
Decoloration of Rhodamine B Aqueous Solution by Ultrasound
Assisted Pulse Discharge
Yu Fang
*
, Daiki Hariu, Takuya Yamamoto and Sergey Komarov
Graduate School of Environmental Studies, Tohoku University, 6-6-02 Aza Aoba, Aramaki, Aoba-ku Sendai, Japan
Keywords: Rhodamine B, decoloration, pulse discharge, cavitation, energy efficiency.
Abstract: Research on the decoloration of refractory dye, Rhodamine B (RhB) by ultrasound assisted pulse discharge
process has been carried out. The effects of ultrasound on the pulse discharge type, decoloration rate and
energy efficiency of pulse discharge were investigated in various electrical conductivity of the solution. The
proposed technique extends the treatable range of solution electric conductivity and shows a significant
improvement on RhB decoloration, and the energy efficiency of pulse discharge was promoted. In addition,
the RhB decoloration in the presence of H
2
O
2
was studied. Results show that RhB decoloration has been
inhibited by additive H
2
O
2
.
1 INTRODUCTION
Wastewater from textile, food, leather,
pharmaceutical, and paper industries are one of the
major water pollutant sources. Finding efficient
methods to disposal those colored wastewaters has
become an important issue for environmental
protection as well as those industries (Lee et al.,
2013). Rhodamine B (RhB) is a highly water soluble
refractory organic compound, which is a widely used
xanthene dye for industry purposes. It is harmful to
animals and human beings, which would cause
irritation to eyes, skin and respiratory tract.
(Merouani et al., 2010). Conventional methods to
remove RhB and similar refractory dye pollutant are
absorptions on activated carbon, reverse osmosis or
coagulation by chemical agents. However, those
non-destructive methods can hardly eliminate the
threat of RhB to environment (Behnajady et al.,
2008).
In recent years, advanced oxidation processes
(AOPs) have been widely investigated as a promising
method for organic pollutant removal(Siddique et al.,
2014, Liang et al., 2007). Pulse discharge, one of
major AOPs, shows great potential in pollutant
removal, especially the refractory organic
compounds pollutant (Van de Moortel et al., 2017).
By inducing high energy into reaction zone in a short
time, generation of radicals (·OH, O·, and HO
2
·),
shock waves, UV irradiation and direct pyrolysis of
pollutant could be achieved. Degradation efficiency
of RhB by ·OH radical attack has been proved in
pulse discharge process(Sugiarto et al., 2003).
However, conventional pulse discharge in liquid
requires much higher input voltage than that in air. In
addition, liquid discharge is also very sensitive to the
environment. High solution conductivity leads to
discharge type change from spark type to streamer
type, which shows undesirable decoloration
efficiency on RhB (Nakagawa et al., 2003).
Ultrasound has also been investigated as an AOP
for wastewater treatment. Ultrasound irradiation
induces generation of numerous cavitation bubbles in
which it is transmitted. After the nucleation and
compression-refractions cycles, those microbubbles
collapse when they reach a critical size(Fang et al.,
2018a). High temperature (6000K) and pressure
(1000atm) of bubble collapse in the small volume
induces the generation of radicals. Moreover, the
diameter of cavitation bubbles usually lies in the
range of tens of microns. Recent research shows that
micrometer scale bubbles in water can help generate
spark type discharge with a lower input voltage
(Bruggeman and Leys, 2009, Medodovic and Locke,
2009). Therefore, it is a promising approach to utilize
cavitation bubbles to obtain desirable spark
discharge, consequently, higher pollutant removal
efficiency.
In this research, a new technique which combines
pulse discharge and ultrasound is proposed to
enhance the decoloration of RhB. This research
306
Fang, Y., Hariu, D., Yamamoto, T. and Komarov, S.
Decoloration of Rhodamine B Aqueous Solution by Ultrasound Assisted Pulse Discharge.
