
Reversible Thermochromic and Phase Change Composites Based on
Dicarboxylic Acid
Lu Wang
1,2
, Xiuwen Wu
1*
, Huanhuan Wang
1,2
, Bingxu Hou
1,3
, Can Cai
1,2
and Jinlin Zhang
1
1
School of Science, China University of Geosciences, Beijing 100083, PR China
2
School of Energy Resources, China University of Geosciences, Beijing 100083, PR China
3
School of Earth Sciences and Resources, China University of Geosciences, Beijing 100083, PR China
Keywords: Thermochromism, Phase change materials, Visualising phase change phenomena, Ultraviolet absorption
Abstract: Reversible thermochromic and phase change dual functional composites were synthesized with crystal violet
lactone as the leuco dye, the mixture of stearic acid and decanoic acid as the developer and phase change
materials, and cetyl alcohol as the solvent by a process of grinding, mixing, stirring and heating. The samples
have a well reversible thermochromic and phase change properties. The enthalpies reached up to about 200
J/g. The phase change temperatures and color change temperatures are about 40°C – 50°C. The absolute
differences between the phase change temperatures and color change temperatures of the optimum samples
were ≤1.92°C, meaning that to visualise phase change phenomena with a colour indicator is feasible. The
samples also exhibited an excellent ultraviolet absorption property, meaning they could be used in ultraviolet
protection aspect besides using as the phase change materials and colour indicators.
1 INTRODUCTION
Recently, reversible thermochromic and phase
change dual functional composites have been
reported by some authors (Li et al., 2018). On the one
hand, due to the significant changes in their
absorption and fluorescent properties in response to
external stimulation, giving them numerous potential
applications such as thermal indicators, optical
storage devices and other luminescent switches
(Seyfouri and Buddhi, 2017; Kumar et al., 2017; Jin
et al., 2017; Carmona et al., 2010; Berdahl et al.,
2008; Raditoiu et al., 2016; Oswald et al., 2014; Jeong
et al., 2018; Oh et al., 2016; Ma et al., 2001; Ma et al.,
2002; Mapazi et al., 2017; Liu et al., 2017; PospíŠil
and Nešpurek, 2000; Malherbe et al., 2010; Wu et al.,
2014; Shobo and Mawire, 2017; Yu et al., 2017). On
the other hand, phase change materials based on the
heat absorption or release exhibited some outstanding
advantages in some areas, for example, solar heat
storage or industrial waste heat recovery (Zalba et al.,
2003; Sharma and Buddhi, 2005; Amin et al., 2016).
Most good results about phase change materials,
focused on building energy efficiency, solar heating
systems, air-conditioning systems, photovoltaic
systems, temperature adaptable greenhouse, thermo-
regulating fibers, smart textile materials, and so on,
have been reported (Sharma et al., 2009; Sharma et
al., 2014; Sarı, 2005; Kant et al., 2016; Sarı et al.,
2015; Sarı et al., 2004; Costa et al., 2009; Gandolfo
et al., 2003; Kim et al., 2017; Hasl and Jiricek, 2014;
Dimaano et al., 2002). There are many studies on
phase change materials or thermochromic materials,
but the materials having the reversible
thermochromic and phase change properties at the
same time have been less studied (Li et al., 2018; Wu
et al., 2014). The phase change materials in Li et al.
(Li et al., 2018) or Wu et al. (Wu et al., 2014) studies,
were single fatty alcohol, which led to definitive and
nonadjustable phase change temperatures, and this
would narrow their application. The phase change
temperatures could be adjusted by binary fatty acid in
some ratio (Sarı, 2006; Keleş et al., 2005; Wang and
Meng, 2010; Ding et al., 2017). The focus in this
study is to prepare reversible thermochromic and
phase change materials with adjustable phase change
temperatures by binary fatty acid, and to visualise
phase change phenomena with a colour indicator. The
samples were prepared by using crystal violet lactone
(CVL) as a leuco dye, the mixture of stearic acid (SA)
and decanoic acid (DA) as the developer, and cetyl
alcohol (CA) as the solvent. The similar study has not
been reported till now. The as-sythesized samples
Wang, L., Wu, X., Wang, H., Hou, B., Cai, C. and Zhang, J.
