
Immobilization of Horseradish Peroxidase on Modified Electrospun
Nanofibrous Membrane for 2,4-Dichlorophenol Removal
Ning Wei
1,2
, Ran Xu
1,2*
and Rongzhi Tang
1,2
1
State Key Laboratory of Pollution Control and Resources Reuse, College of Environmental Science and Engineering,
Tongji University, Shanghai 200092, PR China
2
Shanghai Institute of Pollution Control and Ecological Security, Shanghai200092, P.R. China
Keywords: 2,4-Dichlorophenol, Horseradish Peroxidase Immobilization, Electrospinning, Nanofibrous Membrane,
Water Treatment
Abstract: In this study, a facile and simple method was used to modify the hydrophobic PAN/PVdF membranes into
hydrophilic ones. Results showed that the PAN/PVdF membranes have been modified successfully and the
water contact angle changed from 87.135° to 0°. Horseradish peroxidase (HRP) was immobilized onto the
modified PAN/PVdF membranes through covalent binding and the maximum enzyme loading was
approximately 440 mg/g under optimal conditions (after 8 h at pH 8.0 and 25
o
C). The effects of pH and
temperature on the relative activity of free and immobilized HRP were studied. Under the optimum
conditions of pH and temperature, immobilized HRP was greatly improved in operational and storage
stability. 2,4-DCP removal experiments showed that the immobilized HRP and free HRP had a similar
removal efficiency (87% and 93%, respectively). However, the immobilized HRP had an excellent
reusability. HRP-PAN/PVdF could still remove 47.6% of 2,4-DCP after 7 repeated runs, which could
overcome the inherent drawbacks of free enzymes-hard separation and non-reusability.
1 INTRODUCTION
Phenols, especially chlorinated ones, are considered
as persistent organic pollutants among different
kinds of pollutants in aquatic ecosystems mainly due
to their harmful effects on organisms even at very
low concentrations (Antizar-Ladislao and Galil,
2004) (Khenifi et al., 2009). Chlorophenols are one
of the most important industrial materials and widely
used in the production of insecticides, herbicides,
wood treatment agents and flame retardants (Zhang
et al., 2004). A typical example is 2,4-
dichlorophenol (2,4-DCP), which is regarded as a
priority pollutant by European Union (No, 2001) and
United States Environmental Protection Agency
(Keith and Telliard, 1979). It is mainly used in the
applications of higher chlorophenols manufacturing
and other productions of Cl-based herbicides
(Ormad et al., 2001). People who drink water
containing 2,4-DCP for long time might suffer from
headache, hyperpyrexia, sicchasia, anepithymia, and
even death. The fate and transport of 2,4-DCP in
aqueous media are rather complicated mainly due to
its high solubility and low air-water partition
coefficients (Khenifi et al., 2009). Biochemical
technology, mainly including activated sludge
process, biomembrane process and biological
fluidized bed process, is the most widely used
method for the treatment of organiccontaminations
in water. However, some disadvantages which
cannot be overcame still exist in the methods above.
For example, production of huge amount of sludge,
poor ability to resist impact load and high cost
greatly limits the application of activated sludge
technology. In addition, secondary pollution and
operation difficulties may also be the restrictions on
the methods above. However, enzyme immobilized
on nanofibrous membranes, which may overcome
those shortcomings to a certain extent, was
considered to be a great candidate for the removal of
chlorophenols due to their excellent properties of
large surface area, easy separation and reutilization
as well as high removal efficiency towards organic
pollutants. In our previous studies, we have found
that the removal of the organic pollutants by
immobilized enzyme on nanofibrous membranes
was mainly attributed to two aspects: the adsorption
of the membranes and the biodegradation by the
Wei, N., Xu, R. and Tang, R.
Immobilization of Horseradish Peroxidase on Modified Electrospun Nanofibrous Membrane for 2,4-Dichlorophenol Removal.
DOI: 10.5220/0008188902830292
In The Second International Conference on Materials Chemistry and Environmental Protection (MEEP 2018), pages 283-292
ISBN: 978-989-758-360-5
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
283

enzyme (Xu et al., 2015c). Meanwhile, the organic
pollutants could be removed from water in a short
time. For example, horseradish peroxidase (HRP)
immobilized on chitosan/poly (vinyl alcohol)-
nanocrystalline cellulose nanofibrous membrane
could remove 98.34% 3,3’,5,5’-tetrabromobisphenol
in 2 h (Xu et al., 2015b). In another case, 24% of
diclofenac was absorbed by the electrospun carriers
while the total removal efficiency is 100% after 6 h
(Xu et al., 2015c). Bio-enzymes are considered to be
great candidates for pollutants removal due to their
high specificity, high efficiency and eco-
friendliness, in which HRP is a typical
representative. It is characterized by its tolerance to
pH, temperature and other outside interferences and
is adopted as the experimental enzyme (Nicell et al.,
1993).
Polyacrylonitrile (PAN) (Qin et al., 2007;Liu et
al., 2014;Selloum et al., 2014;Pan et al., 2015)
and polyvinylidene fluoride (PVdF) (Kim et al.,
2004) (Gao et al., 2006)are two commonly used
polymers to prepare electrospun nanofibers. Studies
(Yin et al., 1998) (Yang and Liu, 2003) found that a
suitable incorporation of PVdF will increase the
toughness of the PAN nanofiber, thus increasing the
applicability of the nanofibrous membranes for
future application. However, the PAN/PVdF
membrane is not easy to absorb aquatic pollutants
for its hydrophobicity.
In this study, we aimed to use a simple method to
modify the commonly used PAN/PVdF membrane
in order to change its property from hydrophobicity
to hydrophilicity and apply it as a carrier for HRP to
remove 2,4-DCP in water with operational and
storage stability. The immobilized HRP was used for
the treatment of 2,4-DCP in water. The research had
important scientific and practical significance for the
application of enzyme catalytic technology in
environmental engineering.
