
Study on the Genetic Diversity of Reticulitermes Aculabialis
Sha Zhao
1, 2*
, Ya Wang
1
, Beibei Bie
1
, Le Xu
1
and Hong Bai
1
1
School of Medicine, Xi’an Pei hua University, Xi ’an, 710125, P.R. China
2
Genetics laboratory, College of life sciences, Northwest University, Xi’an, 710069, P.R. China
Keywords: Microsatellite primer, Genetic diversity, Environmental protection
Abstract: Termites are a kind of insect pest that is harmful to the environment. The genetic diversity of two natural
populations in Xi'an and Nanjing was investigated using 6 pairs of highly polymorphic microsatellite markers.
The results showed that the average number of alleles in Reticulitermes aculabialis populations of Xi'an and
Nanjing was 2.075 and 2.011, respectively; the average number of effective alleles (Ne) was 1.747 and 1.705,
respectively; and the average observed heterozygosity (Ho) was 0.483 and 0.445, respectively. The expected
mean value of heterozygosity (He) was 0.366 and 0.344, respectively, while the average value of the diversity
index (I) was 0.566 and 0.534, respectively. All the above results indicated that the genetic diversity and the
degree of genetic variation within Xi'an and Nanjing populations were similar, both at a moderate but
relatively low level. Additionally, the genetic structure of the populations in Xi 'an and Nanjing was analysed
by GenALEx software, showing that there was significant genetic differentiation (Fst=0.409) between the two
populations, and the gene flow between the populations was relatively low (Nm = 0.375).
1 INTRODUCTION
Termites belong to Arthropoda: Insecta: Isoptera,
which are the most primitive of the social insects.
There are 2,935 species of termites in the world
(Chand et al., 2018), belonging to 9 families and 283
genera. China has one of the highest abundances of
termite species. The termite fauna of China is
composed of 476 described species belonging to 44
genera in 4 families (Zhong and Liu, 2002).
At present, breeding termites causes a wide range
of hazards, such as house destruction, dam breakage,
ship sinking, bridge collapse, forest tree health
damage and cultural relic damage; they directly
interfere with people's normal life order (Rust and Su,
2012). The harm is usually isolated; thus, it does not
draw much attention. Humans roughly understand the
seriousness of the hazard but ignore its important
relationship with environmental protection.
As a social insect, the distribution of genetic
diversity at the population level is associated with the
genetic structure at the colony level. However, due to
its complex life history, it is difficult to analyse the
genetic diversity and breeding system by collecting
reproductive subterranean termites, and it is difficult
to further study their biological characteristics and
nest structure (Vargo, 2003).
With the development of molecular technology,
microsatellite genetic markers have been used to
detect the population genetic diversity and genetic
differentiation of termites. The first microsatellite
study of termites was by Vargo, who published 9
microsatellite markers and related primer sequences
for R. flavipes (Vargo, 2000). In the same year, Vargo
and Henderson published 12 microsatellite markers
and their related primer sequences for Coptotermes
formosanus (Vargo and Henderson, 2000).
Reticulitermes aculabialis, belonging to
Rhinotermitidae, is a harmful termite in China and is
distributed in 18 provinces and autonomous regions
in China (Xing, Cui and Cheng, 1998). According to
our investigation, Reticulitermes aculabialis is the
main termite species that endanger garden plants in
Shaanxi and Jiangsu. In recent years, the termite
damage in the northwest region has become
increasingly serious, and the damage in some areas is
close to the south bank of the Yangtze River (Li et al.,
2010).
Considering the significant impact of termite
breeding hazards on the environment, this study
intends to use the microsatellite primer marker SSR
to investigate the genetic diversity of two different
populations of Reticulitermes aculabialis. The results
may serve as a support for the termite reproductive
Zhao, S., Wang, Y., Bie, B., Xu, L. and Bai, H.
Study on the Genetic Diversity of Reticulitermes Aculabialis.
DOI: 10.5220/0008188402550259
In The Second International Conference on Materials Chemistry and Environmental Protection (MEEP 2018), pages 255-259
ISBN: 978-989-758-360-5
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
255
evolution mechanism and provide effective
prevention and control strategies for environmental
protection.