DOI: 10.5220/0008189303060310
In The Second International Conference on Materials Chemistry and Environmental Protection (MEEP 2018), pages 306-310
ISBN: 978-989-758-360-5
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
focuses on the assistance of cavitation bubbles on
pulse discharge in liquid. To achieve this goal, test on
effects of ultrasound on discharge type was
performed firstly, and then RhB decoloration
experiments were carried out by ultrasound, pulse
discharge and ultrasound assisted pulse discharge
respectively. In addition, the effects of irradiative
H
2
O
2
solution were also investigated.
2 EXPERIMENTAL SETUP AND
METHODOLOGY
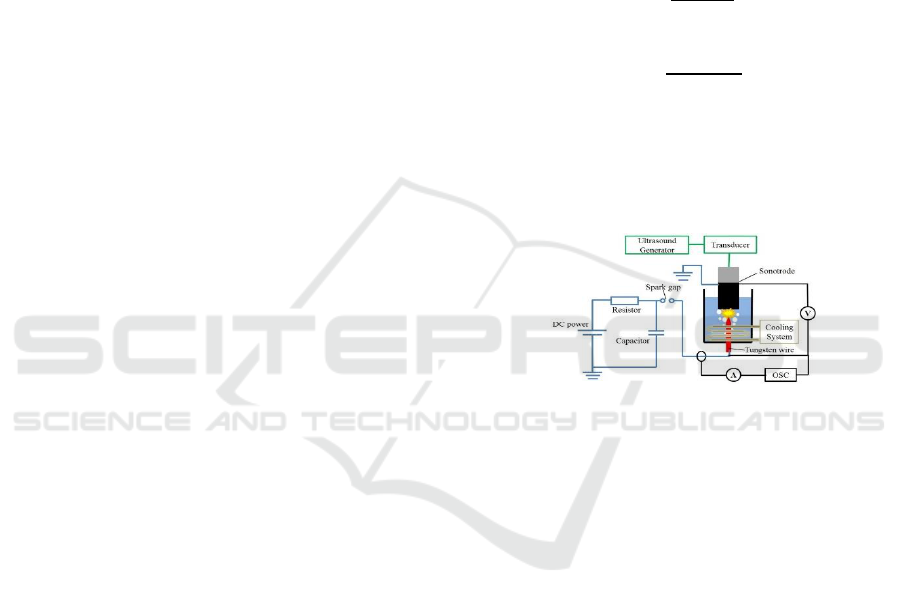
The experimental setup, as shown in Figure 1,
consists of an ultrasound generation system, main
reactor, pulse generation circuit, an electrical
analytical system and cooling device. An ultrasound
generator (TELSONIC, Switzerland) with adjustable
vibration amplitude was applied to irradiate
ultrasound waves at a frequency of 20 kHz into a
water bath through a sonotrode connected with a
piezoceramic transducer. The peak-to-peak
amplitude of the sonotrode tip was ranged from 40 to
75 m (p-p). Notice that the threshold vibration
amplitude exceeding which causes developed
cavitation in water is 4~5 m (p-p)(Komarov et al.,
2013). The reactor comprises of a cylindrical
sonotrode (Diam. 48 mm), a needle shape high
voltage electrode made of tungsten wire (Diam. 1
mm), and a cylindrical vessel (Diam. 140 mm, height
190 mm) made of acrylic resin. The sonotrode is
composed of two cylindrical parts, one is made of
ceramic to prevent the ultrasound generator from
high voltage damage, and the other one is made of
titanium serving as the sonotrode tip and grounded
electrode. Dimensions of both parts were adjusted to
resonance conditions. The distance between
electrodes is an important parameter influencing the
pulse discharge efficiency. In this research, the
distance was set to 4 mm according to preliminary
experiments in optimizing the performance of spark
type discharge unit.
Pulse generation circuit is showed by the blue line.
A DC power supply (0~+40 kV) was used to charge a
capacitor (1000 pF), and then it was discharged into
the reactor through a spark gap discharge unit.
Voltage and current signals between the electrode
during the plasma discharge were collected by an
oscilloscope through a 1000:1 reduction ratio high
voltage probe (PINTEC, China) and a coil current
probe (IWATSU, Japan) respectively. A
water-cooling coil was submerged in the reactor to
maintain the water temperature at a level of 202
o
C.
