
The Antioxidant Activity Analysis of the Ethanolic Extract of Banana
Peel (Musa paradisiaca forma typica) with DPPH Method
Novia Ariani
1
, Laela Hayu Nurani
2
1
Akademi Farmasi ISFI Banjarmasin, Jl. Flamboyan III No. 7B Banjarmasin, Kalimantan Selatan, Indonesia
2
Faculty of Pharmacy, Universitas Ahmad Dahlan, Jl. Prof. Dr. Soepomo, S.H. Janturan, Yogyakarta, Indonesia
Keywords: Banana peel, Flavonoid, Antioxidant, DPPH, IC
50
Abstract: Oxidative stress is one of the triggers of various degenerative diseases and metabolic syndrome. Antioxidants are
compounds that exhibit the activities of neutralizing and scavenging radical molecules, which induce the process
of oxidative reactions in the body. One of the many antioxidant compounds found in plants is avonoids. Banana
peels are known to contain avonoid compounds. This study aimed to determine the antioxidant activity of the
ethanolic extract of banana peel (Musa paradisiaca forma typica). The ethanolic extract of banana peel (Musa
paradisiaca forma typica) was prepared using maceration with 96% ethanol as the solvent. The product was
concentrated in a vacuum rotary evaporator and water bath. The antioxidant activity test was performed with the
DPPH method using various concentrations of extract, namely 1, 2, 3, and 4 ppm. This research found that the
ethanolic extract of banana peel (Musa paradisiaca forma typica) had an IC
50
value of 4.4 ppm. The ethanolic
extract of banana peel (Musa paradisiaca forma typica) has a very strong antioxidant activity.
Banana plants are fruit-producing plants widely
available in Indonesia, and one of them is the Kepok
banana (Musa paradisiaca forma typica). Regarding
plantation area and commodity production in Indonesia,
bananas occupy the rst place among the other types of
fruits. Nevertheless, their utilization in the community is
so far limited to the fruits alone. They can be consumed
either directly or indirectly after being processed rst
into snack foods, but either way, the banana peel is
disposed of as a waste product without adequate options
of optimum application (Khorudin, 2016).
Chemical compounds, existing with different
properties in many plants, are spread throughout
the plant’s organs. Banana peel contains avonoid
compounds whose properties include the potential for
antioxidants (Atun et al., 2007). It also contains many
carbohydrates, minerals such as potassium and sodium,
and cellulose. Based on a phytochemical analysis of
banana peel extract, Salau and Ajani (2012) afrm that
banana peels contain secondary metabolites, such as
saponins, tannins, alkaloids, avonoids, phlobatannins,
anthraquinones, and quinones, that have antibacterial
activity (Fadhilah et al., 2014).
Flavonoids are active compounds that can have
benecial properties, for instance, they function as
antioxidants (Sjahid, 2008; Sousa et al., 2004) and
exhibit anti-dermatosis (Rajendra et al., 2004) chemo-
preventive, anticancer (Galati and O’Brien, 2004),
antiviral (Wei et al., 2004), antibacterial and anti-
inammatory activities (Sjahid, 2008). Horry and Jay in
Harborne (1993) isolate and identify several avonoid
compounds from the banana peel of M. acuminata
species. These compounds are cyanidin, delphinidin,
petunidin, and malvidin-3-ramnosil-1,6-glucoside.
This study aimed to determine the antioxidant
activity of the ethanolic extract of banana peel (Musa
paradisiaca forma typica). Musa paradisiaca forma
typica is still considered one family with Musa
acumianta, which chemotaxonomically has similar
secondary metabolite compounds.
2.1 Materials
The tools and materials used in the research were a
UV-Visible Spectrophotometer (Thermo Scientic
TM
),
2 MATERIALS AND METHODS
1
INTRODUCTION
44
Ariani, N. and Nurani, L.
The Antioxidant Activity Analysis of the Ethanolic Extract of Banana Peel (Musa paradisiaca forma typica) with DPPH Method.