Reversible Thermochromic and Phase Change Composites Based on Dicarboxylic Acid.
DOI: 10.5220/0008186501150122
In The Second International Conference on Materials Chemistry and Environmental Protection (MEEP 2018), pages 115-122
ISBN: 978-989-758-360-5
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
115

could be applied inenergy storage, colour indicators,
memory materials, and so on.
2 EXPERIMENTAL SECTION
2.1 Materials
SA with a mass fraction purity of 98.0% and CA with
a mass fraction purity of 98.0% were supplied by
Xilong Chemical Co.Ltd (China). DA with a mass
fraction purity of 98.5% was supplied by Sinopharm
Chemical Reagent Co., Ltd (China) and CVL with a
mass fraction purity of 95.0% was supplied by Tokyo
Chemical Industry Co. Ltd (China). All the reagents
were used without further purification as received.
2.2 Sample Preparation
Mixtures of CVL, stearic acid, decanoic acid and
cetyl alcohol in the mass ratio area 1: (5 – 8): (2 – 5):
(10 – 50) (Table 1) were firstly ground in a mortar for
15 min, and then transferred into a beaker. The solid
mixtures were heated to become the transparent
solutions, and then cooled automatically in the air to
form the solid samples. All the experiments were
repeated at least three times, and the experimental
data were given in average.
2.3 Characterization
Thermochromic properties tests were done as
follows. The samples were heated and cooled in air
between 20°C and 70°C to check the colour change
and the respective temperatures during the sample
melting and solidifying. The sample colour was
recorded by a digital photo. The colour change
temperatures were detected by an electronic
thermometer. To further test the thermochromic
properties of the samples, absorption bands were
analyzed by a UV-VIS-NIR Spectrometer
(UV3600PLUS, Japan) in the wave length area 200
nm – 850 nm. The scanning rate was medium, and
scanning step was 1 nm.
The sample thermal stability was determined
using a thermal gravimetric analyzer (American STA
Q5000 IR, TGA) at a scan rate of 10°C/min from
25°C to 500°C under a nitrogen atmosphere. A
typical mass of the samples used in the TGA analysis
was 2.5 mg – 4.2 mg.
The enthalpies, phase change temperatures and
thermal cycle stability of the samples were measured
using differential scanning calorimetry (American
Q2000, DSC). All experiments were carried out in
heating and cooling rates of 5°C/min in the
temperature area of −20°C – 80°C under a constant
flow of dry nitrogen (100 ml/min). DSC calibration
was done with certified Indium standard reference
material. The phase change temperatures were taken
at the intersection of an extrapolated base line and
tangent to the heat flow curve drawn at the inflection
point of the appropriate side of the peak. Enthalpies
of the samples were obtained by a numerical
integration of the area between the heat flow curve
and the extrapolated base line. The reproducibility
error of the calorimeter was within ±1%, and the
temperature ±0.01°C.