2 MATERIALS AND METHODS
2.1 Materials
Polyacrylonitrile (PAN, Mw=150,000), N,N-
dimethylformamide (DMF), Coomassie brilliant
blue (G250), citrate phosphate buffer solution
(CPBS), 2,2’-azinobis-(3-ethylbenzthizaoline-6-
sulphonate) (ABTS), 1,1’-carbonyldiimidazole
(CDI), were obtained from Sigma-Aldrich.
Polyvinylidene fluoride (PVdF) was purchased from
Arkema, China. Sodium hydroxide (NaOH), horse
radish peroxidase (HRP, RZ~3), tetrahydrofuran
(THF) were obtained from Sinopharm Chemical
Reagent Co. Ltd, China. Deionized water was used
in all experiments. All chemicals used were of
analytical grade.
2.2 Preparation of PAN/PVdF
Nanofibrous Membranes by
Electrospinning
PAN ( 8 g ) was dissolved in DMF( 92 g ) and
stirred for 8 h at 60
o
C, meanwhile PVdF( 5 g ) was
dissolved in DMF( 50 g ) and stirred for 5 h at 60
o
C. Then, 1.5 g of 10 % PVdF was added into 4.5 g
of 8 % PAN solution and stirred for 2 h at 60
o
C to
obtain the spinning solution, and the mixture
solution was poured into a 10 mL plastic injector
with a stainless steel spinneret of 1.2 mm inner
diameter after the air bubbles were completely
removed. The electrospinning conditions were
controlled as follows: a high voltage of 16 kV, a
flow rate of 1.5 mL/h, a tip-to-target distance of 18
cm and a relative humidity of 45 ± 5%. Finally, the
PAN/PVdF nanofibrous membranes were collected
for 4 h on a rotating cylinder wrapped with
aluminum foiland then dried in a vacuum drying
oven for 12 h to get a non-woven format. The
membranes were water insoluble.
2.3 Measurement and Characterization
Scanning electron microscopy (SEM) was used to
observe the morphology of the nanofibers on a field
emission XL-30 SEM system at 20 kV. Fourier
transform infrared-attenuated total reflectance
(FTIR-ATR) spectroscopy equipped with a
germanium crystal was used to assay the functional
groups of the original and modified nanofibers. The
immobilization efficiency and residual activity of
free and immobilized HRP were measured by
Shimadzu UV-1700 spectrophotometer.
2.4 Immobilization of HRP on the
PAN/PVdF Nanofibrous
Membranes
10 mg PAN/PVdF nanofibrous membrane was
immersed into the mixture solution of 10 mL 0.8 M
NaOH and 2 mL ethanol for 2 h to convert part of
the cyano groups into carboxylate. Then take out the
membrane and wash it with anhydrous THF to
remove any residue water. The reaction of carboxyl
groups with CDI was controlled under non-aqueous
conditions. CDI was dissolved in anhydrous THF at
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
284

a concentration of 0.3 M. The reaction lasted for 12
h at 25
o
C. Afterwards, the activated nanofibers were
washed 3 times with THF to remove excess CDI and
by-products, when THF vanished under natural
drying, the membranes were stored before use.
The activated membranes were immersed into an
HRP solution at a concentration of 1 mg/mL (pH 6.0
CPBS) at 25
o
C for 12 h. The effects of time (2, 4, 6,
7, 8,10,12 h) and pH (3,4,5,6,7,8,9) on HRP
immobilization were analyzed. After enzyme
immobilization, the membranes were removed from
the enzyme solution and rinsed with CPBS until no
HRP was detected in the washings, then the
membranes immobilized with HRP were stored at 4
o
C to protect the immobilized HRP from losing
activity for later use.
2.5 Assays of Immobilization Efficiency
and Activity of Free and
Immobilized HRP
The immobilization efficiency of HRP was
calculated by subtracting the loading of HRP
remained in the supernatants and washing buffer
from the total HRP initially added into the solution.
The loading of HRP (mg) and immobilization
efficiency (mg/g) were estimated at 595 nm by a
UV-1700 spectrophotometer from Shimadzu.
Determination of HRP activity was calculated
spectrophotometrically by monitoring the
absorbance change of ABTS at 420 nm on a UV-
1700 spectrophotometer (Shimadzu) (Xu et al.,
2015c). The formula for calculating the HRP activity
is as follows:
U=
∆A×V×10
ε×M×L×t
(1)
Where ΔA is the change in absorbance before and
after the reaction; V is the volume (L) of the liquid
added to the cuvette; M is the mass of the added
HRP (mg); L is the cuvette path (cm); t is Reaction
time (min).
In the immobilization experiment of HRP, the
concept of relative enzyme activity is often used. In
the horizontal gradient experiment, the enzyme
activity under the optimal conditions was taken as
100%, and the enzyme activities of other horizontal
gradients were relative to the activity of 100%
enzyme.
2.6 Stabilities of Free and Immobilized
HRP
Free HRP (1mg/mL) and a certain amount of
immobilized HRP were stored in CPBS at 4
o
C. The
effects of temperature on the activity of free and
immobilized HRP were examined by evaluating the
enzyme activity at pH 4.0 from temperature 20 to 50
o
C. The effects of pH on the activity of free and
immobilized HRP were obtained by evaluating the
enzyme activity at 25
o
C from pH 3.0 to 9.0.
The reusability of immobilized HRP was
determined as follows: the immobilized HRP was
used 10 times within a day at the optimum
conditions.
The storage stability of immobilized HRP was
determined as follows: After each reaction, the
immobilized HRP was washed with CPBS (pH 4.0)
to remove any residual substrate. The storage
stabilities of free and immobilized HRP were
obtained by calculating the residual activity of the
enzyme every 3 days at 4
o
C within 30 days.