DOI: 10.5220/0008239100440047
In Proceedings of the 1st Muhammadiyah International Conference on Health and Pharmaceutical Development (MICH-PhD 2018), pages 44-47
ISBN: 978-989-758-349-0
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
DPPH (1,1-diphenyl-2-picrylhydrazyl), and Kepok
banana (Musa paradisiaca forma typica) from Jaro
Village, Tabalong Regency, South Kalimantan.
2.2 Methods
The ethanolic extract of Kepok banana (Musa
paradisiaca forma typica) was obtained from
extraction by maceration method. The antioxidant
activity testing was conducted by creating a stock
solution with a concentration of 1,000 ppm using 96%
ethanol as the solvent and then preparing a series of
solutions with different concentrations, namely 1 ppm,
2 ppm, 3 ppm, and 4 ppm. Each concentration was
added with the DPPH (1,1-diphenyl-2-picrylhydrazyl)
solution, and its absorbance was measured with a UV-
Vis spectrophotometer at a wavelength of 515 nm.
2.3 Data Analysis
The stages analysis of antioxidant activity of the
ethanolic extract of banana peel that is as follows:
2.3.1 The Calculation of Antioxidant Activity
The antioxidant activity was expressed in percent (%)
and calculated with the formula (1).
Antioxidant Activity (%)
(Abs of control-Abs of sample )/
(Abs of control)
= x 100%
(1)
2.3.2 The Calculation of IC50 (Inhibitor
Concentration)
The IC
50
of the ethanolic extract of banana peel was
estimated with linear regression using the formula (2).
y = bx + a (2)
where:
y: % inhibition
x: % radical damping
a: sample’s concentration
b: % antioxidant activity
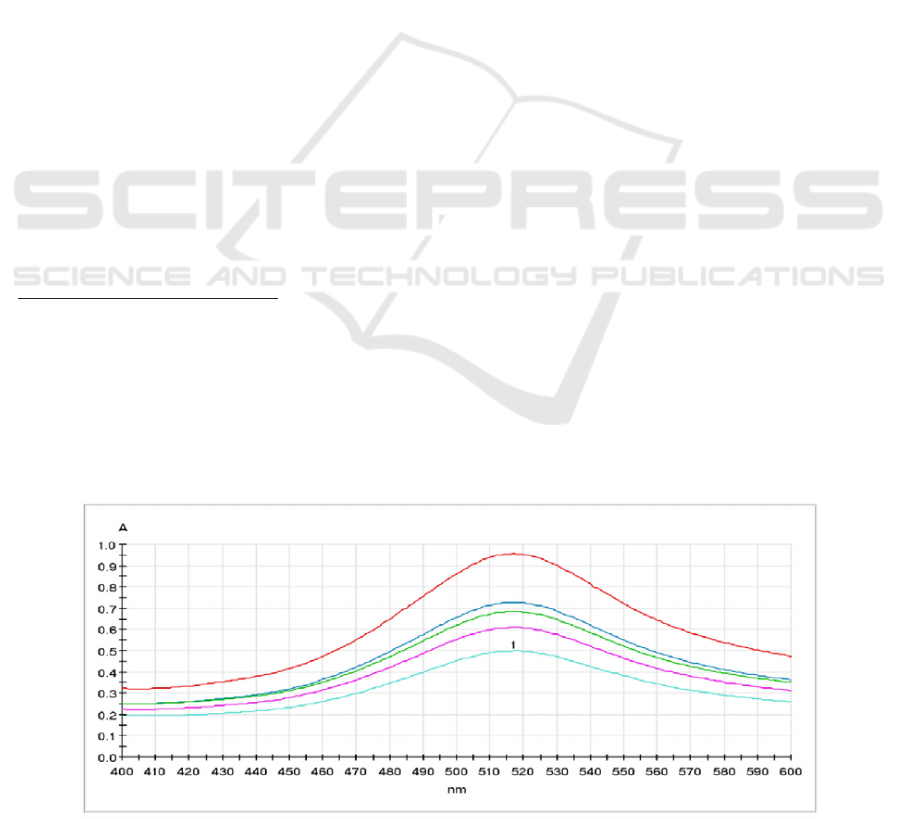
This research was conducted in several stages. The rst
stage determined the maximum wavelength, which
aimed to identify the maximum absorbance of DPPH.