High purity RhB was purchased, and 5 mg/L RhB
concentrations solutions were prepared using
distilled water. H
2
O
2
solution (500ml) with 30%
concentration was purchased from Wako, Japan.
Each experiment of RhB degradation lasted for 12
minutes, and solution samples were taken each 3
minutes. RhB concentration was determined from the
absorbance measured by a spectrophotometer
(AS-ONE ASV11D, Japan) at 554 nm wavelength.
After the treatment, the decoloration rate ξ and
energy efficiency η was calculated as follows:
ξ =
Δ
[
𝑅ℎ𝐵
]
[
𝑅ℎ𝐵
]
0
η =
VΔ
[
𝑅ℎ𝐵
]
𝐸𝑓𝑇
where
[
𝑅ℎ𝐵
]
0
is the initial RhB concentration, V
is the solution volume, Δ
[
𝑅ℎ𝐵
]
is the concentration
change of RhB, E is the energy of fully a charged
capacitor, 𝑓 is the pulse discharge frequency, T is
the treatment time.
Figure 1: Sketch of the experimental setup.
In this research, the output voltage was 25 kV, pH
was about 5 (RhB aqueous solution without any acid
or alkaline addition). The solution conductivity was
adjusted using a NaCl solution.
3 RESULTS AND DISCUSSION
3.1 Effects of Ultrasound on Pulse
Discharge Type
Pulse discharge type plays a vital role in liquid
discharge technique for pollutant removal, especially
for refractory organic pollutants (Sugiarto and Sato,
2001). In the proposed process, the electrodes,
which comprises a tungsten wire and sonotrode, can
be regarded an as a needle-plate electrode
arrangement. Moreover, in a needle-plate pulse
liquid discharge system, discharge type can be
varied into spark discharge, streamer discharge and
mixing discharge (spark and streamer discharge
type). These discharge types mainly concerned with
Decoloration of Rhodamine B Aqueous Solution by Ultrasound Assisted Pulse Discharge
307
the output voltage of the power supply, distance
between electrodes, electric conductivity of solution
and the pressure. In this research, the output voltage,
electrode distance, and pressure were fixed. Thus,
electric conductivity was considered as the only
factor to discharge type changes.
Spark discharge usually appears as a single
plasma channel between electrodes with high energy
density. The formation of this plasma channel
induces strong electron clusters, UV irradiation, and
shock waves into liquid around the channel, which
remarkably promotes exciting ionizing water
molecular. Consequently, the generation of radicals
is improved.
In the case of streamer discharge, it has many
intense but weak channels growing from one
electrode (usually from a needle electrode in
needle-plate system). Streamer discharge generates
higher pulse current and stronger UV light than
spark discharge. However, the low energy density of
streamer discharge induced limit ionization and
excitation gives low radicals yield(Medodovic and
Locke, 2009).
Mixing discharge type in this research means a
combination discharge type (spark discharge and
streamer discharge), that long plasma channel
between electrodes and short, intense channels exist
at the same time. These channels are more stretched
than in the other cases. This discharge type gives the
best degradation efficiency on phenol than spark and
streamer type, presented by Sugiarto (Sugiarto and
Sato, 2001).
In this research, Table 1 shows the discharge type
changes of pulse discharge and ultrasound assisted
pulse discharge in various electric conductivity. This
test was carried out with an electric conductivity
range of 5~1000 μS/cm. Different discharge types
can be identified with digital data from oscilloscope
and observation. For solely pulse discharge, spark
and mixing discharge, which is believed to be
effective in pollutant removal, was limited in 5~70
μS/cm, while for ultrasound assisted pulse discharge
process, it was remarkably expanded to 1000 μS/cm,
almost increased by 13 times.
With the assistance of ultrasound, numerous
cavitation microbubbles were generated between
electrodes, which help decrease the breakdown
threshold and help plasma propagate through the gas
phase inside bubbles. Bubble surface takes an
important role in pulse discharge. When applying an
electric field on a liquid-gas phase, the charge will
accumulate on the surface of bubbles (Gershman et
al., 2007, Yamabe et al., 2005). Due to the huge
bubble surface of numerous cavitation bubbles, the
discharge will be generated through bubbles instead
of water medium, and a spark type discharge can be
achieved even in higher electrical conductivity
solution.
