciences (SKIC-MHS 2018), pages 172-177
ISBN: 978-989-758-438-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
include azadirachtin, paraisin, alkaloid, and volatile
oil containing sulfides. Neem leaves also contain 4
natural compounds that have pesticidal properties
namely azadichtin, salanin, meliatriol, and nimbin
(Subiyakto, 2009).
In the previous article we have showed that
neem leaves extract 800 mg/bw inhibit the growth of
T. evansi upto 80.5% in rats (Rattus norvegicus)
(Fahrimal et al. 2017). Effect of neem leaf extract
administration on the liver of T. evansi infected rats
is presented this article.
2. MATERIALS AND METHODS
Trypanosoma evansi isolates used were the
collection of the Parasitological Laboratory of
Veterinary Faculty of Syiah Kuala University.
Experimental animals used were 24 rats randomly
assigned into 6 treatment groups. Negative control
(K0) and positive control (K1) were consecutively
rats given aquadest and intraperioneally infected
with T. evansi 5x10
4
. Group K2, K3, K4, and K5
were rats infected with T. evansi 5x10
4
and
administrated with neem leaf extract of 50, 100, 400,
and 800 mg/kgBW per oral for 3 days, respectively.
On day 4 all rats were sacrified by ether euthanation
for liver collection. Livers were put in 10% NBF
solution (pH 6.5-7.5) and subjected for standard
histopathological preparation using haematoxylin
and eosin staining. The preparates were
microscopically observed using a binocular
microscope (Olympus CX21, Japan) with 400 and
1000 magnification and documented using a photo
microscope (Olympus BX41, Japan). Data obtained
were analyzed by ANOVA and Duncan test.
3. RESULTS AND DISCUSSION
Microscopic observation indicated that liver of T.
evansi infected rats administrated with neem leaf
extract ranged from 50-800 mg/kgBW showed
haemorrhage, hyperemia, inflammation cells
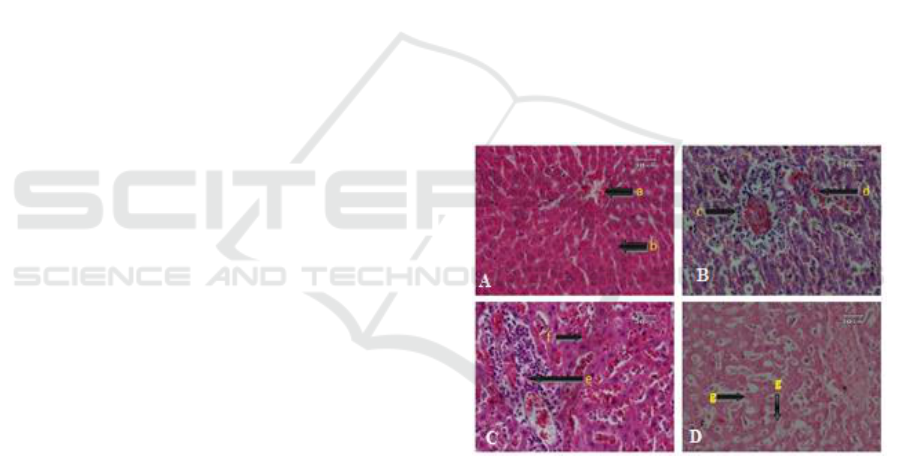
infiltration, degeneration, and necrosis (Figure 1).
Results of statistical analysis of the observed
histological parameters using Duncan test are
presented in Table1.
Figure 1. Histopatological profile of livers of
control (A) and T. evansi infected (B-D) rats
treated with with neem leaf extracts. a. Centralis
vein, b. Normal hepatocytes c. Hyperemia, d.
Haemorrhage, e. leukocyte infiltration, f.
degeneration, and g. necrosis (HE, 400x).