The maximum wavelength represents maximum
sensitivity; and, therefore, it can produce the greatest
absorbance value (Kusumawardhani, Sulistyarti and
Veteran, 2015). The maximum wavelength obtained in
this stage was 517 nm (Figure 1). The second stage was
the measurement of the sample’s absorbance. It started
with measuring the absorbance of the control solution
(DPPH), followed by the absorbance of samples from
the lowest to the highest concentration. The results
showed that extracts with the highest concentration had
the lowest absorbance value and the greatest percentage
of antioxidant resistance. The results of the absorbance
measurements can be seen in Table 1.
The absorbance measurement results were
continued with the calculation of the antioxidant
activity (%) (Table 2). Then, the nal step was
classifying the resultant antioxidant activity based
on the IC
50
values. IC
50
is the concentration required
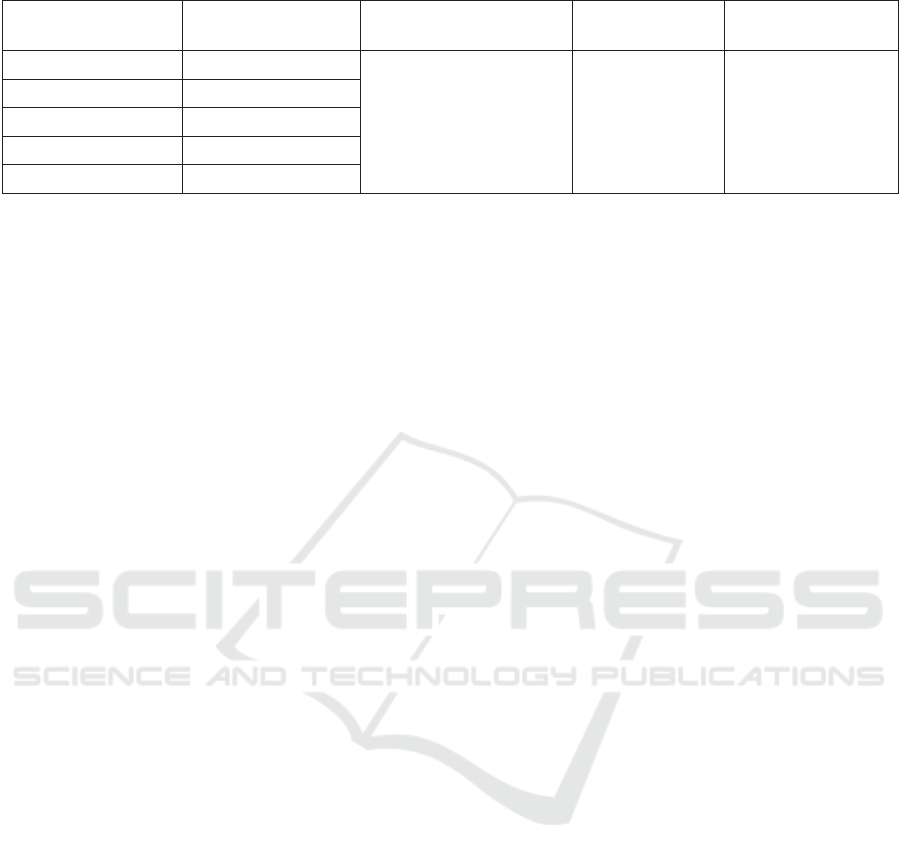
to reduce DPPH by 50%. In this research, it was
determined using a linear regression equation (Figure
2). A smaller IC
50
value would result in higher
antioxidant activity, meaning that the compound can
counter DPPH as a free radical effectively (Kristiana,
Ariviani and Khasanah, 2012). As proposed by
Figure 1: The maximum wavelength of DPPH.
3 RESULTS AND DISCUSSION
The Antioxidant Activity Analysis of the Ethanolic Extract of Banana Peel (Musa paradisiaca forma typica) with DPPH Method
45
Molyneux (2004), the antioxidant activity was
classied based on the IC
50
value (Table 3).