2.7 Removal of 2,4-DCP by Free and
Immobilized HRP
Fifty mL of 20 mg /L 2,4-DCP in PBS was used as
substrate, 5 mg of free or immobilized HRP and 0.8
mmol/L H
2
O
2
were used for the removal of 2,4-
DCP. After 3 h, the upper solution was filtered
through syringe filters (membrane of nylon) with the
size of 0.45 μm and the residue concentration of the
2,4-DCP was measured using UV-1700 based on the
standard methods for the assay of phenols.
The effect of pH on the removal of 2,4-DCP was
investigated at 25
o
C, pH 3-8. The effect of initial
H
2
O
2
on the 2,4-DCP removal was carried out with
the concentration range of 0.2 mM to 1.2 mM. A
PAN/PVdF membrane modified by NaOH was used
as a pure carrier to study the adsorption capacity of
the membrane.
The removal of 2,4-DCP by HRP-PAN/PVdF
includes two aspects: adsorption of the membrane
and biodegradation of HRP immobilized on
PAN/PVdF. The biotransformation by the
immobilized HRP was calculated by the following
Eq. (2):
C
b
= C
r
-C
a
(2)
C
r
, C
a
, and C
b
are the concentrations of the 2,4-
DCP removed, absorbed, and biotransformed by
HRP-PAN/PVdF NFM, respectively.
Immobilization of Horseradish Peroxidase on Modified Electrospun Nanofibrous Membrane for 2,4-Dichlorophenol Removal
285

Figure 1: Water contact performances (a) comparison of PAN/PVdF membrane before and after treatment (b) water contact
angle of PAN/PVdF membrane (c) water contact angle of PAN/PVdF membrane after treatment.
2.8 Data Analysis
Non-linear regression analysis using first-order
model, Eq. (3)-(5) (Xu et al., 2015c), was used to
estimate the first order rates (k), the time required to
obtain 50% of substrate degradation/adsorption
(t1/2), and the 2,4-DCP removal efficiency after
(REt).
C
t
= C
0
exp(-kt)
(3)
C
0
and C
t
are the substrate concentrations at the
beginning of the run and at the time (t), and k is the
first-order rates (k), and the time required to obtain
50% of the substrate degradation/adsorption (t
1/2
),
and the 2,4-DCP removal efficiency after t (RE
t
).
t
1/2
= ln2/k
(4)
RE
t
=(C
0
-C
t
)/C
0
×100
(5)
3 RESULTS AND DISCUSSION
3.1 Characterization of the
Electrospun PAN/PVdF
Nanofibrous Membranes
The hydrophobicity of the pristine PAN/PVdF
membrane was a great limitation to its application.
Therefore, the PAN/PVdF membranes were treated
with 10 wt% NaOH and a certain amount of ethanol
beforehand to improve its hydrophilic performance.
Figure 1(a) shows the different water contact
performances of pristine PAN/PVdF and PAN/PVdF
membrane after treated with NaOH. It can be seen
that the color of the nanofiber membrane changes
from white to reddish brown after modification with
an aqueous solution of sodium hydroxide and
ethanol. It was found the water contact angle of
PAN/PVdF membrane was 87.135
o
(see Figure 1b),
while that of the membrane after NaOH treatment
was 0
o
(see Figure 1c). The smaller the water contact
angle, the greater the hydrophilicity. It means the
membrane surface had been changed from
hydrophobic to totally hydrophilic after NaOH
treatment. This change could be attributed to the
existence of carboxyl groups derived from cyano
groups.
The membranes were activated under non-
aqueous condition by CDI to form N-acylimidazoles
of high reactivity, followed by enzyme
immobilization through conjugation with amino
groups of HRP.
Figure 2 shows the SEM photographs of the
nanofibrous membranes before and after HRP
immobilization. The nanofibers’ surface was smooth
and their average diameter ranged from 200 to 300
nm. The HRP immobilized on the membranes made
the surface of the fiber coarser than those without
HRP. Several big beads appeared in the image may
be some fragments of the fiber or salt crystals.
Figure 2: SEM images of the nanofibrous membrane (a)
before and (b) after immobilization.
FTIR was used to characterize the PAN/PVdF
nanofibrous membrane (NFM), NFM after NaOH
treatment, and NFM immobilized with HRP. Figure
3a shows peaks at 1402.8, 1276.7 and 474.6 cm
-1
,
which are corresponding to CF
2
bending, CF
2
stretching and CF
2
wagging, respectively. Compared
Figure 3b with Figure 3a, new peaks at 1561.8 cm
-1
and 1665.7 cm
-1
indicate the existence of carboxylic
and formamide groups. The appearance of these
hydrophilic groups can also support the change of
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
286
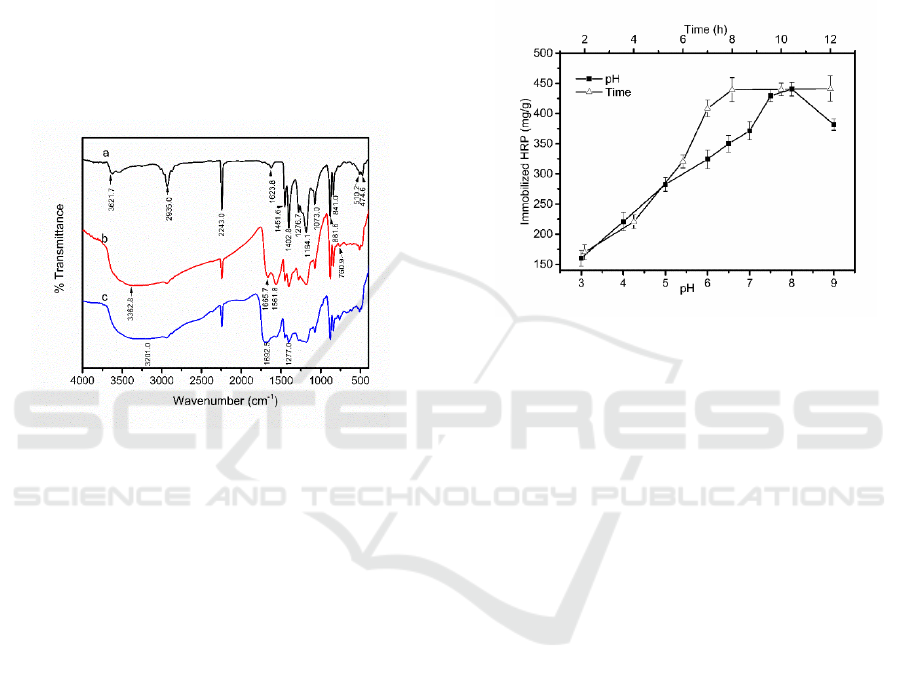
membrane surface from hydrophobic to totally
hydrophilic after NaOH treatment. which depicted in
Figure 1. It can be seen that Figure 3c has a strong
adsorption band at 1692.5 cm
-1
, representing the
stretching vibration of C=O. Meanwhile, a peak at
1277.0 cm
-1
appeared which could be due to the
combination of C-N stretching vibration and N-H
bending vibration. In addition, the peak at 3201.0
cm
-1
indicates the stretching vibration of N-H. These
new peaks are produced during the immobilization
of HRP on the surface of the fiber membrane. As a
result, it can be confirmed that HRP molecules has
been successfully immobilized on PAN/PVdF NFMs
through chemical bonding.