3 RESULTS AND DISCUSSION
3.1 Thermochromic Properties
To synthesize the samples which have both high
latent heats in their phase change processes and
obvious color change phenomena, and the same
temperature area in their phase change processes and
thermochromic processes, a series of the raw material
ratios were used in preparing the samples. After some
preliminary experiments and found that the solvent
amount affects the sample color obviously. The
sample showed weak color and leaded to the
thermochromic phenomena were not easy to be
observed when the excessive solvent was used. But
the thermochromic phenomena did not occur when
the less solvent was used. The effect of the raw
material ratios to the thermochromic temperatures
was listed in Table 1, and the thermochromic
phenomena were recorded by the digital photos
(Figure 1). Thermochromic temperatures are usually
related with the melting or solidifying temperatures,
but they are not the same. The initial color change
temperatures in the melting processes and the
temperatures when the samples restored their initial
colors in the solidifying processes are in the
temperature areas of 40.90°C –48.90°C and 39.60°C–
46.00°C, and the melting and solidifying
temperatures are in the areas of 40.00°C–48.00°C and
34.20°C–39.60°C, respectively, when the raw
material (CVL, (SA+DA) and CA) mass ratio
changed from 1: (8+2): 10 to 1: (5+5): 50. No obvious
relationship was observed between the
thermochromic temperatures with the raw material
mass ratio. The samples SDC8210, SDC8220,
SDC6420 and SDC5520 could not restored their
initial colors in their solidifying processes when the
CA mass percentage ≤64.5%. The sample melting
and solidifying temperatures, normally related with
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
116
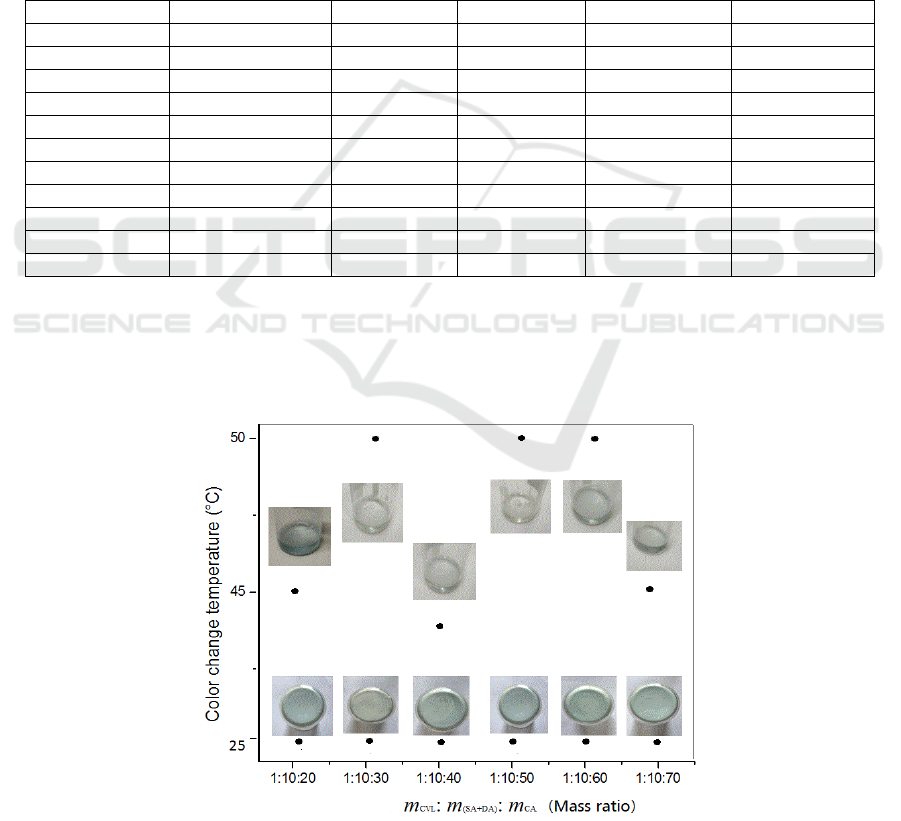
the phase change temperatures and they could be
tuned by changing the mass ratio of SA and DA which
were well studied in the previous study (Wu, 2016),
showed a decrease tendency with the SA and DA
mass ratio decreasing, this is in agreement with the
results reported before (Sarı, 2006; Keleş et al., 2005;
Wang and Meng, 2010; Ding et al., 2017), and
reasonable because DA melting temperature (31.5°C)
was lower than SA (60°C). The thermochromic
phenomena could be observed in the raw material
(CVL, (SA+DA) and CA) mass ratio area 1: 10: 30 –
1: 10: 70, and the mass ratio corresponding to the
most obvious color change was 1:10:50. All the
thermochromic phenomena showed a good repeat
ability, suggesting that the samples, except those of
the CA mass percentage ≤64.5%, could be applied as
color indicators of phase change phenomena if the
thermochromic temperatures and phase change
temperatures were much the same by selecting the
appropriate raw material ratios. For further explaining
the ideal thermochromic phenomena, a sketch about
sample color shown after many thermal cycles was
shown in Figure 2. When a sample is in a liquid state,
the CVL ring closes, and the sample is colorless.