Based on the linear regression equation, the R
2
was 0.9606, and the IC
50
value was 4.44 ppm (Table
4). This IC
50
value indicates that the banana peel
extract exhibits excellent activity and ability to absorb
free radical from DPPH compound. Therefore, it can
be used as an additional therapy or prevention in
increasing the body’s antioxidants and, consequently,
the free-radical scavenging activity. The antioxidant
activity of banana peel extract was visible from the
color change in the DPPH solution. The original
DPPH solution was purple (violet), and this color
faded after its reactions with the extract solution. This
change occurred because of DPPH reduction, i.e.,
a process where the antioxidant compounds in the
extract donate protons or hydrogen to DPPH resulting
in the formation of new stable or non-reactive radicals
(1,1-diphenyl-2-picrylhydrazyl).
The antioxidant activity originates from the
secondary metabolites contained in the banana peel
extract, namely alkaloids, avonoids, tannins, and
saponins (Ariani and Riski, 2018). Flavonoids are
strong antioxidants that can reduce free radicals
and produce avonoid compounds (Middleton,
Theoharides, and Kandaswami 2000).
Free radicals are highly reactive and harmful
substances that can damage the tissues of organs and
cause various diseases. Since antioxidants can inhibit
free radicals and increase endurance simultaneously,
their presence are crucial in countering the effects of
free radicals in the body (Winarsi, 2011).
This research also offers another benet, namely
the optimization of the use and utilization of Kepok
banana peel as antioxidants. Therefore, the most
favorable utilization of bananas can include not
only the fruit but also the peel to reduce waste
production.
Table 1: The absorbance values of the samples used in the
antioxidant activity testing.
No Samples Absorbance
1 DPPH 0.966
2 DPPH + Extract 1 ppm 0.735
3 DPPH + Extract 2 ppm 0.691
4 DPPH + Extract 3 ppm 0.614
5 DPPH + Extract 4 ppm 0.500
Table 2: The antioxidant activity (%) of the ethanolic extract
of banana peels.
No Samples % Antioxidant
Activity
1 DPPH + Extract 1 ppm 24.00
2 DPPH + Extract 2 ppm 28.50
3 DPPH + Extract 3 ppm 36.47
4 DPPH + Extract 4 ppm 48.29
Table 3: The classication of antioxidant activity based on
IC50 value (Molyneux, 2004).
Antioxidant Activity IC
50
Value
Very strong <50 ppm
Strong 50-100 ppm
Average 100-200 ppm
Weak >200 ppm
Figure 2: The XY graph showing the correlation between the sample’s concentrations and the percentage of antioxidant
activity.
MICH-PhD 2018 - 1st Muhammadiyah International Conference on Health and Pharmaceutical Development
46
The ethanolic extract of the raw banana peel (Musa
paradisiaca forma typica), originating from Jaro
Village, Tabalong Regency, South Kalimantan, has
very strong antioxidant activity with an IC
50
value of
4.44 ppm. This study proves that banana peel extract
can be used as an additional therapy or prevention
in increasing the body’s antioxidants, which play a
signicant role in free-radical scavenging.
ACKNOWLEDGMENT
The authors would like to thank the Director of
Pharmacy Academy (Akademi Farmasi) of ISFI
Banjarmasin for the Lecturer Research Grant in
2017 and the permission to claim it as a Research
Institution.
REFERENCES
Ariani, Novia, and Akhmad Riski. 2018. “Aktivitas Ekstrak
Etanol Kulit Buah Pisang Kepok Mentah (Musa
Paradisiaca Forma Typica) Terhadap Pertumbuhan
Candida Albicans Secara In Vitro.” Jurnal
Pharmascience 05(01): 39–44.
Atun, Sri et al. 2007. “Identication And Antioxidant
Activity Test Of Some Compounds From Methanol
Extract Peel Of Banana (Musa Paradisiaca Linn.).”
Indonesian Journal of Chemistry 7(1): 83–87.