Figure 3: FTIR graphs of (a) pristine PAN/PVdF
membrane (b) membrane after NaOH treatment (c)
membrane immobilized with enzyme.
3.2 Effects of pH and Time on HRP
Immobilization
Figure 4 shows the effects of time and pH on the
immobilization efficiency of HRP. Both pH and
time have significant influences on the
immobilization. It can be seen from Figure 4 that
extreme pH conditions greatly restricted the
immobilization efficiency. This is not only because
extreme pH condition will destroy the structure of
the enzyme, but more importantly because the mode
of interaction between the enzyme and the carrier
(eg, the orientation of the enzyme) depends on the
ionic strength and pH (Xu et al., 2016). The
optimum pH for HRP immobilization was between
7.5 and 8.0. It was in accordance with the results in
our previous study (Xu et al., 2015a). The
immobilization efficiency improved with the
increase of time until it levelled off after 8 h. It
might be resulted from the saturation of immobilized
HRP owing to steric constraints because the enzyme
occupies a certain volume of space (Cristóvão et al.,
2011). The maximum HRP loading was 440.2 mg/g
after 8 h at pH 8.0 and 25
o
C. This immobilization
efficiency was relatively higher than that reported
before (Takahashi et al., 2001) (Lai and Lin, 2005)
(Xu et al., 2013). The high HRP loading may be
attributed to the high specific surface area of the
PAN/PVdF membrane and the suitable
immobilization method which may retain high
residue activity of HRP.
Figure 4: Effect of pH (12 h at 25 oC with pH
3,4,5,6,7,8,9) and time (2, 4, 6, 7, 8,10,12 h at 25 oC with
pH 6.0 CPBS) on the HRP immobilization efficiency.
3.3 Characterization of Free and
Immobilized HRP
Stabilities are important indexes to measure the
properties of the immobilized enzymes for their
further industrial applications. Figure 5(a) and (b)
shows the effects of pH and temperature on the
relative activity of free and immobilized HRP. Free
HRP reached optimum activity at pH 4.0 and 30
o
C
while immobilized ones shifted to pH 7.0 and 40
o
C.
The enzyme activity of immobilized HRP was
higher than that of free ones in the pH range from
3.0 to 3.5 and5.0 to 9.0, demonstrating that the
immobilized HRP was less sensitive to the pH
conditions during the testing period. For example,
the relative activity of immobilized HRP at pH 9
was 50.2%, which was significantly higher than that
of free HRP (10.1%). It could be attributed to the
buffering effect provided by the support of
membrane (Liu et al., 2013). Additionally, the
immobilized HRP showed greater relative activity
than free HRP, especially at temperature higher than
40
o
C. As temperature increased, the relative activity
of immobilized HRP decreased significantly slower
than that of free HRP, indicating that the
immobilized HRP had a higher temperature stability.