Protective Effect of Neem (Azadirachta Indica) Leaf Extract on Liver of Trypanosoma evansi Infected Rats (Rattus norvegicus)
173
Table 1. The changes in the livers of T. evansi infected rats treated with neem leaf extracts
Treatment
Parasitemia
inhibition
(%)
*
Normal
Cell
Haemorrhage
Hyperemia
Leukocyte
Infiltration
Degeneration
Necros
is
K0
0
63.65 9.59
a
0.00 0.00
a
0.00 0.00
a
0.00 0.00
a
0.00 0.00
a
1.55
0.82
a
K1
0
27.25 7.32
b
9.30 7.81
bc
2.35 1.57
b
2.75 2.17
b
6.30 1.64
b
32.30
10.47
b
K2
14.64
15.15
4.87
bc
10.60 3.82
b
3.70 1.55
b
3.90 0.99
b
6.95 0.25
b
43.80
12.93
b
K3
23.78
12.90 4.26
c
17.75 2.36
c
3,15 0.57
b
4.75 0.47
b
10.15 1.60
c
45.00
10.35
b
K4
58.68
21.25 2.45
b
6.30 5.41
b
2,25 0.68
b
2.55 0.41
b
1.070.50
d
31.25
10.89
b
K5
80.50
20.00 1.36
b
9.05 3.10
b
2,55 1.28
b
2.70 0.99
b
1.00 0.71
d
32,55
3.59
b
*
Fahrimal et al. (2017)
Note: Different notation a, b, c, and d shows significant difference (p<0.05) between treatment groups
K0: negative control (rats given aquadest)
K1: positive control (rats infected by T. evansi)
K2: T. evansi infected rats given neem leaf extract 50 mg/kgBW
K3: T. evansi infected rats given neem leaf extract 100 mg/kgBW
K4: T. evansi infected rats given neem leaf extract 400 mg/kgBW
K5: T. evansi infected rats given neem leaf extract 800 mg/kgBW
Data in Table 1 shows that in addition to had no
hemorrhage, hyperemia and leukocyte infiltration,
the rats in the K0 (negative control) had the highest
numbers of normal hepatocytes and the lowest
necrosis compared to T. evansi infected rats, either
untreated (positive control K1) or treated with
different doses of neem leaves extracts (K2, K3, K4
and K5). Infection of T. evansi (K1) significantly
reduced the numbers of normal hepatocytes and
resulted in moderate hemorrhage, hyperemia,
leukocyte infiltration, lipid degeneration and
necrosis. The administration of neem leaves extract
ranged from 50-100 mg/kgBW could not protect
liver of rats from negative effects of T. evansi
infection, as shown by increased degree of
hemoraghe, hyperemia, leukocyte infiltration, lipid
degeneration and necrosis. Neem leaves extracts 400
and 800 mg/kg BMW, on the other hands, had
protective effects on the liver of T. evansi infected
rats, as indicated by higher numbers of normal
hepatocytes, reduced hemoraghe, hyperemia,
leukocyte infiltration and necrosis. These findings
are in agreement with those reported by Astuti et al.
(2012) that T. evansi infection causes hepatocytes
necrosis, lipid and hydrophic degeneration,
pycnosis, light kariolysis, and hyperemia in mice.
Other studies in T. evansi infected goats also found
various damages in the livers such as hepatocytes
necrosis (Lazuardi, 2008; Shehuet al., 2006), sentro-
perilobulary lipid degeneration and PMN infiltration
around centralis vein (Lazuardi, 2008).
Reduced normal hepatocytes in T. evansi
infected rats are predicted caused by the deleterious
effects of the high prevalence of the parasite on the
tissues. Destruction at hepatocyte structure might
increase migration of phagocyted inflammation cells
and macrophages travel in blood vessels to damage
tissues. Inflamation cells and macrophages produce
free radicals that could result in cellular damages.
During inflamation occurs proliferation of Kupffer
cells and leukocytes increase, causing the increase of
macrophages (Contranet al., 1994). Studies in
buffalo showed that T. evansi infection is not only
caused congestion, intralesional trypanosomes in
blood vessel and extramedullary hematopoiesis in
the liver but also non specific lesions – edema,
congestion and hemosiderosis – in the lungs
(Verdillo et al., 2012).