Fadhilah, Fairuz et al. 2014. “Antibacterial Effects of Banana
Pulp Extracts Based on Different Extraction Methods
against Selected Microorganisms.” Asian Journal of
Biomedical and Pharmaceutical Sciences 4(36): 14–19.
Galati, Giuseppe, and Peter J. O’Brien. 2004. “Potential
Toxicity of Flavonoids and Other Dietary Phenolics:
Signicance for Their Chemopreventive and Anticancer
Properties.” Free Radical Biology and Medicine 37(3):
287–303.
Harborne, J.B. 1993. The Flavonoids. London: Chapman
& Hall.
Khorudin, Ahmad. 2016. “Uji Aktivitas Antioksidan
Fraksi Kloroform Ekstrak Etanol Kulit Pisang Raja
(Musa Paradisiaca Var. Raja) Dengan Metode DPPH
(1.1difenil-2-Pikrilhidrazil) Beserta Identikasi
Senyawa Flavonoid.” Universitas Wahid Hasyim.
Kristiana, Herlina Dwi, Setyaningrum Ariviani, and Lia
Umi Khasanah. 2012. “Ekstraksi Pigmen Antosianin
Buah Senggani (Melastoma Malabathricum Auct. Non
Linn) Dengan Variasi Jenis Pelarut.” Jurnal Teknosains
Pangan 1(1).
Kusumawardhani, Nury, Hermin Sulistyarti, and Jalan
Veteran. 2015. “Penentuan Panjang Gelombang
Maksimum Dan pH Optimum Dalam Pembuatan Tes
Kit Sianida Berdasarkan Pembentukan Hidrindantin.”
Kimia Stiudent Journal 1(1): 711–17.
Middleton, EJR, TC Theoharides, and C Kandaswami.
2000. “The Effects of Plant Flavonoids on Mammalian
Cells: Implications for Inammation, Heart Disease,
and Cancer.” Pharmacological reviews 52(4): 673–751.
Molyneux, Philip. 2004. “The Use of the Stable Free Radical
Diphenylpicryl-Hydrazyl (DPPH) for Estimating
Antioxidant Activity.” Songklanakarin Journal of
Science and Technology 26(December 2003): 211–19.
Sjahid, L.R. 2008. “Isolasi Dan Identikasi Flavonoid Dari
Daun Dewandaru (Eugenia Uniora L.).” Universitas
Muhammadiyah Surakarta.
Rajendra, N., C. Anandi, S. Balasubramanian, and K.
V. Pugalendi. 2004. “Antidermatophytic Activity
of Extracts from Psoralea Corylifolia (Fabaceae)
Correlated with the Presence of a Flavonoid
Compound.” Journal of Ethnopharmacology 91(1):
21–24.
Salau, B.A., and E.O. Ajani. 2012. “Methanolic Extract of
Musa Sapientum (L Var. Paradisiaca) Sucker Improves
Lipid Proles in Alloxan Induced Diabetic Rats.” Asian
Journal if Biological Sciences.
Sousa, Eliandra de et al. 2004. “Hypoglycemic Effect and
Antioxidant Potential of Kaempferol-3,7-O-(Alpha)-
Dirhamnoside from Bauhinia Forcata Leaves.”
Journal of Natural Products 67(5): 829–32.
Wei, Feng et al. 2004. “Antiviral Flavonoids from the Seeds
of Aesculus Chinensis.” Journal of Natural Products
67(4): 650–53.
Winarsi, Hery. 2011. Antioksidan Alami Dan Radikal
Bebas. Yogyakarta: Kanisius.
Table 4: The calculation of the IC50 of the Kepok banana peel extract.
Concentrations
(ppm)
% Antioxidant
Activity
Linear Regression
Equation
IC
50
Classication
1 24.00
y = 8.0833x + 14.11
R² = 0.9606
4.44 ppm Very Strong
2 28.50
3 36.47
4 48.29
4 48.29
4 CONCLUSIONS
The Antioxidant Activity Analysis of the Ethanolic Extract of Banana Peel (Musa paradisiaca forma typica) with DPPH Method
47