When a sample is in a solid state, the CVL ring opens,
and the sample color is blue.
Table 1: Effect of raw material ratios to sample melting-solidifying temperatures and color change temperatures.
Sample
R
a
T
m
b
(°C)
T
s
b
(°C)
T
cm
c
(°C)
T
cs
c
(°C)
SDC8210
1: (8+2): 10
43.40
34.20
–
–
SDC8220
1: (8+2): 20
48.00
35.33
–
–
SDC8230
1: (8+2): 30
45.30
37.51
48.90
40.81
SDC8240
1: (8+2): 40
43.00
35.72
43.30
41.53
SDC8250
1: (8+2): 50
48.00
36.90
48.21
46.00
SDC6420
1: (6+4): 20
45.70
34.51
–
–
SDC5520
1: (5+5): 20
43.31
35.54
–
–
SDC8260
1: (8+2): 60
43.22
37.21
48.70
43.00
SDC8270
1: (8+2): 70
43.01
39.60
45.02
39.60
SDC6450
1: (6+4): 50
40.00
35.91
40.90
40.50
SDC5550
1: (5+5): 50
42.20
35.53
44.53
41.04
a
Mass ratio of CVL: (xSA+yDA): zCA.
b
Sample melting temperatures and solidifying temperatures.
c
Sample initial color change temperatures in melting processes and temperatures when samples restored their initial
colors in solidifying processes.
Figure 1: Digital photos of samples in heating and cooling processes.
Reversible Thermochromic and Phase Change Composites Based on Dicarboxylic Acid
117
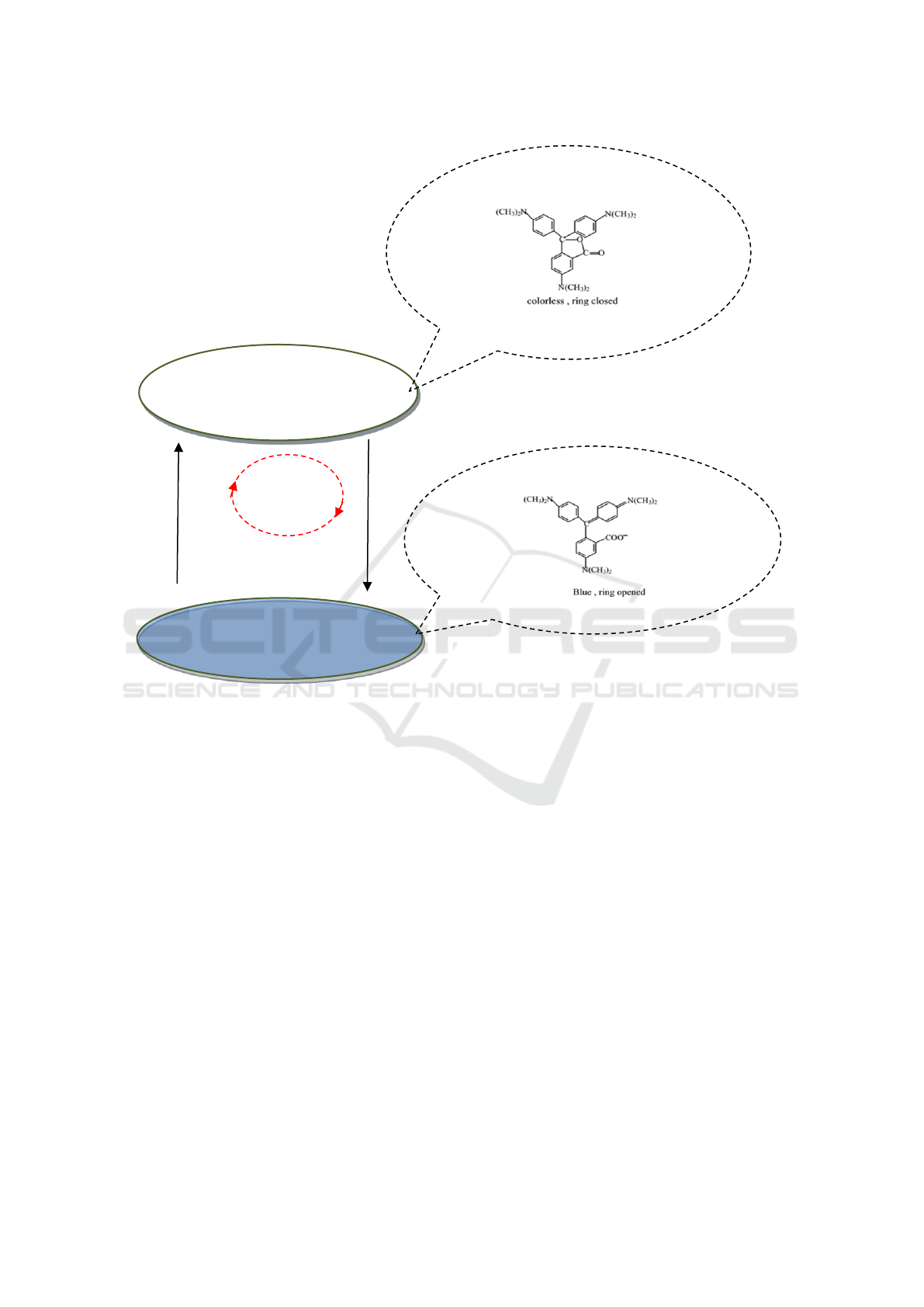
Figure 2: Sketch of sample color shown after many thermal cycles.
3.2 Phase Change Properties
The phase change parameters of all the samples,
except SDC8210, SDC8220, SDC6420 and
SDC5520 for the undetected thermochromic
phenomena or bad thermochromic repeatability, were