2 MATERIALS AND METHODS
2.1 Experimental Materials
Samples of Reticulitermes aculabialis were collected
from 60 natural colonies in Xi'an and Nanjing, for a
total of 240 experimental samples that were used as
experimental materials. The experimental materials
collected from the field were immediately immersed
in absolute ethanol and stored at -20 °C until use
(Table 1).
2.2 Experimental Instruments and
Reagents
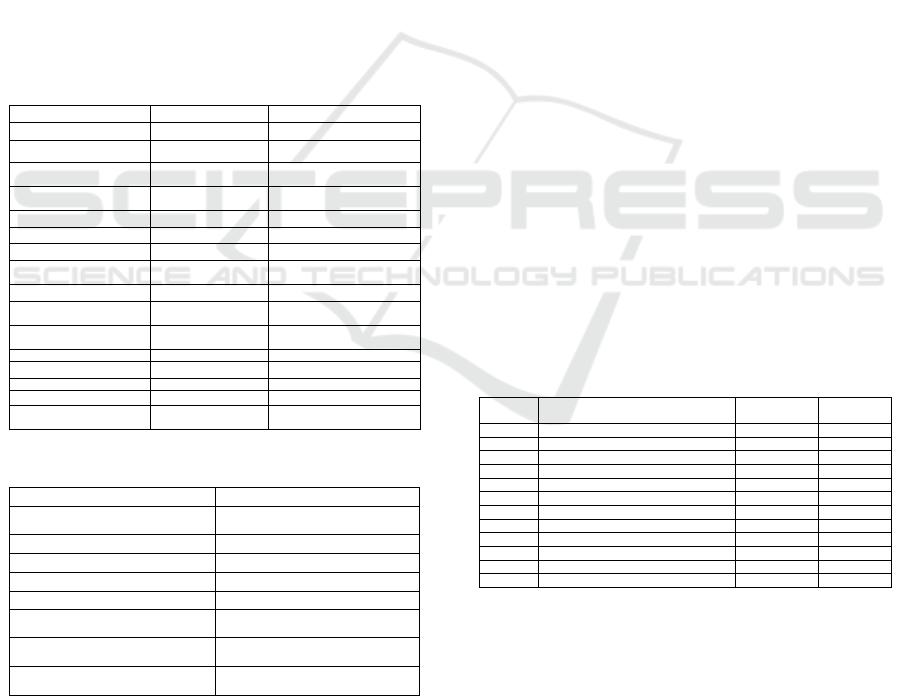
Table 1: Instruments and equipments used in the
experiment.
Names of equipments
Models of equipments
Sources of experimental equipments
Water bath
HH-6
Guohua Electric Company
PCR amplification instrument
Mastercycler nexus gradient
Eppendorf, Germany
Ultra-clean workbench
AIRTECH
Suzhou Yida Boland Purification
Laboratory System Equipment
Pressure steam sterilization pot
ZDX-35
Shanghai Shen'an Medical
Instrument Factory
UVP gel imager
Bio-rad
Thermo Fisher Scientific
Vertical electrophoresis tank
DYCZ-24B
Beijing Liuyi Instrument Factory
Horizontal electrophoresis tank
DYCP-31DN
Beijing Liuyi Instrument Factory
Oscillator
Qilinbeier QL-901
Qilinbeier Instrument Manufacturing
Co., Ltd.
Pure water system
Arium611
Sartorius, Germany
Electric constant temperature
drying oven
TXQ-LQ-18SI
Shanghai Senxin Instrument
Co., Ltd.
Decolorization shaker
Qilinbeier TS-1 orbital
Shaker
Qilinbeier Instrument Manufacturing
Co., Ltd.
Refrigerator
BCD-208k/A CJN
Qingdao Haier Co., Ltd.
Micropipette
Eppendorf
Eppendorf, Germany
Analytical Balances
METTLER TOLEDO
Switzerland
High speed refrigerated centrifuge
Eppendorf 5430R
Eppendorf, Germany
Vortex mixer
QL-901
Qilinbeier Instrument Manufacturing
Co., Ltd.
Table 2: Names and Sources of experimental reagents.
Names of experimental reagents
Sources of experimental reagents
Blood genomic DNA Extraction Kit
(centrifugal column type)
Shanghai Shenggong Biological
Engineering Co., Ltd.