Table 1: Discharge type changes of P (pulse discharge) and
UP (ultrasound assisted pulse discharge) in various ranges
of electric conductivity of solution (/μS·cm
-1
).
5~30
30~70
70~1000
P
Spark
Mixing
Streamer
UP
Spark
Spark
Mixing
3.2 Effects of Ultrasound Assistance on
RhB Decoloration
Decoloration tests of RhB were carried out to
evaluate the performance of the proposed process.
Due to the decomposition of RhB molecular, the
electric conductivity of solution will continuously
increase. To avoid discharge type changing caused
by electric conductivity, the decoloration tests were
limited in 12 minutes, with an average of 10 μS/cm
growth in electric conductivity.
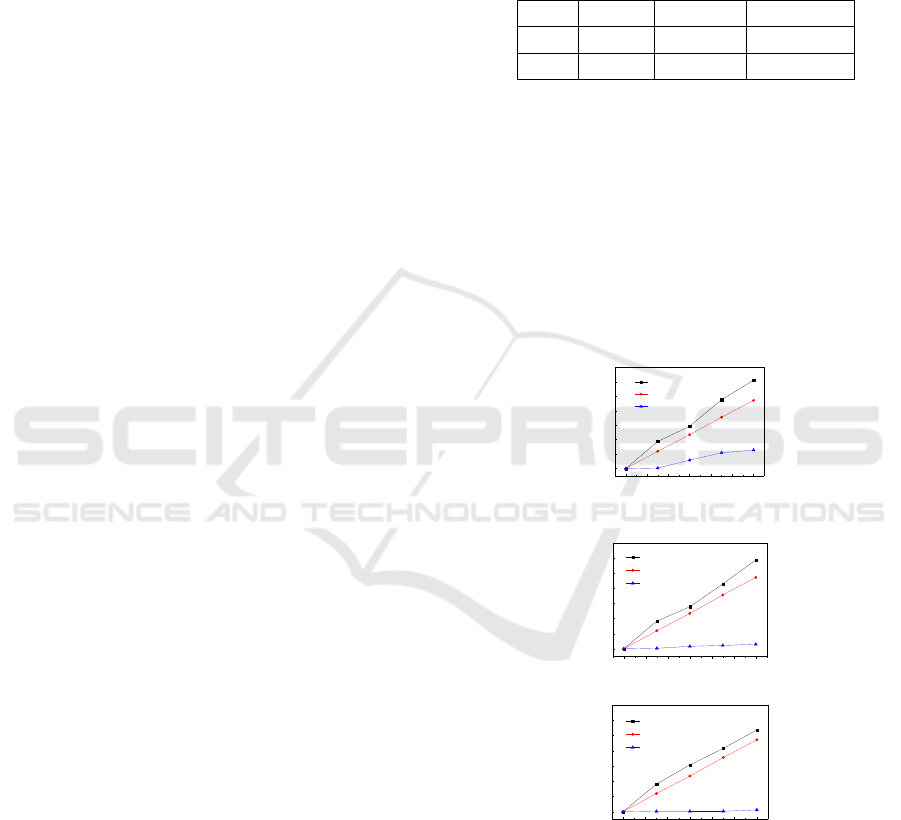
0 2 4 6 8 10 12
0
5
10
15
20
25
30
35
Decoloration rate
/ %
Time / minute
(a)
UP
U
P
0 2 4 6 8 10 12
0
5
10
15
20
25
30
35
Time / minute
(b)
Decoloration rate
/ %
UP
U
P
0 2 4 6 8 10 12
0
5
10
15
20
25
30
35
UP
U
P
Time / minute
(c)
Decoloration rate
/ %
Figure 2: RhB decoloration rate of various methods (UP:
ultrasound assisted pulse discharge. U: ultrasound singly.