listed in Table 2, and the DSC curves were shown in
Figure 3. The melting and solidifying enthalpies are
in the areas of 190.3 J/g – 208.6 J/g, and 184.4 J/g –
214.2 J/g, respectively. The enthalpies increased and
then showed a decrease tendency when the mass
percentage of CA increased from 73.17% to 86.42%,
with a turn point at 81.97%. The phase change
temperatures are in the areas of 40.25°C – 47.96°C
and 41.67°C – 44.31°C, respectively, in the melting
and solidifying processes, and showed an increase
tendency in the melting process with the CA mass
percentage increasing from 73.17% to 86.42% when
the mass ratio of SA and DA was 8: 2. The reason
about the increase tendency is that the CA as both of
an solvent and phase change material has a relatively
higher phase change temperature (49.6°C) compared
with that of the mixture of SA and DA. When the
mass ratio of SA and DA decreased from 8: 2 to 5: 5,
the phase change temperatures are in the areas of
40.25 °C – 47.75 °C, and 42.33 °C – 44.08 °C,
respectively, in the melting and solidifying processes.
But no obvious changing tendency in the phase
change temperatures was found related with the mass
ratio of SA and DA. The optimum mass ratio of CVL,
DA, SA and CA, evaluated by the enthalpies, the
phase change temperatures and the thermochromic
temperatures, are 1: 6: 4: 50 and 1: 8: 2: 50,
corresponding to the samples SDC6450 and
SDC8250. The phase change parameters of these two
samples are as follows.