2×Taq PCR Mix
Xi'an Runde Biotechnology Co., Ltd.
ddH
2
O
Xi'an Runde Biotechnology Co., Ltd.
600 bp DNA Marker I
Xi'an Runde Biotechnology Co., Ltd.
Microsatellite primer
Xi'an Runde Biotechnology Co., Ltd.
Goldview
Shanghai Shenggong Biological
Engineering Co., Ltd.
Ethanol
Shanghai Shenggong Biological
Engineering Co., Ltd.
TEMED
Shanghai Shenggong Biological
Engineering Co., Ltd.
2.3 Sample Genome DNA Extraction
Genomic DNA was extracted using a DNA extraction
kit (Table 2). The sample was detected by
electrophoresis on a 10 g/L agarose gel. The
concentration was measured by an ultraviolet
spectrophotometer and stored at 4 °C (Table 1).
2.4 Microsatellite Primers, PCR
Reaction Parameters and Genotype
Products
Six pairs of microsatellite primers were selected;
three pairs of primers, Ra132, Ra141 and Ra144,
were selected by the laboratory and proved to have
good polymorphism. The other three pairs of primers
Rs03, Rs76 and Rs78 also proved to have good
polymorphism (Vargo, 2000; Dronnet et al., 2004).
The primer sequences and related parameters are
shown in Table 3. The microsatellite primers were
fluorescently labelled using semi-automatic
fluorescent microsatellite markers, and the extracted
whole genome DNA was amplified by PCR with the
Mastercycler nexus GXS1 using synthetic fluorescent
primers. The reaction solution was as follows: 7.5 μl
of Mix (Table 2), 0.6 μl of forward and reverse
primers, 3 μl of template DNA, and ddH2O to bring
the total solution to 15 μl . The amplification
procedure was as follows: 94°C pre-denaturation for
5 min, 30 cycles of 94°C denaturation for 30 s,
suitable annealing temperature for 30 s, 72°C
extension for 30 s, and a 72°C extension for 10 min.
A total of 3 μl of the amplified PCR product was
taken for agarose gel electrophoresis, and high-
quality PCR products were selected and sent to
Shanghai Biotech for testing on an ABI3700
automatic analyser.
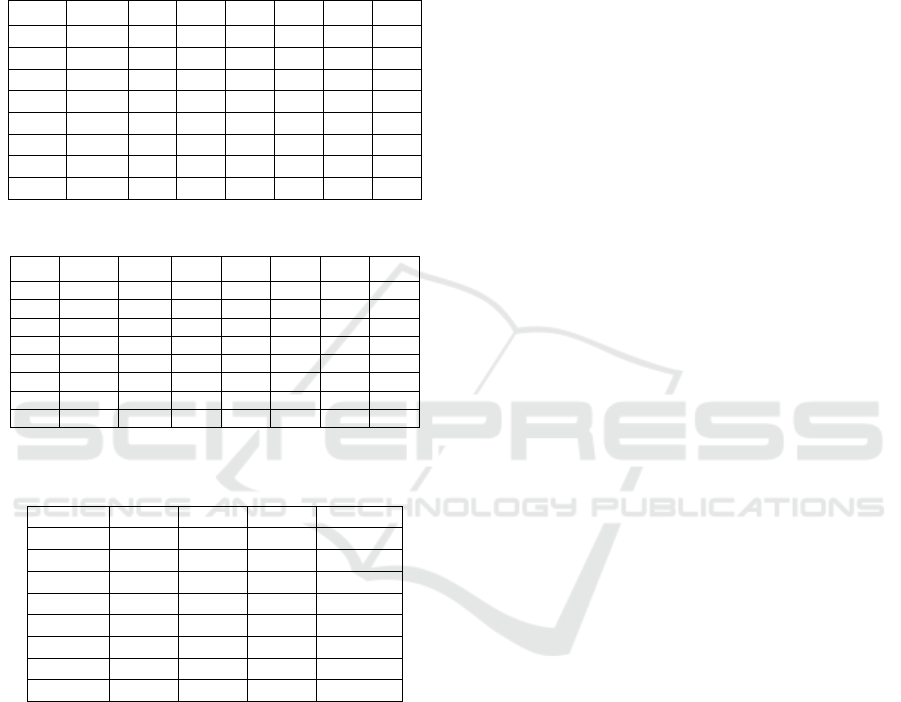
Table 3: Six pairs of microsatellite loci primers.
