
Fabrication of CuO Nanoflakes at Gas-liquid Interface Via Chemical
Bath Deposition with High Photocatalytic Activity
Xi Lin and Fengqiang Sun
*
School of Chemistry and Environment, South China Normal University, Guangzhou 510006, PR China
Keywords: CuO, gas-liquid interface, chemical bath deposition, photocatalytic activity
Abstract: Fabricating materials at gas-liquid interface is a kind of novel method and has aroused a great attention.
Herein, we introduce a new chemical bath deposition method to fabricate CuO nanoflakes directly at the
gas-liquid interface of a solution of CuSO
4
and NH
3
·H
2
O. The structure and morphology are characterized by
X-ray diffraction and scanning electron microscopy. The as-prepared CuO nanoflakes exhibited excellent
visible light photocatalytic activity in degradation of RhB in the presence of a little amount of H
2
O
2
under
tungsten halogen lamp. The method is easily-manipulated, low-cost, environment friendly and promising for
the fabrication of micro/nano-structured semiconductor functional materials including the photocatalyst.
1 INTRODUCTION
Copper oxide (CuO) is a typical p-type
semiconductor with narrow band gap, excellent
optical and electronic properties, mechanical stability
and has been widely applied in fields of
photocatalysis (Du et al., 2019), gas sensors (Hou et
al., 2018), supercapacitors (Dong et al., 2016) and so
on. Hydrothermal method (Andana et al., 2017),
thermal oxidation of metallic Cu (Zhang et al., 2016)
were used to fabricate CuO, but both of them were
consist of high cost, complicated manipulations.
Therefore, exploring a facile method to fabricate CuO
was essential.
Surface science has played an important role in
chemical research (Borders et al., 2018). Fabrication
of materials at gas-liquid interface is an important
branch, which has aroused a great attention because
of its novelty (Hsieh et al., 2016). Generally,
photochemical method (Hoshyarmanesh et al., 2016),
self-assembly method (Long et al., 2017) were used
to fabricate materials on gas-liquid interface.
Compared with above methods, chemical bath
deposition (CBD) can fabricate materials with high
performance because of its homogeneity,
controllability and high production (Yang et al.,
2016). However, as far as we know, fabricating
materials at gas-liquid interface via CBD has not
been reported yet.
In this work, we develop a new chemical bath
deposition method to fabricate CuO nanoflakes
directly at the gas-liquid interface of a solution of
CuSO
4
and NH
3
·H
2
O. The as-prepared CuO
nanoflakes exhibited excellent visible light
photocatalytic activity in degradation of RhB. The
method is easily-manipulated, low-cost,
environmental friendly.
2 EXPERIMENT SECTION
2.1 Materials
CuSO
4
.
5H
2
O (AR, 99%), hydrogen peroxide (AR)
and RhB (AR) were purchased from DaMao
Chemical Reagent Factory (Tianjin, China).
Ammonia (AR) was purchased from Guangzhou
Chemical Reagent Factory (Guangzhou, China).
2.2 Preparation of CuO Nanoflakes
The CuO nanoflakes were prepared by CBD method.
A typical procedure is described as following: Firstly,
2.5 g of CuSO
4
.
5H
2
O was dissolved in 200 mL of
distilled water in a beaker. Secondly, 8 mL of 25%
ammonia solution was gradually dropped into the
mixed solution by a pipette and a piece of filter paper
was put at the bottom of the beaker. The mixed
solution was heated at 90 ℃ in a water bath. CuO
208
Lin, X. and Sun, F.
Fabrication of CuO Nanoflakes at Gas-liquid Interface Via Chemical Bath Deposition with High Photocatalytic Activity.
DOI: 10.5220/0008187702080211
In The Second International Conference on Materials Chemistry and Environmental Protection (MEEP 2018), pages 208-211
ISBN: 978-989-758-360-5
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
nanoflakes could form at the gas-liquid interface after
1 hour. The product was picked up by a glass sheet
and washed by distilled water, following by drying at
60 ℃ for 12 hours. The dried product was finally
ground into powder before storage.
2.3 Materials Characterization
The morphologies of CuO nanoflakes were
investigated via scanning electron microscopy (SEM,
Carl Zeiss Gemini 500), The chemical composition
was characterized via X-ray powder diffraction
(XRD, BRUKER D8 ADVANCE D/max2200, with
Cu-Kα radiation) in which the diffraction peaks and
the indices of crystallographic plane were
characterized by MDI Jade 6.5. The UV–Vis diffuse
reflectance spectra (DRS) of all samples were
measured with Shimadzu UV-2700
spectrophotometer.