Thermal
cycles
Sample in liquid state
Sample in solid state
Melting
CVL
CVL
Solidify
ing
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
118
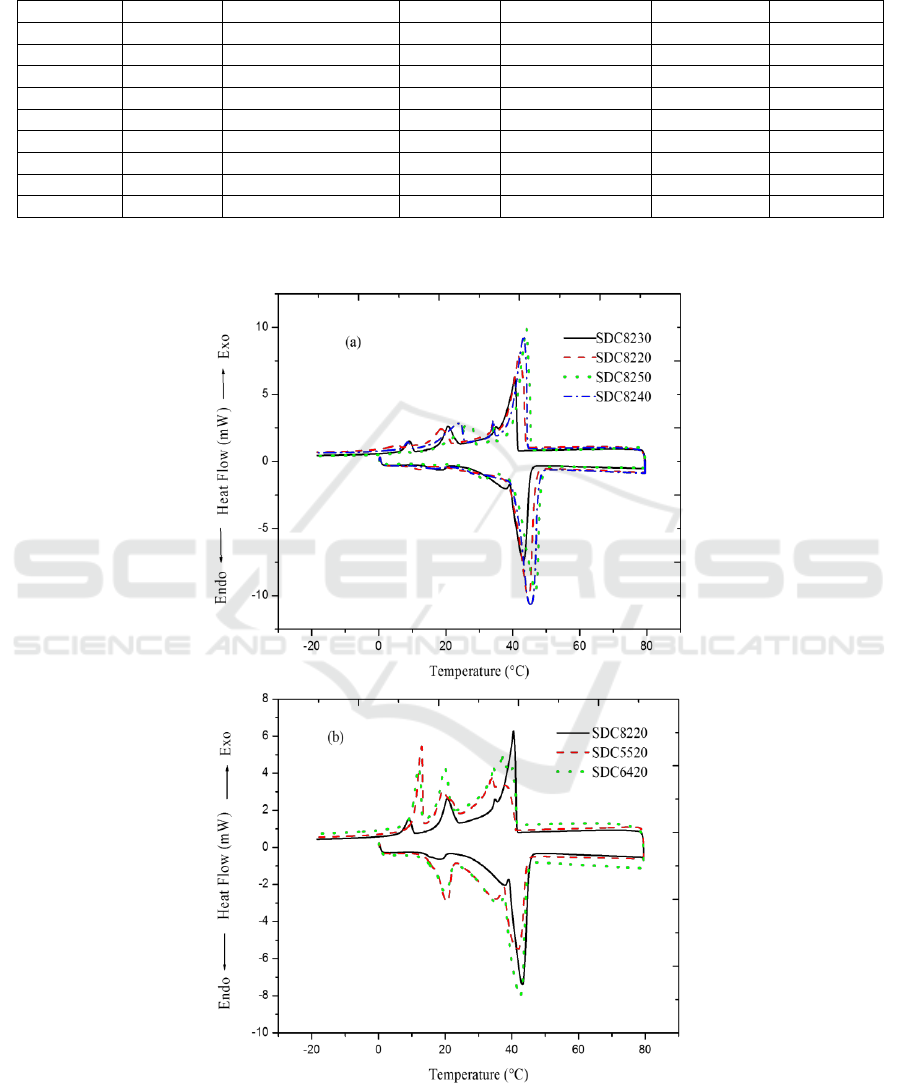
Table 2: Sample phase change properties.
Sample
T
pm
a
(°C)
ΔT
m
=T
cm
-T
pm
(°C)
T
ps
b
(°C)
ΔT
s
=T
cs
-T
ps
(°C)
△H
m
a
(J/g)
△H
s
b
(J/g)
SDC8230
44.63
+4.27
41.67
−0.87
191.7
184.4
SDC8240
45.17
−1.87
44.31
−2.81
203.7
194.3
SDC8250
47.75
+0.45
44.08
+1.92
207.7
214.2
SDC6420
42.38
+2.22
37.44
–
197.1
184.8
SDC5520
41.84
+2.66
37.86
–
196.4
185.4
SDC6450
40.25
+0.65
42.33
−1.83
208.6
202.1
SDC5550
41.09
+3.41
43.15
−2.15
205.2
196.8
SDC8260
46.87
+1.83
43.04
−0.04
190.3
194.1
SDC8270
47.96
−2.96
43.64
−4.04
198.6
193.6
a
Phase change temperature and enthalpy in melting process.
b
Phase change temperature and enthalpy in solidifying process.