Primer
Primer sequences
Core repeat unit
The annealing
temperature
Rs03
TCCTGACTGTACAAAGAAAAGTGG
(CT)9
58.2℃
TGGCATCAAGCTACGTATTCA
Rs76
AATCCGGGGAATTTCTTGAC
(AGTT)8
56.9℃
CTGCATAACGATGTCTGCGT
Rs78
GCTTCTCAAGAAGGACTGTGC
(AGTT)7
56.9℃
GCCCCAGTTGAGATATGGAA
Ra132
GATTGGTTTCCTCCGAATCA
(TTA)14
58.2℃
AAAGACTACTGCCACCGGG
Ra141
CACATTTGAGGTTCGCAAGA
(TTA)8
59.7℃
GCCAGAAGGCCAATTACAGA
Ra144
CAAATAGAGCTCCGTGTTTCG
(TTAG)7
56.9℃
CCATAGAAACCTCCGAAAGG
2.5 Statistical Analysis of Data
After data collection using GeneMarker 2.2.0
software, the length of the amplified product was read
and calibrated for statistical analysis. The data format
was converted using GenAlEx software based on an
Excel macro calculation. GenAlEx6 software was
used to transform the data format used by a different
software package (Rod and Petere, 2006). The genetic
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
256

diversity and genetic structure index of each
population, including percentage of diversity bands
(PPL), number of observed alleles (Na), number of
effective alleles (Ne) (Nei, 1973), observed
heterozygosity (Ho), Nei's genetic diversity index
(He) and Shannon information index (I) (Lewontin,
1995) for each pair of primers was calculated using
Popgene32 software and Excel software. The
polymorphic information content (PIC) of each
microsatellite locus was calculated using Cervus
software referenced by Smith (Jsc et al., 1997) and
other methods. Application of GenAlEx 6.5 (Peakall
et al., 2012) software was used to calculate genetic
variation fixed index F-statistics (FIT, FIS, FST)
(Wright, 1978) and gene flow between populations
(Nm) (Whitlock and Mccauley, 2010).
3 RESULTS AND ANALYSIS
3.1 Analysis of SSR Genetic Diversity
in Each Population of
Reticulitermes Aculabialis
In this study, six pairs of SSR primers were used to
analyse the genetic diversity of populations in Xi 'an
and Nanjing. The calculation of genetic diversity
parameters was performed using GenAlEx software
(Table 3 and 4). The results showed that the
polymorphic information content (PIC) ranged from
0.293 to 0.739, and the average polymorphic
information content was 0.472. Of the six
microsatellite loci, highly polymorphic seats with
PIC values greater than 0.5 were observed in 2 of the
6 microsatellite loci. The PIC values of Rs 03, Rs 76,
Ra 132 and Ra 141 were between 0.25 and 0.5,
indicating that the six microsatellite loci used in this
study showed moderately high polymorphism in the
population of Reticulitermes aculabialis from Xi'an.
The number of alleles (Na) was 1.710 to 3, with an
average of 2.075, and the number of effective alleles
(Ne) was 1.431 to 2.441, with an average of 1.747,
indicating that the alleles (Na) in this population were
not evenly distributed and that the availability of
alleles (Na) was low. The observed heterozygosity
(Ho) values were 0.363 to 0.774, and the average
observed heterozygosity was 0.483. The expected
heterozygosity values (He) were 0.252 to 0.558, and
the average expected heterozygosity was 0.366.
The larger the observed heterozygosity value, the
greater the degree of genetic variation within the
population; thus, the genetic variation within the
population was at a moderately low level. The
diversity index values (I) were 0.379 to 0.939, with
an average value of 0.566, which was a medium but
relatively low level, indicating that there was a certain
diversity within the population, but the diversity was
not high.
In the population of Reticulitermes aculabialis in
Nanjing, the polymorphic information content (PIC)
ranged from 0.260 to 0.721, and the average
polymorphic information content was 0.483. Highly
polymorphic seats at PIC values greater than 0.5 were
observed in 2 of the 6 microsatellite loci, while Rs 03,
Rs 76, Ra 132, Ra 141 had PIC values between 0.25
and 0.5, showing moderate polymorphism (Table 5).