P: pulse discharge singly) in (a) 20, (b) 60 and (c) 200
μS/cm solutions.
Figure 2 shows the effects of ultrasound
assistance on RhB decoloration in various electric
conductivity solutions. Treatments by ultrasound and
pulse discharge singly were also investigated for
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
308
comparisons. Ultrasound assisted pulse discharge
gives the highest decoloration rate rather than
another two approaches, and the decoloration
slightly decreases in a higher electrical conductivity
solution. No significant effects of electrical
conductivity can be found on the decoloration by
ultrasound singly, while the decoloration rate almost
decreases to zero when conductivity above 60 μS/cm.
In 20 μS/cm solution, it will cost much energy to
breakdown water medium. When solution
conductivity increase, pulse discharge type usually
transfers from spark type to streamer type, which has
more intense plasma channels but low energy
density (Šunka, 2001). In this case, much energy
was consumed into current heat instead of RhB
decomposition. The sole ultrasound gives relatively
high decoloration rate even the amplitude was set at
40 m (p-p), the minimum of an ultrasound generator.
This is because even with the minimum amplitude of
sonotrode, a strong cavitation field could be
generated, and caused effective decomposition of
RhB molecular.
In addition, irradiation of ultrasound in liquid
will cause a macro steady flow due to the
soundwave attenuation in water medium, named
acoustic streaming, which helps improve the mass
transfer in reactor, consequently yield a better
decoloration rate (Fang et al., 2018b).
Table 2 shows the data of energy efficiency of
each approach.
p’
is the mean energy efficiency of
improved pulse discharge by ultrasound, which was
calculated as follows:
𝜂
𝑃
′
= 𝜂
𝑈𝑃
− 𝜂
𝑈
Where 𝜂
𝑈𝑃
is energy efficiency of ultrasound
assisted pulse discharge, 𝜂
𝑈
is the energy efficiency
of ultrasound. It can be found that in energy
efficiency of pulse discharge was significantly
improved by ultrasound, especially in higher
electrical conductivity solution.As di scussed in
previous part, the existence of cavitation bubbles
between electrodes help plasma to propagate in the
gas phase inside bubbles, thus more energy could be
used for RhB decoloration. Moreover, discharge
types in 60 and 200 μS/cm solution were changed by
cavitation bubbles to spark discharge and mixing
discharge respectively. Spark discharge and mixing
discharge give a much higher radical yield, and
induce extreme physical conditions (strong shock
wave and 3000~5000K high temprature), which
improve the decoloration of RhB. While for streamer
discharge, much energy was consumed into current
heat, consequently decreased energy efficiency.
Compare with other liquid discharge process for dye
decoloration (Malik, 2010), especially for those RhB
decoloration researches, ultrasound assisted pulse
discharge shows a promising energy efficiency, such
as Anto presented 0.081 g/kWh for spark discharge
and 0.025 g/kWh for streamer discharge (Sugiarto et
al., 2003).
Table 2: Energy efficiency (g/kWh) in P (solely pulse
discharge) and P
’
(improved pulse discharge) with different
solution electrical conductivities.
20 μS/cm
60 μS/cm
200 μS/cm
P
0.191
0.048
0.013
P
’
0.267
0.257
0.107
3.3 Effects of Additive H
2
O
2
Solution
H
2
O
2
is a commonly used method to combine with
AOP process to help generate radicals to decompose
pollutants (Mehrdad and Hashemzadeh, 2010,
Mehrdad et al., 2011). In this part, effects of
different concentrations of H
2
O
2
were investigated
with ultrasound assisted pulse discharge method.