Figure 3: DSC curves of samples.
Reversible Thermochromic and Phase Change Composites Based on Dicarboxylic Acid
119
The Phase change temperatures and latent heats in
melting and solidifying processes: 40.25°C and
42.33°C, 208.6 J/g and 202.1 J/g of SDC6450,
47.75°C and 44.08°C, 207.7 J/g and 214.2 J/g of
SDC8250. The absolute differences between the
phase change temperatures and color change
temperatures were ≤1.92°C, meaning that the original
experimental goal was achieved, and visualising
phase change phenomena with a colour indicator is
feasible. The extra relative weak peaks existing in
Figure 3 caused by the phase seperation, and this
could be weaken by prolonging the mixing time our
mixing method (Li et al., 2018; Wu, 2016).
3.3 Thermal Stability
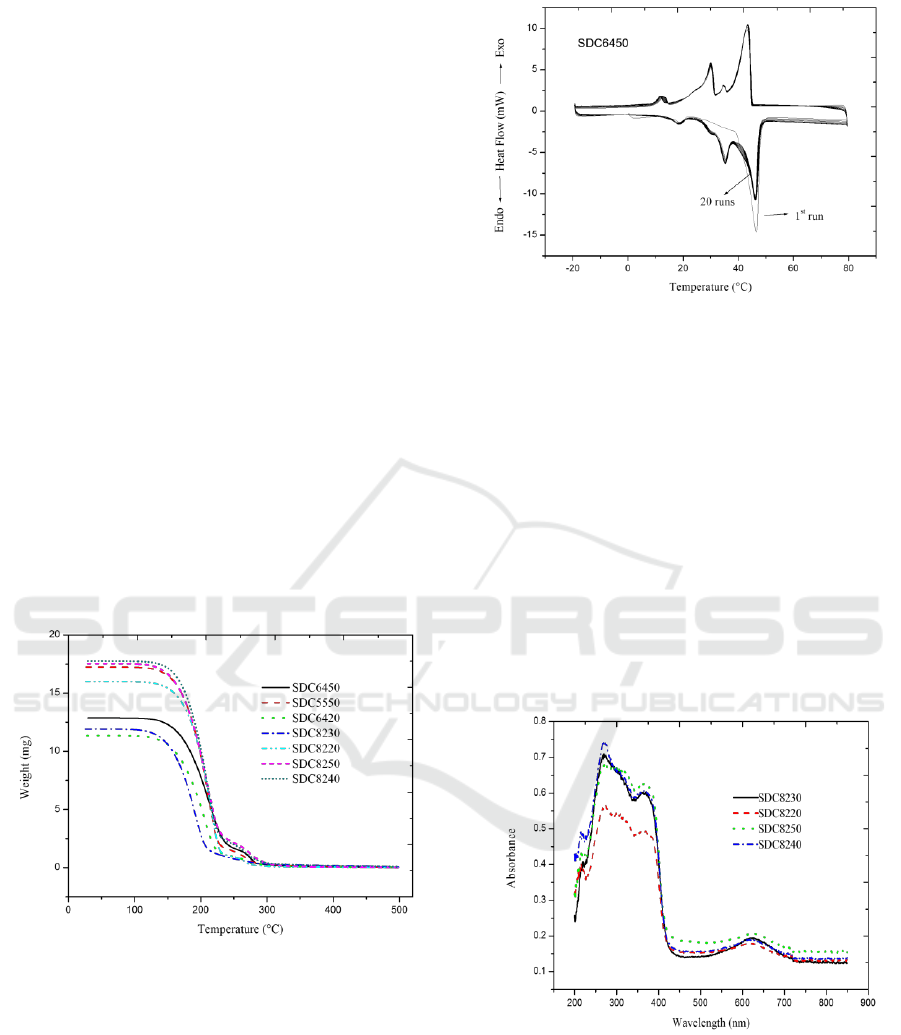
The thermal stability of the samples were evaluated
by the thermogravimetric analysis and the parameters
of the sample thermochromism and phase change
processes after some thermal runs. The
thermogravimetric curves of the samples were
presented in Figure 4. The curves of the samples were
horizontal when the temperatures were ≤130°C and
≥280°C, with a sharp weight loss in the temperature
area of 130°C – 225°C, indicating that the samples are
thermal stable at ≤130°C, or in their phase change
processes.