2.4 Measurement of Photocatalytic
Activity
Firstly, 0.025 g of as-prepared CuO nanoflakes
catalyst was added in 100 mL of RhB aqueous
solution in a beaker and the solution was ultrasonic
treated until the catalyst was dispersed and 4 mL of 3%
H
2
O
2
was added. The mixture was sequentially
stirred for 30 minutes in dark in order to achieve
absorption equilibrium. Secondly, two 150 W
commercial tungsten halogen lamps with 420 nm
cut-off filter were employed as visible light source
and a condenser pipe was immersed into the solution
to keep the photocatalytic reactions occur at room
temperature. Thirdly, 5 mL of mixed solution was
taken out every 20 minutes from beaker and
centrifuged at a speed of 4000 r/min for 2 minutes to
remove any solids after the photocatalytic reaction
started. The absorbance of the clarified liquid was
measured by UV–Vis spectrophotometer (SP-752,
Shanghai Spectrum, China) at a wavelength of 553
nm, which is consistent with the maximum
absorption wavelength of RhB. The
photodegradation rate η (%) was calculated by the
following formula:
η= C
t
/ C
0
× 100%
where η is the photodegradation rate (%), C
0
is
concentration of RhB after the absorption, and C
t
is
concentration of RhB at time t during photocatalytic
reaction.
3 RESULTS AND DISCUSSION
3.1 Composition and Morphology
Characteristic
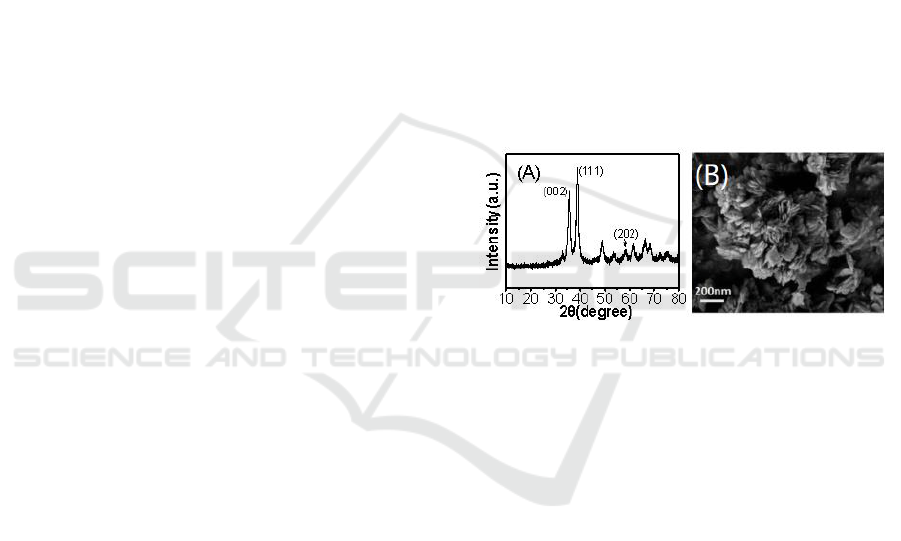
Figure 1A shows the XRD pattern of CuO formed at
the gas-liquid interface. Diffraction peaks at 35.5
o
,
38.7
o
and 2θ=58.0
o
correspond to the (002), (111) and
(202) planes of tenorite CuO (JCPDS card No.
48-1548) (Ahmadi and Siadati, 2018). The high
intensity of the peaks reveals that the CuO has high
crystallinity. No other diffraction peaks were
observed in the XRD pattern, meaning that the
as-fabricated sample had high purity. Figure 1B
shows the morphology of the as-fabricated CuO
nanoflakes. It could be clearly observed that the
sample was composed of uniform and rhombus
nanoflakes, which was corresponding to its tenorite
crystal system mentioned above and their average
thickness was 20 nm.
Figure 1: XRD spectrum (A) and SEM image (B) of CuO
nanoflakes.
3.2 Growth Mechanism
Briefly, when ammonia was added into the CuSO
4
solution, it could combine Cu
2+
ions by coordination
action to gradually produce [Cu(NH
3
)
4
]
2+
ions (Eq. 1).