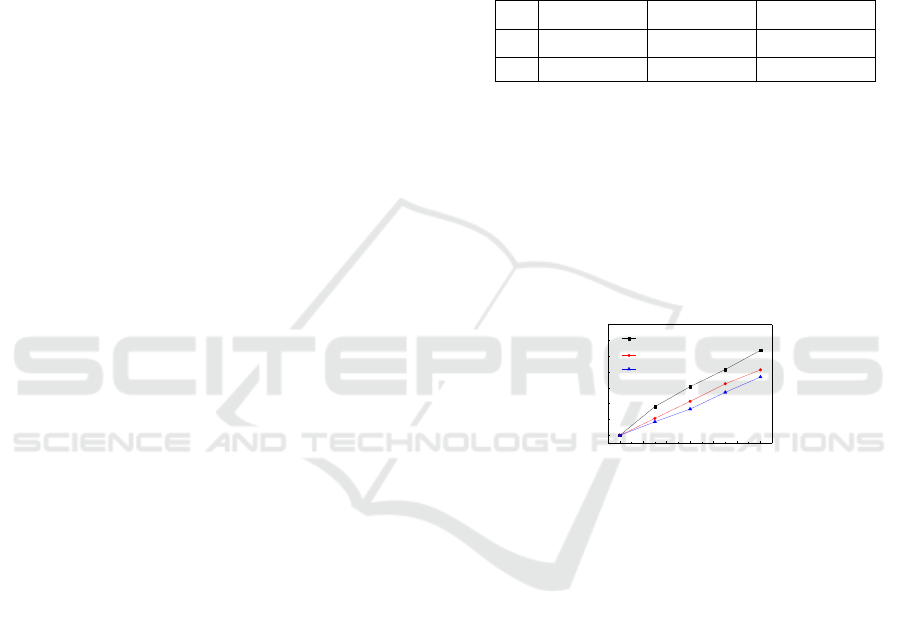
The result is shown in Figure 3.
0 2 4 6 8 10 12
0
5
10
15
20
25
30
35
No H
2
O
2
15 mg/L
45 mg/L
Time / minute
Decoloration rate
/ %
Figure 3: RhB decoloration rate as a function time of time
with various concentrations of H
2
O
2
.
It is clear that the decoloration rate decreases in
higher H
2
O
2
concentration. Similar results have been
reported in related researches (Behnajady et al.,
2008, Merouani et al., 2010). However, in these
researches decreasing trend of RhB decoloration
appears after a relatively large dosage of the additive
H
2
O
2
solution.
It is known that solely H
2
O
2
solution shows very
limit decoloration on RhB. OH radical is mainly
responsible for RhB decoloration of those AOPs
techniques. In this research, because of the short life
time and diffusion distance of OH radicals,
decoloration reaction is mainly conducted on the
interface between liquid phase and gas phase
(induced by cavitation and plasma evaporation).
However, H
2
O
2
would also take part of the interface,
after reaching a certain saturation limit, there would
be no enough interface area for the decoloration
reaction. The acoustic streaming will also improve
Decoloration of Rhodamine B Aqueous Solution by Ultrasound Assisted Pulse Discharge
309

H
2
O
2
diffusion to the interface. Consequently, the
decoloration rate was decreased. On the other hand,
due to a large amount of ·OH radical generated in
proposed process, more OH radicals were scavenged
by H
2
O
2
instead of attacking RhB molecular. The
inhibitory effect of H
2
O
2
could be explained as
follows:
H
2
O
2
+ ·OH → H
2
O + HOO·
In this case, the scavenging effect of H
2
O
2
shows
more influences rather than releasing ·OH radical.
Thus, the decoloration will decrease with the
increasing additive H
2
O
2
.
4 CONCLUSIONS
The present work has shown that RhB can be
effectively removed from aqueous solution by
proposed ultrasound assisted pulse discharge.
Solution electrical conductivity for desirable
discharge type is widely extended. Energy efficiency
of discharge is significantly improved by ultrasound,
especially in higher electrical conductivity of
solution. Additive H
2
O
2
solution shows an inhibitive
effect on RhB decoloration in proposed method.
Additive H
2
O
2
performs as a inhibitor in the
experiments.
ACKNOWLEDGMENTS
This work is supported by China Scholarship
Council.
REFERENCES
Behnajady, M., Modirshahla, N., Tabrizi, S. B. &
Molanee, S. 2008. Ultrasonic degradation of
Rhodamine B in aqueous solution: influence of
operational parameters. Journal of Hazardous
Materials, 152, 381-386.