It may be attributed to the protecting effect provided
Immobilization of Horseradish Peroxidase on Modified Electrospun Nanofibrous Membrane for 2,4-Dichlorophenol Removal
287
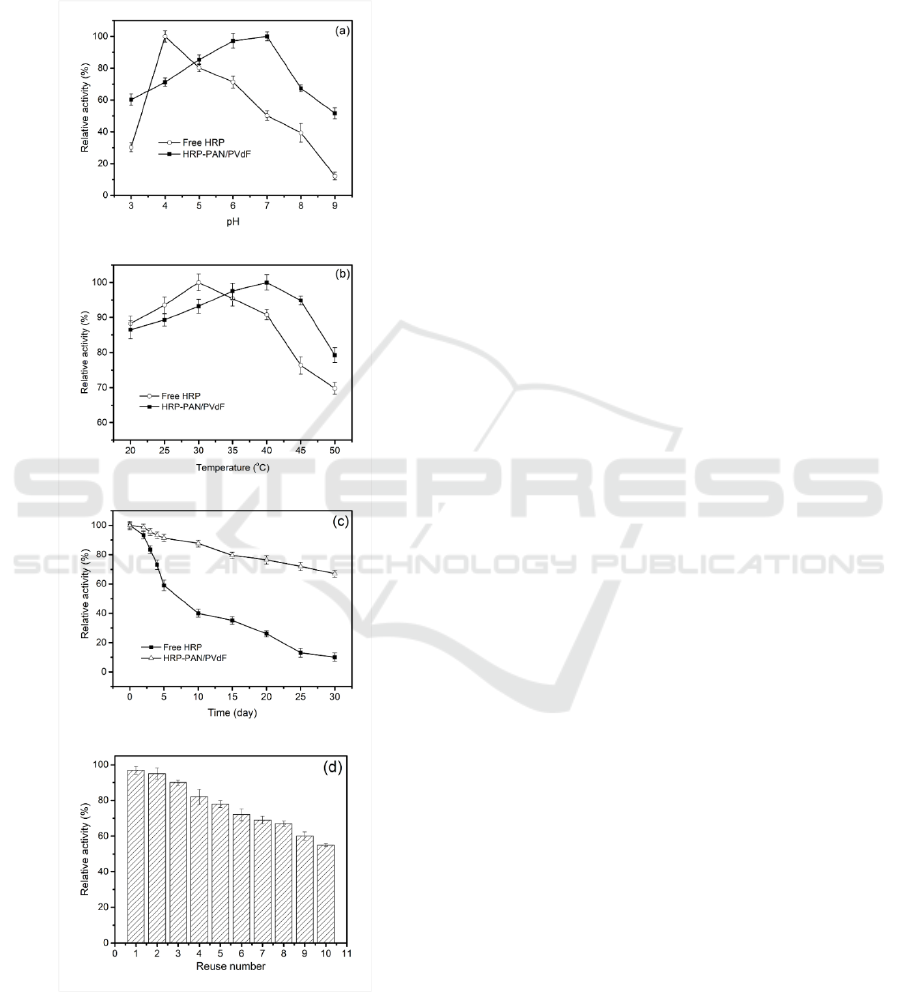
by the immobilization support at high temperatures
when enzyme deactivation occured (Osma et al.,
2010). The enzyme rigidity increased through the
immobilization process demonstrated by an
increased thermal stability against denaturation
(Abdel-Naby, 1993).
Figure 5: pH (a) (at 25
o
C with pH 3-9), temperature (b)
(at 20-50
o
C with pH 4), storage (c) (optimum conditions)
and operational stabilities (d) (optimum conditions) of free
HRP and HRP-PAN/PVdF.
Generally, enzymes are unstable in solution, and
their activities would decrease as storage period
increases. In contrast, immobilized enzyme can
overcome this disadvantage. Figure 5c shows the
activity of free HRP decreased significantly faster
than immobilized HRP (p<0.05). For example, after
30 days, the immobilized HRP still retained an
activity of 66.9%, while for free HRP, the relative
activity was only 10.0%. Therefore, immobilized
HRP was more stable than free HRP. It was
attributed to the limited conformational changes in
enzyme molecules in the matrix of fibrous
membrane (Xu et al., 2015b).
Immobilized enzyme can be more easily
separated from the reaction solution compared with
free enzyme, which would greatly decrease the cost
of enzyme for further application (Quintanilla-
Guerrero et al., 2008). It was found from Figure 5d
that HRP-PAN/PVdF retained 55% of its initial
activity after 10 cycles of reuse. The loss in enzyme
activity may be related to the inactivation of enzyme
caused by denaturation of the protein as well as the
breakage of the membrane (Huang et al., 2008).
3.4 Removal of 2,4-DCP by Free and
Immobilized HRP
Figure 6 shows the effect of pH and H
2
O
2
initial
concentration on the removal efficiency of 2,4-DCP
by free HRP, PAN/PVdF NFM as well as HRP-
PAN/PVdF. As illustrated in Figure 6(a), the
adsorption of the PAN/PVdF membrane removed
approximately 20% of 2,4-DCP in the experiment
about the effect of pH, and no significant variation
(p>0.05) was observed as pH value changed, which
could due to the strong stability of the PAN/PVdF
membrane under different pH conditions. The
removal efficiency of 2,4-DCP reached a maximum
of 90% at pH 7.0 by immobilized HRP and 89.2%
by free HRP at pH 4.0. The difference in optimum
pH conditions for 2,4-DCP removal by free and
immobilized HRP was consistent with the pH
stability results. Furthermore, immobilized HRP
showed high removal efficiency towards 2,4-DCP
than free HRP, especially under alkaline conditions.
It may be explained by the protection of the carrier.
Figure 6(b) shows that the maximum 2,4-DCP
removal efficiency reached as high as 94.9% by free
HRP at an initial H
2
O
2
concentration of 0.4 mM and
94% by immobilized HRP at an initial H
2
O
2
concentration of 0.6 mM. As initial H
2
O
2
concentration changed, there was no significant
change (p>0.05)in adsorption rate of 2,4-DCP by
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
288
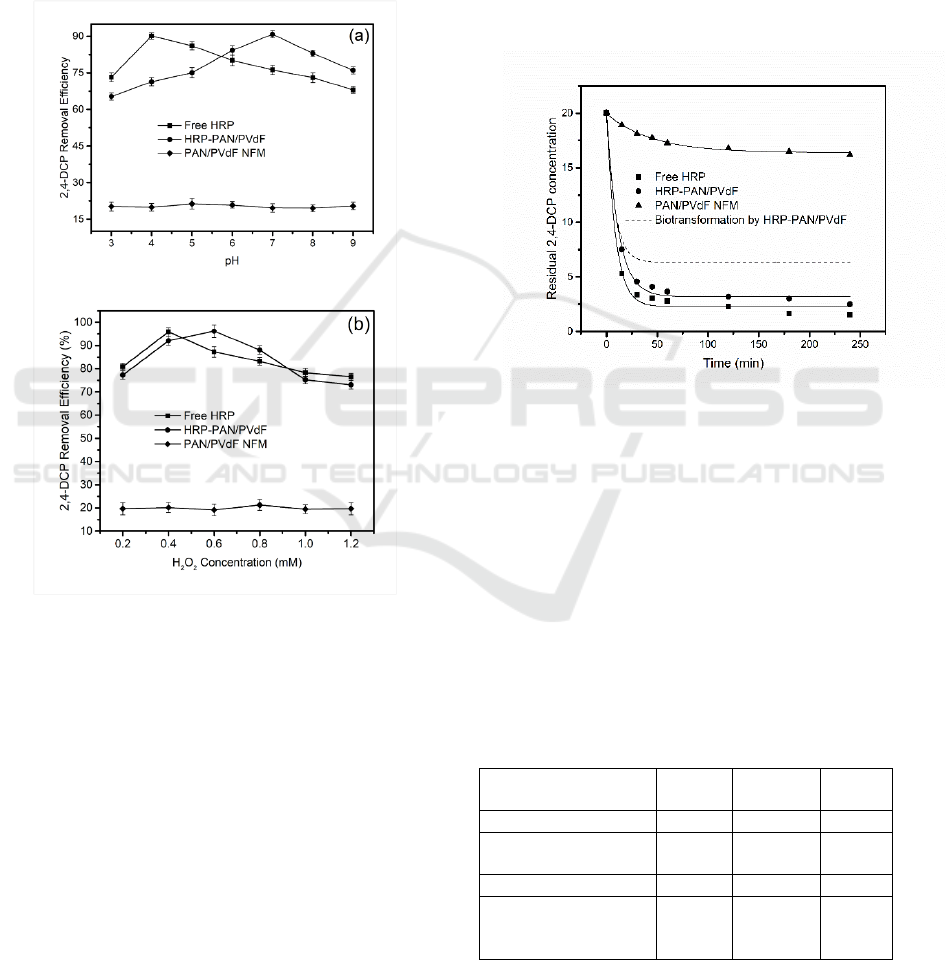
PAN/PVdF NFM. In addition, superabundant H
2
O
2
had adverse effect on the removal of 2,4-DCP. It
may be attributed to the following reasons: Firstly,
excess HRP may react with the intermediates and