The six microsatellite loci used in this study also
showed moderately high polymorphism in the
population of Reticulitermes aculabialis from
Nanjing. The number of alleles (Na) was 1.552 to
2.793, and the mean was 2.011. The number of
effective alleles (Ne) was 1.326 to 2.337, and the
mean was 1.705. The difference between the number
of effective alleles (Ne) and the number of alleles
(Na) was not very large, indicating that the alleles in
the population are more evenly distributed. The
observed heterozygosity values (Ho) were 0.190 to
0.638, the average observed heterozygosity was
0.445, the expected heterozygosity values (He) were
0.190 to 0.502, and the average expected
heterozygosity was 0.344. Compared with the Xi'an
population, the genetic variation between the two
populations was similar. The polymorphism index
values (I) were 0.335 to 0.850, with an average value
of 0.534, indicating a moderately low level of genetic
variation and genetic diversity in the population.
3.2 Genetic Differentiation of
Populations of Reticulitermes
Aculabialis
GenAlEX software was used to calculate the total
gene diversity index (FIT), the inbreeding coefficient
(FIS) between individuals within the population and
the genetic differentiation between populations
Coefficient (FST) for 240 samples of termites in Xi'an
and Nanjing. Additionally, analysis of the genetic
structure of the termite population was conducted.
The results are shown in Table 6. Six
microsatellite loci showed negative values in the total
gene diversity range (FIT) of the population and
negative values of the inbreeding coefficient (FIS),
indicating that there was excess heterozygosity. The
differentiation coefficient (FST) was 0.311~0.504;
the maximum was detected at the Rs 03 site, and the
minimum was detected at the Ra 141 site, with an
average value of 0.409 (> 0.25). The gene flow (Nm)
Study on the Genetic Diversity of Reticulitermes Aculabialis
257
was 0.246~0.553, with an average value of 0.375 (<
1), indicating that the gene flow between different
geographical populations was obstructed, the level of
gene exchange between populations was relatively
low, and the genetic differentiation was relatively
large.
Table 4: Genetic diversity of R. aculabialis in Xi’an.
Locus
Sample
Na
Ne
I
Ho
He
PIC
RS03
124
2.000
1.772
0.578
0.452
0.387
0.486
RS76
124
1.903
1.605
0.500
0.484
0.336
0.393
RS78
124
2.097
1.742
0.589
0.460
0.386
0.511
Ra132
124
1.742
1.489
0.409
0.363
0.274
0.410
Ra141
124
1.710
1.431
0.379
0.363
0.252
0.293
Ra144
124
3.000
2.441
0.939
0.774
0.558
0.739
Mean
124
2.075
1.747
0.566
0.483
0.366
0.472
SE
0
0.054
0.042
0.024
0.025
0.015
0.062
Table 5: Genetic diversity of R. aculabialis in Nanjing.
Locus
Sample
Na
Ne
I
Ho
He
PIC
RS03
116
1.552
1.326
0.289
0.190
0.190
0.496
RS76
116
2.172
1.760
0.606
0.534
0.390
0.496
RS78
116
2.034
1.656
0.537
0.414
0.346
0.553
Ra132
116
1.931
1.744
0.587
0.517
0.409
0.375
Ra141
116
1.586
1.409
0.335
0.379
0.228
0.260
Ra144
116
2.793
2.337
0.850
0.638
0.502
0.721
Mean
116
2.011
1.705
0.534
0.445
0.344
0.483
SE
0
0.058
0.044
0.027
0.026
0.017
0.064
Table 6: Results of F-statistics analysis and gene flow
(Nm).