During the subsequent CBD process, the ions would
gradually transformed into CuO (Eqs. 2-4). The
gas-liquid interface acts as a special nucleation site
which induces the formation of CuO nanoflakes by a
self-assembling process (Terasako et al., 2015).
H2CuO2)OH(Cu
H2)OH(CuOH2Cu
NH4Cu])NH(Cu[
OH4])NH(Cu[OHNH4Cu
2
22
2
3
22
43
2
2
4323
2
3.3 Photocatalytic Activity
To analyze the photocatalytic activity of CuO
nanoflakes (CuO flake), the degradation reactions of
RhB after the addition of CuO nanoflakes
commercial CuO powder (C-CuO), P25 with H
2
O
2
(1)
(3)
(4)
(2)
Fabrication of CuO Nanoflakes at Gas-liquid Interface Via Chemical Bath Deposition with High Photocatalytic Activity
209
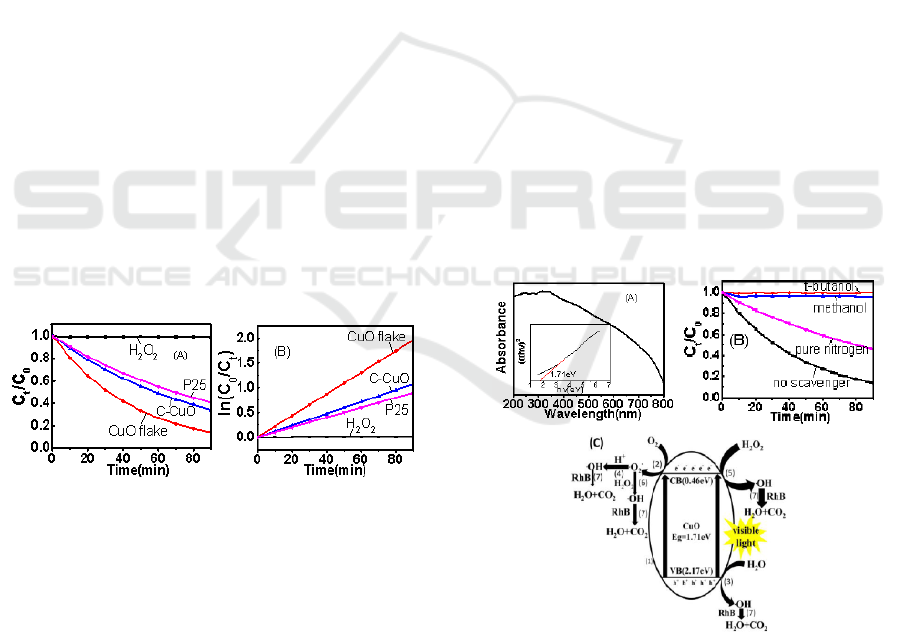
and
pure
H
2
O
2
without catalyst were observed and the
degradation rate of RhB were calculated and
compared. Before the reaction, the catalysts were
added into RhB aqueous solution and the solution
was ultrasonic treated until the catalyst was well
dispersed. The mixture was then kept in dark for 30
minutes to build an absorption−desorption
equilibrium. The result indicated that the sample
nearly had no discernible adsorption for the RhB
aqueous solution. The results of degradation rate of
RhB in 90 minutes were shown in Figure 2A. The
RhB aqueous solution with CuO flake catalyst and
3%H
2
O
2
added had obtained excellent
photodegradation rate and the corresponding value
could reach 85.9%, while the solutions with C-CuO
and P25 had degradation rate of 65.9% and 59.3%,
respectively. Furthermore, concentration of RhB
aqueous solution was almost constant when pure
H
2
O
2
was added, which reveals that pure H
2
O
2
had
poor activity. The curve of apparent reaction rate
(ARR) constant of CuO nanoflakes, C-CuO, P25 with
H
2
O
2
and
pure
H
2
O
2
added in RhB aqueous solution
and the corresponding ARR constant were calculated
and poltted in Figure 2B. It could be obviously
observed that the curve of ARR constant followed the
pseudo-first-order kinetics. The corresponding
equation was suggested as followings: ln(C
0
/C
t
) = kt
(Sudrajat et al., 2018), where k represents the
apparent reaction rate (ARR) constant. The
corresponding ARR constant of catalyst was 0.0218
min
-1
for CuO nanoflakes, 0.0119min
-1
for C-CuO,
0.0100min
-1
for P25, and 0.0001min
-1
for pure
H
2
O
2.