Bruggeman, P. & Leys, C. 2009. Non-thermal plasmas in
and in contact with liquids. Journal of Physics D:
Applied Physics, 42, 053001.
Fang, Y., Shimizu, S., Yamamoto, T. & Komarov, S.
2018a. Generation of OH Radical by Ultrasonic
Irradiation in Batch and Circulatory Reactor. IOP
Conference Series: Earth and Environmental Science,
120, 012019.
Fang, Y., Yamamoto, T. & Komarov, S. 2018b. Cavitation
and acoustic streaming generated by different
sonotrode tips. Ultrasonics Sonochemistry, 48, 79-87.
Gershman, S., Mozgina, O., Belkind, A., Becker, K. &
Kunhardt, E. 2007. Pulsed electrical discharge in
bubbled water. Contributions to Plasma Physics, 47,
19-25.
Komarov, S., Oda, K., Ishiwata, Y. & Dezhkunov, N.
2013. Characterization of acoustic cavitation in water
and molten aluminum alloy. Ultrason Sonochem, 20,
754-61.
Lee, H., Park, S. H., Park, Y.-K., Kim, B. H., Kim, S.-J. &
Jung, S.-C. 2013. Rapid destruction of the rhodamine
B using TiO 2 photocatalyst in the liquid phase
plasma. Chemistry Central Journal, 7, 156.
Liang, J., Komarov, S., Hayashi, N. & Kasai, E. 2007.
Improvement in sonochemical degradation of
4-chlorophenol by combined use of Fenton-like
reagents. Ultrasonics Sonochemistry, 14, 201-207.
Malik, M. A. 2010. Water purification by plasmas: Which
reactors are most energy efficient? Plasma Chemistry
and Plasma Processing, 30, 21-31.
Medodovic, S. & Locke, B. 2009. Primary chemical
reactions in pulsed electrical discharge channels in
water. Journal of Physics D: Applied Physics, 42,
049801.
Mehrdad, A. & Hashemzadeh, R. 2010. Ultrasonic
degradation of Rhodamine B in the presence of
hydrogen peroxide and some metal oxide. Ultrasonics
sonochemistry, 17, 168-172.
Mehrdad, A., Massoumi, B. & Hashemzadeh, R. 2011.
Kinetic study of degradation of Rhodamine B in the
presence of hydrogen peroxide and some metal oxide.
Chemical engineering journal, 168, 1073-1078.
Merouani, S., Hamdaoui, O., Saoudi, F. & Chiha, M.
2010. Sonochemical degradation of Rhodamine B in
aqueous phase: effects of additives. Chemical
Engineering Journal, 158, 550-557.
Nakagawa, Y., Mitamura, S., Fujiwara, Y. & Nishitani, T.
2003. Decolorization of rhodamine B in water by
pulsed high-voltage gas discharge. Japanese journal of
applied physics, 42, 1422.
Siddique, M., Farooq, R. & Price, G. J. 2014. Synergistic
effects of combining ultrasound with the Fenton
process in the degradation of Reactive Blue 19.
Ultrasonics sonochemistry, 21, 1206-1212.
Sugiarto, A. T., Ito, S., Ohshima, T., Sato, M. & Skalny, J.
D. 2003. Oxidative decoloration of dyes by pulsed
discharge plasma in water. Journal of Electrostatics,
58, 135-145.
Sugiarto, A. T. & Sato, M. 2001. Pulsed plasma
processing of organic compounds in aqueous solution.
Thin solid films, 386, 295-299.
Šunka, P. 2001. Pulse electrical discharges in water and
their applications. Physics of plasmas, 8, 2587-2594.
Van De Moortel, N., Van Den Broeck, R., Degrève, J. &
Dewil, R. 2017. Comparing glow discharge plasma
and ultrasound treatment for improving aerobic
respiration of activated sludge. Water research, 122,
207-215.
Yamabe, C., Takeshita, F., Miichi, T., Hayashi, N. &
Ihara, S. 2005. Water treatment using discharge on the
surface of a bubble in water. Plasma Processes and
Polymers, 2, 246-251.
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
310