result in a less active form of HRP during the
process of 2,4-DCP catalytic oxidation (Cai and
Tien, 1992). Secondly, an overdose of H
2
O
2
may
participate in irrelevant reactions (Maloney et al.,
1986).Therefore, a suitable amount of H
2
O
2
is a key
factor for 2,4-DCP removal in an reaction system.
Figure 6: Effect of pH (a) (4h with 20 mg/L 2,4-DCP, 5
mg of free or immobilized HRP and 0.8 mmol/L H2O2)
H2O2 initial concentration (b) on the removal efficiency
of 2,4-DCP by free HRP and HRP-PAN/PVdF. (4h with
20 mg/L 2,4-DCP, 5 mg of free or immobilized HRP and a
constant pH)
Figure 7 shows the removal efficiency of 2,4-
DCP in a 4 h batch experiment. The degradation
efficiency of 2,4-DCP by free HRP reached
approximately 83% in the first 30 min and 93% after
a 3 h treatment. By contrast, the removal efficiency
of 2,4-DCP by HRP-PAN/PVdF reached 77% in the
first 30 min and 87% after 3 h. According to the Eq.
(2), we could easily calculate that about 68% of 2,4-
DCP was biotransformed by HRP-PAN/PVdF.
Although the enzyme molecules immobilized on
PAN/PVdF NFM were relatively less than that of
free HRP, the removal efficiency towards 2,4-DCP
did not show significant difference. It was due to the
combined effects of biodegradation and adsorption
by HRP-PAN/PVdF NFM. The adsorption of 2,4-
DCP on the nanofiber membrane carrier was
concentrated in 1 hour after the start of the reaction,
and there was no significant increase after 1 hour.
This indicates that the adsorption of 2,4-DCP on the
carrier is in the initial stage of the reaction, after
which the adsorption-desorption equilibrium is
reached. Figure 7 also shows that the kinetics of 2,4-
DCP removal followed a first-order reaction.
Figure 7: Removal kinetics of 2,4-DCP by free HRP (4h
at 25
o
C with pH 4.0 and 0.4 mM H
2
O
2
initially) and HRP-
PAN/PVdF (4 h at 25
o
C with pH 6.0 and 0.6 mM H
2
O
2
initially).
Table 1 showed the biodegradation rate of HRP
immobilized on NFMs was slower than that of free
HRP. It may be explained by spatial limitations for
substrate diffusion and protein flexibility after
enzyme immobilization on the carrier (Bai et al.,
2006) (Sari et al., 2006), as well as the fact that the
immobilized HRP was partly inactivated during the
immobilization process.
Table 1: Value of k, t
1/2
, and RE
240
of free HRP, HRP-
PAN/PVdF NFM, and NFM towards 2,4-DCP.
Sample k/(min) t
1/2
(min)
RE
240
(%)
Free HRP 0.122 5.68 92.35
HRP-PAN/PVdF
NFM
0.087 7.97 87.55
PAN/PVdF NFM 0.022 31.51 19.00
Biotransformation by
HRP-PAN/PVdF
NFM
0.112 6.19 68.55
Immobilization of Horseradish Peroxidase on Modified Electrospun Nanofibrous Membrane for 2,4-Dichlorophenol Removal
289
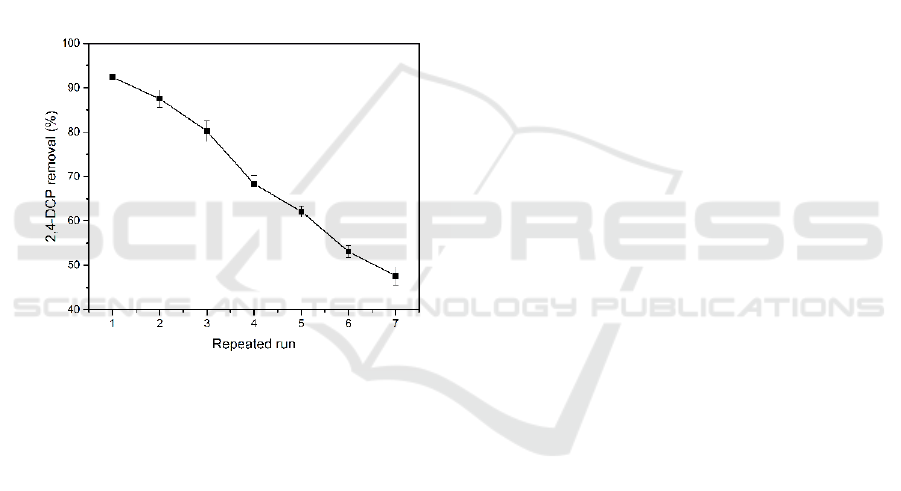
3.5 Reusability of HRP-PAN/PVdF
NFM
Immobilization of the enzyme can overcome the
inherent drawbacks of free enzymes-hard separation
and non-reusability. Figure 8 shows that HRP-
PAN/PVdF could still remove 47.6% of 2,4-DCP
after 7 repeated runs, which is much higher than
some other researches (Magri et al., 2007). The
decrease of the 2,4-DCP removal efficiency could be
explained by the adsorption of the reaction products
as well as the appearance of some undissolved
substances like the damaged components of the
PAN/PVdf membrane. The adsorption prevented the
contact between enzyme and substrate and the
undissolved substances slowed down the flow
velocity (Durán et al., 2002).