Locus
FIS
FIT
FST
Nm
RS03
-0.114
0.447
0.504
0.246
RS76
-0.404
0.125
0.377
0.413
RS78
-0.193
0.339
0.446
0.311
Ra132
-0.288
0.278
0.440
0.318
Ra141
-0.541
-0.061
0.311
0.553
Ra144
-0.333
0.172
0.379
0.409
Mean
-0.312
0.217
0.409
0.375
SE
0.062
0.073
0.028
0.044
4 DISCUSSION
4.1 Analysis of SSR Genetic Diversity
in Two Populations of
Reticulitermes Aculabialis
Analysis of the SSR genetic diversity of two
populations in Xi'an and Nanjing showed the average
number of alleles per SSR locus (Na=2.011~2.075),
the average number of effective alleles (Ne=
1.705~1.747), the average observed heterozygosity
(Ho=0.445~0.483) and the average expected
heterozygosity (He=0.344~0.366), indicating that
there was a certain degree of genetic diversity in both
populations. As a very important indicator for
measuring the genetic diversity of a population, the
expected heterozygosity of microsatellites between
0.3 and 0.8 indicates that a population has higher
genetic diversity (Nei and Takezaki, 1996).
The
average expected heterozygosity of Reticulitermes
aculabialis in Xi'an and Nanjing was 0.355, which is
greater than 0.3. The two loci of Rs 76 and Rs 78 used
in this experiment were also involved in the genetic
diversity of R. grassei, R. santonensis and R. flavipes.
The expected heterozygosity of the two loci was
0.38~0.73 and 0.46~0.85, respectively, which are
both higher than the expected heterozygosity of Rs 76
and Rs 78 of Reticulitermes aculabialis in Xi'an and
Nanjing. Therefore, this result indicates that the
genetic diversity of Reticulitermes aculabialis in
Xi'an and Nanjing is generally rich, which has a
positive influence on the environment. This may be
due to inbreeding pressures, genetic mutations, small
populations or human factors.
4.2 Analysis of the Genetic Structure of
Reticulitermes Aculabialis
Populations
The key indicator reflecting the genetic
differentiation between populations is the genetic
differentiation coefficient (Fst). In general, a large
range of continuously distributed populations will
lead to a gradual decrease in gene flow as a result of
increased geographical distance leading to genetic
differentiation (Barton, 2001; Barton and Etheridge,
2010). Wright (1978) proposed that when 0 < Fst <
0.05, the genetic differentiation of the population is
non-existent. When 0.05 < Fst < 0.15, the population
has moderate genetic differentiation (Wright, 1978) .
When 0.15 < Fst < 0.25, the population has high
genetic differentiation. In this study, the genetic
differentiation coefficient (Fst) was 0.311~0.504, and
the average value was 0.409, indicating that the
genetic differentiation between the two populations
was great. Compared to the Chinese honeybee
population (Fst=0.002~0.037) in Qinba Mountain,
the genetic differentiation between Xi'an and Nanjing
is higher (Wang, et al., 2004) .
The extent of gene flow (Nm) is an important
factor affecting genetic differentiation among
populations. Inter-gene communication can reduce
the degree of differentiation by increasing the genetic
variation between populations (Wright, 1974).
Therefore, when the gene flow (Nm) is greater than
one, the genetic differentiation caused by genetic drift
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
258

and selection can be effectively inhibited, and
eventually the population tends to become consistent
(Whitlock, 1999). However, when the gene flow
(Nm) between populations is less than one, genetic
differentiation may occur due to the obstruction of
gene flow among sub-populations (Chen, et al.,
2004).
In this experiment, the gene flow (Nm) of each
microsatellite locus ranged from 0.226 to 0.553, with
an average of 0.375, which revealed that the gene
exchange between the two populations of
Reticulitermes aculabialis in Xi'an and Nanjing was
weak or non-existent, resulting in a higher genetic
differentiation among populations.
The reason for the low gene exchange between the
two populations was that the termites could not
migrate over a wide range due to the short and limited
flight time and poor flight ability, which limited the
genetic communication among the populations.
Additionally, urban areas were densely populated,
and human activities were frequent. Inter-city eco-
tourism and urbanization construction led to
environmental fragmentation, leading to isolated
nesting and breeding of termites, which in turn
affected genetic communication among populations.
ACKNOWLEDGEMENTS
This work was financially supported by scientific
research project in school-level of Xi’an Pei hua
University (PHKT18064).
REFERENCES
Barton, N. H., Etheridge, A. M., 2010. Véber A. A new
model for evolution in a spatial continuum[J]. Electron
J Probab, 15(7): 162-216.