Figure 2: The photocatalytic activities of CuO nanoflakes,
commercial CuO powder, P25 with H2O2 and pure H2O2
without catalyst (A) The degradation rates of RhB in 90
minutes; (B) The curve of apparent reaction rate (ARR)
constant of CuO nanoflakes, C-CuO, P25 with H2O2 and
pure H2O2 without catalyst.
3.4 Mechanism of Photocatalysis
The optical property of CuO nanoflakes was
measured by ultraviolet-visible (UV-Vis) diffuse
reflectance spectra (DRS) and the result was shown
in Figure 3A. It can be observed in Figure 3A that the
absorption edge of the pure CuO sample is at
wavelength of about 725 nm and the corresponding
energy band gap (Eg) is ∼1.71 eV. The DRS results
indicate that CuO nanoflakes possessed narrow band
gap, which lead to higher photocatalytic activity. In
order to determine the effect of hole (h
+
), hydroxyl
radical (·OH), superoxide radical (·O
2
-
), we
additionally drop methanol, which was used as h
+
scavenger and t-butanol, which was used as OH
scavenger into the RhB aqueous solution, also, pure
nitrogen, which was considered as ·O
2
-
removal agent
was bubbled through the RhB aqueous solution in the
control photocatalytic experiments. As shown in
Figure 3B, compare with the solution without any
scavenger, the addition of methanol or t-butanol had
almost completely prevented the degradation of RhB,
while pure nitrogen was bubbled through the solution,
the degradation rate of RhB was obviously decreased.
Therefore, as Figure 3C indicated, holes and
electrons were generated when the semiconductor
was irradiated by UV light or visible light, during the
reaction, the photo-generated electrons react with
dissolved oxygen
and thus lead to the generation of
superoxide radicals, the superoxide radicals further
react with photo-generated holes, H
+
,
to form
hydroxyl radicals. Furthermore, the hydroxyl radicals,
which are conducive to the degradation of RhB, are
generated by the reaction of H
2
O
2
and
photo-generated electrons or superoxide radicals
(Wang et al., 2016, Li et al., 2018) and the reaction of
photo-generated holes and H
2
O (Huang et al., 2015,
Ma et al., 2018, Zhang et al., 2012).
Figure 3: (A) UV–vis diffuse reflectance spectra (DRS) and
band gap energy(inset) of CuO nanoflakes; (B)
Photodegradation dynamics of the RhB aqueous solution in
the presence of CuO nanoflakes, H
2
O
2
and t-butanol, CuO
nanoflakes, H
2
O
2
and methanol, CuO nanoflakes, H
2
O
2
and
pure nitrogen, CuO nanoflakes and H
2
O
2
, respectively; (C)
Schematic of proposed mechanism of the RhB aqueous
solution degradation in the presence of CuO nanoflakes and
H
2
O
2
.
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
210

4 CONCLUSIONS
In summary, CuO nanoflakes at gas-liquid interface
was fabricated by a facile CBD method. The product
was composed of uniform and rhombus nanoflakes
with high crystalline. The CuO nanoflakes exhibited
excellent photocatalytic activity with the addition of
H
2
O
2
and the corresponding photodegradation rate
was 85.9%. The results indicated that fabricating
materials on gas-liquid interface via chemical bath
deposition was a promising method.
ACKNOWLEDGEMENTS
This work was co-supported by the National Natural
Science Foundation of China (No. 21571068), the
Natural Science Foundation of Guangdong Province
(No. 2015A030313387) and the Science and
Technology Program of Guangzhou (No.
201607010301).
REFERENCES
Ahmadi, M., Siadati, M.H., 2018. Synthesis, mechanical
properties and wear behaviour of hybrid
Al/(TiO
2
+CuO) nanocomposites. Journal of Alloys and
Compounds. 769, 713-724.
Andana, T., Piumetti, M., Bensaid, S., Veyre, L. et al.,
2017. CuO nanoparticles supported by ceria for
NOx-assisted soot oxidation: insight into catalytic
activity and sintering. Applied Catalysis B:
Environmental. 216, 41-58.
Borders, T., Wang, L., Fushimi, R., Redekop, E. et al., 2018,
Pulse response analysis using the Y-produce: A data
science approach. Chemical Engineering Journal. 192,
46-60.