Figure 8: Variation of the 2,4-DCP removal rate by HRP-
PAN/PVdF. (at 25
o
C with pH 6.0 and 0.6 mM H
2
O
2
initially)
4 CONCLUSIONS
One novel method for horseradish peroxidase
immobilization was developed in this study. The
PAN/PVdF membranes fabricated by
electrospinning were converted from hydrophobic
into hydrophilic ones and were successfully applied
for HRP immobilization to retain high activity.
Under the optimum immobilization conditions, the
maximum enzyme loading of PAN/PVdF
nanofibrous membranes were 440 mg/g. Meanwhile,
the enzyme could retain high relativity after
immobilization. Compared with free HRP, the
immobilized HRP has better pH, thermal, storage
and operational stability. The work range of pH and
temperature was extended as well. Free HRP and the
immobilized HRP were applied in the removal of
2,4-DCP. Results showed that the removal
efficiency of the immobilized HRP for 2,4-DCP was
87%, while that of free HRP was 93%. The removal
efficiency of the immobilized enzyme as good as
free HRP. It could be concluded from the
experiments that the degradation of immobilized
HRP was first-order reaction and the removal of the
pollutants could be attributed to the adsorption of
nanofibrous membrane and the biodegradation of
HRP. HRP immobolized on PAN/PVdF membranes
also had better reusability. Therefore, HRP
immobilized on modified PAN/PVdF membrane
could be deemed as a promising material for future
applications in aquatic organic pollutants removal.
ACKNOWLEDGEMENTS
This work was funded by the National Natural
Science Foundation of China (21777119) and
Sichuan Science and Technology Program
(2018TJPT0017).
REFERENCES
Abdel-Naby, M. A., 1993. Immobilization ofAspergillus
niger NRC 107 xylanase and β-xylosidase, and
properties of the immobilized enzymes. Applied
biochemistry and biotechnology, 38, 69-81.
Antizar-Ladislao, B. & Galil, N. I., 2004. Biosorption of
phenol and chlorophenols by acclimated residential
biomass under bioremediation conditions in a sandy
aquifer. Water Research, 38, 267-276.
Bai, Y.-X., Li, Y.-F. & Wang, M.-T., 2006. Study on
synthesis of a hydrophilic bead carrier containing
epoxy groups and its properties for glucoamylase
immobilization. Enzyme and microbial technology, 39,
540-547.
Cai, D. & Tien, M., 1992. Kinetic studies on the formation
and decomposition of compounds II and III. Reactions
of lignin peroxidase with H2O2. Journal of Biological
Chemistry, 267, 11149-11155.
Crist V O, R. O., Tavares, A. P., BR Gida, A. I., Loureiro,
J. M., Boaventura, R. A., Macedo, E. A. & Coelho, M.
A. Z., 2011. Immobilization of commercial laccase
onto green coconut fiber by adsorption and its
application for reactive textile dyes degradation.
Journal of Molecular Catalysis B: Enzymatic, 72, 6-
12.
Dur N, N., Rosa, M. A., D’Annibale, A. & Gianfreda, L.,
2002. Applications of laccases and tyrosinases
(phenoloxidases) immobilized on different supports: a
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
290

review. Enzyme and Microbial Technology, 31, 907-
931.
Gao, K., Hu, X., Dai, C. & Yi, T., 2006. Crystal structures
of electrospun PVDF membranes and its separator
application for rechargeable lithium metal cells.
Materials Science and Engineering: B, 131, 100-105.
Huang, X.-J., Yu, A.-G. & Xu, Z.-K., 2008. Covalent
immobilization of lipase from Candida rugosa onto
poly(acrylonitrile-co-2-hydroxyethyl methacrylate)
electrospun fibrous membranes for potential bioreactor
application. Bioresource Technology, 99, 5459-5465.
Keith, L. & Telliard, W., 1979., ES&T special report:
priority pollutants: Ia perspective view. Environmental
Science & Technology, 13, 416-423.
Khenifi, A., Zohra, B., Kahina, B., Houari, H. & Zoubir,
D., 2009. Removal of 2, 4-DCP from wastewater by
CTAB/bentonite using one-step and two-step methods:
a comparative study. Chemical engineering journal,
146, 345-354.
Kim, J. R., Choi, S. W., Jo, S. M., Lee, W. S. & Kim, B.
C., 2004. Electrospun PVdF-based fibrous polymer
electrolytes for lithium ion polymer batteries.
Electrochimica Acta, 50, 69-75.
Lai, Y.-C. & Lin, S.-C., 2005. Application of immobilized
horseradish peroxidase for the removal of p-
chlorophenol from aqueous solution. Process
Biochemistry, 40, 1167-1174.