Among factors contribute to this bad impact of
T.evansi in animals are its capability to produces
hemolysins, toxic compounds that might lyse
erythrocytes (Mbaya et al, 2012). Here,
erythrocytes, platelets and reticulocytes adhere to the
surface of trypanosome surfaces via sialic acid
receptors leading to damages to erythrocyte cell
(Shehu et al., 2006). This is because several areas of
discontinuity occur along the surface of erythrocyte
membranes where they adhere to the trypanosomes
(Mbaya et al, 2012).
SKIC-MHS 2018 - The 2nd Syiah Kuala International Conference on Medicine and Health Sciences
174

Haemorrhagioccurred in liver of T. evansi
infected rats was also reported by Bal et al. (2012).
This condition was assumed caused by the high
prevalence of T. evansiin the tissue, resulted in the
change of membrane permeability of blood vessels.
According to Widodoet al. (2012) torn or damage
blood vessels due to high permeability of cell wall
facilitated erythrocytes leakage out from blood
vessels, a clinical condition known as haemorrhage.
Mechanical damage to vascular endothelium has
been reported when tissue-invading trypanosomes
such as the T. brucei group penetrate tissues via the
interstices (Anosa and Kaneko, 1983).
High infiltration of inflammation cells in the
liver might be related to formation of new antigens
by T. evansi to manipulate antibody of the host.
Variable antigenic type (VAT) is the agent encodes
variations of antigenic glycoproteins on the surface
of T. evansi (Wang et al., 2010). Every time T.
evansi grows and develops inside host, it synthesizes
new variant of VAT protein. Immune system of the
host will adjust this change by creating new,
apropriate antibody. This mechanism causes
decreased immunity of the host, making it become
vulnerable to secondary infections that in turn result
in infiltration of inflammation cells (Wang et al.,
2010).
Degeneration is a sign of the initiation of cell
damages from toxins and cells might lose their
normal structure that lead to cell death (Assiam et
al., 2014). Parenchimatous degeneration or albumin
degeneration is the failure of oxidation causes
accumulation of water inside the cells. As
consequences transportation of proteins produced by
ribosomes might be disturbed and cause swollen
cells, cytoplasm turbidity, and granulated cytoplasm
from protein sedimentation (Mitchell et al., 2008).
Hydrofic degeneration is irreversible degeneration
related to the accumulation of lipid and glycogens in
the vacuoles containing water (Kasno, 2000). Lipid
degeneration might occure in the condition of
ischaemia, anaemia, and toxin as well as
overconsumption of lipid and protein (Dannuri,
2009). Lipid degeneration is characterized by high
proportion of lipid in cytoplasm that leads to the
shift of nuclei to the egde of cell and enlarged
sinusoid, and cell necrosis (Oktavianti et al., 2005).
Number of degenerated cells might reduce if
cell become necrosis from toxic effect of high doses
of neem extract given. Amalina (2009) explains that
higher concentration of chemicals generally causes
higher toxicity responses. Necrosis is the death of
cells or tissues in the living organisms characterized
by smaller, more solid nuclei, folded chromatin and
reticular fibrous and eusinophilic/kariolysis cells
(Kasno, 2000) (Figure 1).
Administration of neem leaf extracts in T.
evansi infected rat seems efective in K5 (dose 800
mg/kgBW) where all observed parameters were
better although smaller than those in negative
control (K0). Increased numbers of normal
hepatocyte might be caused by bioactive compounds
that are able to kill or at least inhibit the growth of T.
evansi or to reduce vulnerable effect of toxins
produced by the parasite. Choudhary et al. (2014)
argued that phytochemical analysis indicated that
neem leaf extract contains glycosides, tannin,
flavonoids, and saponin that might function as
hepatoprotectant. Sonyafitri (2006) added that
azadirachtin (C
35
H
44
O
16
) is the most active
compound in neem leaf extract. This limonoid
(triterpenoid) inhibits the growth and development
of T. evansi (Nzelibe et al., 2013).