Barton, N., 2001. The role of hybridization in evolution[J].
Molecular Ecology, 10(3): 551-568.
Chand, R. R., Jokhan, A. D., Charan, H. et al., 2018. Threats
posed by Asian subterranean termites in the Fiji Islands
and their potential controls: a review[J]. New Zealand
Plant Protection, 71: 129-139.
Chen, J., Dong, C., Sun, D. et al., 2004. Genetic variation
analysis of chum salmon populations in Heilongjiang
river based on microsatellite markers[J]. Acta
Hydrobiologica Sinica, 28(6):607-612.
Dronnet, S., Bagneres, A. G., Juba, T. R. et al., 2004.
Polymorphic microsatellite loci in the European
subterranean termite, Reticulitermes santonensis
Feytaud[J]. Molecular Ecology Notes, 4(1): 127-129.
Jsc, S., Ecl, C., Shu, H. et al., 1997. An evaluation of the
utility of SSR loci as molecular markers in maize (Zea
mays L.): comparisons with data from RFLPS and
pedigree[J]. Theoretical and Applied Genetics, 95(1):
163-173.
Lewontin, R. C., 1995. The Apportionment of Human
Diversity[J]. Bmc Evolutionary Biology, ,6: 381-398.
Li, G. X., Rong, D. Z., and Yang, B., 2010. "Introduction
to termite research in China." Journal of Applied
Entomology 117.1-5(2010):360-369.
Nei, M., 1973. Analysis of gene diversity in subdivided
populations[J]. Proceedings of the National Academy
of Sciences of the United States of America, 70(12):
3321.
Nei, M., Takezaki, N., 1996. The root of the phylogenetic
tree of human populations[J]. Molecular Biology &
Evolution, 13(1): 170-177.
Peakall, R., Smouse, P. E., 2012. GenAlEx 6.5[J].
Bioinformatics, 28(19): 2537-2539.
Rod, P., Petere, S., 2006. GENALEX 6: genetic analysis in
Excel. Population genetic software for teaching and
research[J]. Molecular Ecology Notes, 6(1): 288-295.
Rust, M. K., Su, N. Y., 2012. Managing Social Insects of
Urban Importance[J]. Annual Review of Entomology,
57(57):355.
Vargo, E. L., 2000. Polymorphism at trinucleotide
microsatellite loci in the subterranean termite
Reticulitermes flavipes[J]. Molecular Ecology, 9(6):
817–820.
Vargo, E. L., 2003. Hierarchical analysis of colony and
population genetic structure of the eastern subterranean
termite, Reticulitermes flavipes, using two classes of
molecular markers[J]. Evolution, 57(12): 2805-2818.
Vargo, E. L., Henderson, G., 2000. Identification of
polymorphic microsatellite loci in the Formosan
subterranean termite Coptotermes formosanus
Shiraki[J]. Molecular Ecology, 9(11): 1935-1938.
Wang, H., Chang, H., Xu, W. et al., 2004. Genetic analysis
of microsatellite DNA markers in domestic quail and
wild japanese quail populations[J]. Chinese Journal of
Animal & Veterinary Sciences, 20(8):1-6.
Whitlock, M. C., 1999. Neutral additive genetic variance in
a metapopulation[J]. Genetical Research, 74(3):215-
221.
Whitlock, M. C., Mccauley, D. E., 2010. Indirect measures
of gene flow and migration: FST≠1/(4Nm+1)[J].
Heredity, 82(82): 117-125.
Wright, S., 1974. Evolution in Mendelian populations[M].
New directions in police-community relations.
Rinehart Press, 1974: 241-295.
Wright, S., 1978. Evolution and the genetics of populations:
a treatise in four volumes: Vol. 4: variability within and
among natural populations[M]. University of Chicago
Press.
Xing, L., Cui, H., and Cheng, J., 1998. Foraging
populations and territories of Reticulitermes aculabialis
Tsai et Hwang (Isoptera: Rhinotermitidae) in urban
environment[J]. Journal of Zhejiang Agricultural
University.
Zhong, J. H., Liu, L. L., 2002. Termite fauna in China and
their economic importance.[J]. Sociobiology, 40:25-32.
Study on the Genetic Diversity of Reticulitermes Aculabialis
259