Liu, Q., Kong, X., Zhang, C., Chen, Y. & Hua, Y., 2013.
Immobilisation of a hydroperoxide lyase and
comparative enzymological studies of the immobilised
enzyme with membrane‐bound enzyme. Journal of
the Science of Food and Agriculture, 93, 1953-1959.
Liu, S., Fu, J., Ge, M., Tan, L. & Du, W., 2014.
Electrospinning of polyacrylonitrile nanofibers using
strain-hardening spinning solutions. Fibers and
Polymers, 15, 2441-2445.
Magri, M. L., De Las Nieves Loustau, M., Victoria
Miranda, M. & Cascone, O., 2007. Immobilisation of
soybean seed coat peroxidase on its natural support for
phenol removal from wastewater. Biocatalysis and
Biotransformation, 25, 98-102.
Maloney, S. W., Manem, J., Mallevialle, J. & Fiessinge, F.
1986. Transformation of trace organic compounds in
drinking water by enzymic oxidative coupling.
Environmental science & technology, 20, 249-253.
Nicell, J., Bewtra, J., Biswas, N., St. Pierre, C. & Taylor,
K., 1993. Enzyme catalyzed polymerization and
precipitation of aromatic compounds from aqueous
solution. Canadian Journal of Civil Engineering, 20,
725-735.
No, D., 2001. 2455/2001/EC of the European Parliament
and of the Council of 20 November 2001 establishing
the list of priority substances in the field of water
policy and amending Directive 2000/60/EC. Official
Journal of the European Communities, 15, 1-5.
Ormad, M., Ovelleiro, J. & Kiwi, J., 2001. Photocatalytic
degradation of concentrated solutions of 2, 4-
dichlorophenol using low energy light: identification
of intermediates. Applied Catalysis B: Environmental,
32, 157-166.
Osma, J. F., Toca-Herrera, J. L. & Rodr Guez-Couto, S.,
2010. Biodegradation of a simulated textile effluent by
immobilised-coated laccase in laboratory-scale
reactors. Applied Catalysis A: General,
373, 147-153.
Pan, H., Yang, J., Wang, S., Xiong, Z., Cai, W. & Liu, J.,
2015. Facile fabrication of porous carbon nanofibers
by electrospun PAN/dimethyl sulfone for capacitive
deionization. Journal of Materials Chemistry A, 3,
13827-13834.
Qin, X. H., Yang, E. L., Li, N. & Wang, S. Y., 2007.
Effect of different salts on electrospinning of
polyacrylonitrile (PAN) polymer solution. Journal of
applied polymer science, 103, 3865-3870.
Quintanilla-Guerrero, F., Duarte-V Zquez, M., GARC A-
ALMENDAREZ, B., TINOCO, R., VAZQUEZ-
DUHALT, R. & REGALADO, C., 2008. Polyethylene
glycol improves phenol removal by immobilized
turnip peroxidase. Bioresource technology, 99, 8605-
8611.
Sari, M., Akg L, S., Karatas, M. & Denizli, A., 2006.
Reversible immobilization of catalase by metal chelate
affinity interaction on magnetic beads. Industrial &
engineering chemistry research, 45, 3036-3043.
Selloum, D., Chaaya, A. A., Bechelany, M., Rouessac, V.,
Miele, P. & Tingry, S., 2014. A highly efficient
gold/electrospun PAN fiber material for improved
laccase biocathodes for biofuel cell applications.
Journal of Materials Chemistry A, 2, 2794-2800.
Takahashi, H., Li, B., Sasaki, T., Miyazaki, C., Kajino, T.
& Inagaki, S., 2001. Immobilized enzymes in ordered
mesoporous silica materials and improvement of their
stability and catalytic activity in an organic solvent.
Microporous and Mesoporous Materials, 44, 755-762.
Yin, X., Cheng, H., Wang, X., and Yao, Y., 1998.
Morphology and properties of hollow-fiber membrane
made by PAN mixing with small amount of PVDF.
Journal of membrane science, 146, 179-184.
Xu, R., Chi, C., Li, F. & Zhang, B., 2013. Immobilization
of horseradish peroxidase on electrospun microfibrous
membranes for biodegradation and adsorption of
bisphenol A. Bioresource technology, 149, 111-116.
Xu, R., Si, Y., Li, F. & Zhang, B., 2015a. Enzymatic
removal of paracetamol from aqueous phase:
horseradish peroxidase immobilized on nanofibrous
membranes. Environmental Science and Pollution
Research, 22, 3838-3846.
Xu, R., Tang, R., Liu, S., Li, F. & Zhang, B., 2015b. An
environmentally-friendly enzyme-based nanofibrous
membrane for 3, 3 ′ , 5, 5 ′ -tetrabromobisphenol
removal. RSC Advances, 5, 64091-64097.
Xu, R., Tang, R., Zhou, Q., Li, F. & Zhang, B., 2015c.
Enhancement of catalytic activity of immobilized
laccase for diclofenac biodegradation by carbon
nanotubes. Chemical Engineering Journal, 262, 88-95.
Xu, R., Yuan, J., Si, Y., Li, F., & Zhang, B., 2016. Estrone
removal by horseradish peroxidase immobilized on a
nanofibrous support with Fe 3 O 4 nanoparticles.
Yang, M.-C. & Liu, T.-Y., 2003. The permeation
performance of polyacrylonitrile/polyvinylidine
Immobilization of Horseradish Peroxidase on Modified Electrospun Nanofibrous Membrane for 2,4-Dichlorophenol Removal
291

fluoride blend membranes. Journal of membrane
Science, 226, 119-130.
Zhang, J., Shen, H., Wang, X., Wu, J. & Xue, Y., 2004.
Effects of chronic exposure of 2, 4-dichlorophenol on
the antioxidant system in liver of freshwater fish
Carassius auratus. Chemosphere, 55, 167-174.
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
292