In addition to azadirachtin, neem leaf extract
contain alkaloid, terpenoid, quinolide, and phenolic
compounds might act as antiprotozoa (Karira et al.,
2004). Flavonoid dan terpenoid are are chemicals
inhibit the growth and development of T. evansi
(Eliawardani, 2015). Choudhary et al. (2014) have
proven that secondary metabolics contained in neem
leaf extract could reduce and repair alcoholic
induced tissue damages. Result of K5 is also
supported by results obtained by Kale et al. (2003)
that neem leaf extract is able to repair tissue
damages caused by medicines. This is because the
extract contains chemicals function as
hepatoprotective agent (Innih et al., 2014).
Beside hepatoprotective, neem leaf extract is
also hepatotoxic when used in high doses (Kadiri et
al., 1999). Hepatotoxisity is damage of liver caused
by drugs use. According to Robbins et al. (2007) the
occurrence of toxin accumulation causes the damage
of liver and disrupt membrane permeability, osmotic
homeostasis, enzyme and cofactor binding, which in
turn disturb cellular work and function. Katsayal et
al. (2008) added that neem leaf extract, if it was
given in a very high dose (up to 2000 mg/kgBW) for
4 weeks, resulted in a numbers of toxicity effects in
liver. These include infiltration of inflammation
cells, increased Kupffer cells number, hepatocyte
apoptosis and necrosis, and narrower blood vessels.
Astuti et al. (2012) also reported that liver tissue of
mice administrated with mindileaf extract 800
mg/kgBW and infected with T. evansi showed
severe necrosis. Amalina (2009) suggested that
higher concentrations of chemical result in stronger
toxic effects. This is that causes the damage of liver
Protective Effect of Neem (Azadirachta Indica) Leaf Extract on Liver of Trypanosoma evansi Infected Rats (Rattus norvegicus)
175

as indicated by lession affected the change of
cellular function and structure.
4. CONCLUSION
Neem (Azadirachta indica) leaf extract 800
mg/kgBW resulted in the best inhibition on the
damage of the liver of Trypanosoma evansi infected
male rats (Rattus norvegicus) and inhibition of
parasitemia than 50 and 100 mg/kgBW.
ACKNOWLEDEMENT
We would like to sincerely thank all people for their
contribution in this research article. This research
was partially funded by Syiah Kuala University
under the scheme Penelitian professor with contract
number: 288/UN11/SP/PNBP/2018.
REFERENCES
Amalina, N. 2009. Acute toxicity test of Valerian
(Valetiana officinalis) extract on the liver of
Balb/C mice. Karya Scientific paper.
Semarang: Medical Faculty of Diponegoro
University, Semarang.
Anosa, V.O.and Kaneko, J.J. 1983. Pathogenesis of
Trypanosoma brucei infection in deer mice
(Peromyscus maniculatus), light and electron
microscopic studies on erythrocyte pathologic
changes and phagocytosis, American Journal
of Veterinary Research 44 (4): 645-651.
Assiam, N., Setyawati, I., and Sudirga, S.K. 2014.
Effect of dose and treatment duration of
caliandra leaf (Calliandra calothyrsusmeissn.)
on the histological structure of kidney in mice
(Mus musculus L.). Jurnal Simbiosis. 2(2):236-
246.
Astuti, W.N.U.Rr., Rismawati, D., Hidayati, S. and
Suntoro, H.S. 2012. The use of mindi (Melia
azedarch L.) as antiparasit Trypanosoma
evansi and its effect on the structure of hepatic
and renal tissues in mice. Jurnal Kemajuan
Terkini Penelitian Klaster Sains-Teknologi
291-309.
Bal, S.M., Singla, D.L., Kumar, H., Vasudev, A.,
Gupta, K., and Juyal, D.P. 2012. Pathological
studies on experimental Trypanosoma evansi
infection in swiss albino mice. Journal
Parasitic Disease. 36(2):260-264.