Dong, C.J., Xing, X.X., Chen, N., Liu, X., Wang, Y.D.,
2016, Biomorphic synthesis of hollow CuO fibers for
low-ppm-level n-propanol detection via a facile
solution combustion method. Sensor. Actuat. B-Chem.
230, 1-8.
Du, X.D.,Zhang, Y.Q.,Si, F., Yao, C.H., Du, M.M. et al.,
2019, Persulfate non-radical activation by nano-CuO
for efficient removal of chlorinated organic
compounds: Reduced graphene oxide-assisted and
CuO (0 0 1) facet-dependent. Chemical Engineering
Journal.356, 178-189.
Hoshyarmanesh, H., Ghodsi, M., Park, H.H., 2016.
Electrical properties of UV-irradiated thick film
piezo-sensors on superalloy IN718 using
photochemical metal organic deposition. Thin Soild
Films. 616, 673-679.
Hou, L., Zhang, C.M., Li, L., Du, C., Li, X.K. et al., 2018.
CO gas sensors based on p-type CuO nanotubes and
CuO nanocubes: Morphology and surface structure
effects on the sensing performance. Talanta. 188,
41-49.
Hsieh, K., Wang, H. J., Locke, B.R., 2016. Analysis of a
gas-liquid film plasma reactor for organic compound
oxidation. Journal of Hazardous Materials.317,
188-197.
Huang, X.M., Ding, J., Zhong, Q., 2015. Catalytic
decomposition of H
2
O
2
over Fe-based catalysts for
simultaneous removal of NO
X
and SO
2.
Applied
Surface Science. 326, 66-72.
Li, D., Fang, M.J., Jiang, C.L. et al., 2018. Size-controlled
synthesis of hierarchical bismuth selenide nanoflowers
and their photocatalytic performance in the presence
of H
2
O
2
. Journal of nanoparticle research. 20.
Long, J., Yang, Z.H., Zeng, X., Huang, J.H., 2017.
Self-assembly of exfoliated layered double hydroxide
and graphene nanosheets for electrochemical energy
storage in zinc/nickel secondary batteries. Journal of
Powder Source.359, 111-118.
Ma, X.Y., Xiang, Q.J., Liao, Y.L., Wen, T.L., Zhang, H.W.,
2018. Visible-light-driven CdSe quantum
dots/graphene/TiO
2
nanosheets composite with
excellent photocatalytic activity for E. coli disinfection
and organic pollutant degradation. Applied Surface
Science. 457, 846-855.
Sudrajat, H., Hartuti, S., 2018. Structural properties and
catalytic activity of a novel ternary CuO/gC
3
N
4
/ Bi
2
O
3
photocatalyst. Journal of Colloid and Interface
Science. 524, 227-235.
Terasako, T., Murakami, T., Hyodou, A., Shirakata, S.,
2015. Structural and electrical properties of CuO films
and n-ZnO/p-CuO heterojunctions prepared by
chemical bath deposition based technique. Solar
Energy Materials and Solar Cells.132, 74-79.
Wang, Q.L., Li, H.Y., Yang, J. H., Sun, Q., Li, Q.Y., Yang,
J.J., 2016. Iron phthalocyanine-graphene
donor-acceptor hybrids for visible-light-assisted
degradation of phenol in the presence of H
2
O
2
. Applied
Catalysis B: Environmental. 192, 182-192.
Yang, J., Cho, M., Lee, Y.K., 2016. Synthesis of
hierarchical Ni(OH)
2
hollow nanorod via chemical bath
deposition and its glucose sensing performance.
Sensor. Actuat. B-Chem. 222, 674-681.
Zhang, G.Q., Li, Z., Wang, X., Zheng, H.Y., Hao, Z.Q.,
Wang, J.J., 2016. Influence of surface oxygenated
groups on the formation of active Cu species and the
catalytic activity of Cu/AC catalyst for the synthesis of
dimethyl carbonate. Applied Surface Science.390,
68-77.
Zhang, N., Zhang, Y.H., Xu, Y.J., 2012. Recent progress on
graphene-based photocatalysts: current status and
future perspectives. Nanoscale. 4, 5792-5813.
Fabrication of CuO Nanoflakes at Gas-liquid Interface Via Chemical Bath Deposition with High Photocatalytic Activity
211