Biswas, K. I., Dhatyopadhya, R.K., Bandyopadhya,
U. 2002. Biological activities and medical
properties of neem (Azadirachta indica).
Current Science. 8(2):1336-1345.
Choudhary, U., Augustine, B.B., Lahkar, M. and
Mathew, A. 2014. Hepatoprotective effect of
Azadirachta indica (neem) in alcohol-induced
liver damage. World Journal of
Pharmaceutical Research 3(4):1913-1925.
Contran, R.S., Kumar, V., and Robbins, S.L. 1994.
Robbin’s Pathologic Basis of Disease, 5
th
ed.
WB Saunders, Philadelphia.
Damayanti, R., Graydon, R.J., and Ladd, P.W. 1994.
The pathology of experimental Trypanosoma
evansi infection in the Indonesian buffalo
(Bubalus bubalis). Journal of Comparative
Pathology. 110(2): 267-276.
Dannuri, H. 2009. Analysis of alanin amino
tranferase (ALAT), aspartate amino transferase
(ASAT), blood urea, and liver histopathology
of liver and kidney of Sprague-Dawley mice
administrated with angklak. Jurnal Teknologi
dan Industri Pangan. 20(1):1-9.
Eliawardani. 2015. Activity test of Wedelia biflora
leaf estract as antitrypanosoma in white rats
(Rattus norvegicus). Jurnal Medika
Veterinaria. 9(1):1-3.
Fahrimal, Y., Maghfirah, S., Rinidar, R., Azhar, A.,
Asmilia, N., and Erina, E. 2017.
Antitrypanosoma activity of ethanolic extract
of neem leaf (Azadirachta indica) on
Trypanosoma evansi in Rats (Rattus
norvegicus). Jurnal Kedokteran Hewan. 11(1):
27-30.
Innih. S.O., Eze, I.G., Ekpruke, D.C, and Baxter, D.,
Grillo, D. 2014. The effect of aqueous extract
of neem (Azadirachta indica) leaves on liver
functions of wistar rats. A Peer-review.
Journal of Biomedical Science. 13(2):61-66.
Kadiri, S., Arije, A, and Salako, L.B. 1999.
Traditional herbal preparations and acute renal
failure in South West Nigeria. Tropical
Doctor. 29: 244-246.
Kale, B.P., Kothekar, M.A., Tayade, H.P., Jaju, J.B.,
and Mateenuddin, M. 2003. Effect of aqueous
extract of Azadirachta indica leaves on
hepatotoxicity induced by antitubercular drugs
in rats. Indian Journal of Pharmacology
35:177-180.
Karira, P.G., Rukunga, A.W., Wannyonvi, A.W.,
Muregi, F.M., Gathirwa, J.W., and Omar, S.A.
2004. Antiplasmodial activity and toxicity of
extract of plants used in traditional malaria
SKIC-MHS 2018 - The 2nd Syiah Kuala International Conference on Medicine and Health Sciences
176

therapy in Mem and Kifili districts of Kenya.
Journal Ethnopharmacology 34:160-168.
Kasno, P.A. 2008. Pathology of Liver andExtra
Hepatic Bile Ducts. Semarang: Balai Penerbit
Universitas Diponegoro, Semarang.
Katsayal, U.A., Nadabo, A.Y., and Isiorho, V.J.
2008. Effects of methanol extract of
Azadirachta indica leaves on the histology of
liver and kidney of wistar rats. Nigerian
Journal of Pharmaceutical Sciences 7(1):9-14.
Lazuardi, M. 2008. Histological structure of kidney
and liver of trypanosomiasis suffered from
trypanosomiasis after berenil
®
treatment.
Jurnal Media Peternakan. 31(1):14-21.
Luckins, A.G. 1996. Problems associated with
infection caused by T. evansi in Asia. Proc
Seminar on Diagnostic Techniques for
T.evansi in Indonesia. Balitvet, Bogor 10-17.
Mbaya, A., H. Kumshe, and Nwosu, O.C 2012. The
mechanisms of anaemia intrypanosomosis: A
Review, Anemia, D. Silverberg (Ed.), ISBN:
978-953-51-0138-3, InTech, Available from:
http://www.intechopen.com/books/anemia/the-
mechanisms-of-anaemia-in-trypanosomosis-a-
review.
Mitchell, R.N., Kumar, V., Abbas, A.K., and Fausto,
N. 2008. Cellular adaptation, cell injure and
death in: Pocket Book of Pathological Base of
Diseases, EGC, Jakarta.
Nzelibe, C.H., Habila, N., and Agbaji, S.A. 2013.
Sinergy of Azadirachta indica seed and Tridax
procumbens leaf extracts induced death of
Trypanosoma evansi. International Journal of
Traditional and Natural Medicines 3(1):11-18.
Oktavianti, R., Harini, M. and Handajani, S.N. 2005.
Histological structure of rat (Mus musculus
L.) liver after oral administration of aspartam.
Enviro. 5: 30-31.
Robbins, S.L., Cotran, R.S., and Kumar, V. 2007.
Cellular Injure, Adaptation, and death. In:
Textbook of Patology, vol 1. EGC, Jakarta.
Shehu, A.S., Ibrahim, D.G.N., Esievo, N.A.K.,
Mohammed, G. 2006. Pathology of
experimental Trypanosoma evansi infection in
savannah brow buck. Pakistan Journal of
Biological Sciences. 9(3):522-525.
Sonyafitri D. 2006. Investigation of insecticide
ability of neem (Azadirachta indica A. Juss)
leaves and mindi (Melia azedarach L.) leave
extract on the development of storage insect
pest Sitophilus zeamais Motsch. Thesis. Bogor
Agricultural University, Bogor.
Stevenson, P., Okech, G., Mwendia, C., and Sones,
K.R. 2000. Comparison of the
isometamedium- based trypanocidal drug
samorin®, and
veridium® in cattle under field conditions at
Nguruman, Kenya. Acta Tropical 7:195-201.
Subiyakto. 2009. Neem seed extract as plant
pesticide: potential, problems, and
developmental strategy. Jurnal Perspektif.
8(2):108-116.
Sukanto, I.P., Payne, R.C, and Graydon, R. 1988.
Trypanosomiasis in Madura parasitology and
serological survey. Jurnal Penyakit Hewan.
19(13):14-16.
Syarmalina and Laksmitawati, D.R. 2005.
Antibacterial testing of neem (Azadirachta
indica A. Juss) leaf against bacteria.
Proceeding of National Seminar on Medicinal
Plant in Indonesia, Bogor. 274-276.
Wahyuningsih, H.M.S., Mubarika, S., Bolhui,
H.L.R Nooter, K., and Wahyuono, S. 2002. In
vitro cytotoxic of neem (Azadirachta indica A.
Juss) leaf extracts on several type of human
cancer cell lines. J. Kedokteran. 10(3):16-20.
Wang, Y., Wang, M., and Field, M.C. 2010.
Trypanosoma brucei: Trypanosome-specific
endoplasmic reticulum proteins involved in
variant surface glycoprotein expression.
Experimental Parasitology 125:208-221.
Widodo, S., Sajuthi, D., Choliq, C., Wijaya, A.,
Wulansari, R., Lelana, R.P.A. 2012. Clinical
Diagnostic for Small Animal Clinic. IPB
Press, Bogor.
Verdillo, J.C., Lazaro, J.V., Abes, N.S,, and
Mingala, C.N. 2012. Comparative virulence of
three Trypanosoma evansi isolates from water
buffaloes in the Philippines. Exp. Parasitol.
130(2):130-4.
Protective Effect of Neem (Azadirachta Indica) Leaf Extract on Liver of Trypanosoma evansi Infected Rats (Rattus norvegicus)
177