Figure 4: TGA curves of samples.
To evaluate the sample thermal durability,
SDC6450 was chosen as a representative. The DSC
curves of SDC6450 from the 1st to 20th runs were
presented in Figure 5. No obvious changes in the
enthalpies and the phase change temperatures were
detected from the 1st to 20th runs.
3.4 UV-VIS-NIR Spectrometer
Analysis
Figure 6 showed the absorption spectra of the
samples at 25°C. In the visible region, an steamed
bun peak at 550 nm – 650 nm could be seen,
corresponding to yellow and orange light regions.
This was consistent with the sample color (light blue)
appeared in the thermochromic experiments. In the
ultraviolet region, there was a wide and strong
absorption peak in the area 230 nm – 380 nm,
indicating other potential application related with this
property, such as ultraviolet protection equipment.
Figure 6: UV-VIS-NIR spectral curves of samples.
Figure 5: DSC curves of SDC6450 from 1st run to
20th run.
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
120

4 CONCLUSIONS
Reversible thermochromic and phase change dual
functional materials were prepared with the raw
materials CVL, DA, SA and CA by mixing, heating
and cooling processes. The adequate mass ratio of
CVL, (SA+DA) and CA are in the area 1: 10: 30 – 1:
10: 70. The melting and solidifying enthalpies of the
samples reached up to 208.6 J/g and 202.1 J/g
corresponding to the mass ratio of CVL, (SA+DA)
and CA 1: (6+4): 50. The phase change temperatures
of the samples (40.25°C – 47.96°C) were well
matched with the thermochromic temperatures
(39.60°C – 48.90°C). The samples have good thermal
stability in their phase change processes. The absolute
differences between the phase change temperatures
and color change temperatures of the optimum
samples were ≤1.92°C. In addition, the samples also
exhibited an excellent ultraviolet absorption property,
meaning they could be used in ultraviolet protection
aspect.
ACKNOWLEDGEMENTS
This work was supported by Fundamental Research
Funds for the Central Universities of China (NO.
2652017157), and National Undergraduate
Innovation Project of China University of
Geosciences, Beijing (NO. 201711415091,
2018AB069).
REFERENCES
Amin, M., Putra, N., Kosasih, E.A., Prawiro, E., Luanto,
R.A., Mahlia, T.M.I., 2016. Thermal properties of
beeswax/graphene phase change material as energy
storage for building applications. Applied Thermal
Engineering 112 273.
Berdahl, P., Akbari, H., Levinson, R., William, A., 2008.
Weathering of roofing materials – An overview.
Construction & Building Materials 22 (4) 423.
Carmona, N., Bouzas, V., Jiménez, F., Plaza, M., Pérez, L.,
García, M.A., Villegas, M.A., Llopis, J., 2010. Cobalt
(II) environment characterization in sol–gel
thermochromic sensors. Sensors & Actuators B
Chemical 145 (1) 139.
Costa, M.C., Sardo, M., Rolemberg, M.P., Ribeiro-Claro, P.,
Meirelles, A.J., Coutinho, J.A., Krahenbuhl, M.A.,
2009. The solid-liquid phase diagrams of binary
mixtures of consecutive, even saturated fatty acids:
differing by four carbon atoms. Chemistry & Physics of
Lipids 160 (2) 85.
Dimaano, M.N.R., Watanabe, T., 2002. Performance
investigation of the capric and lauric acid mixture as
latent heat energy storage for a cooling system. Solar
Energy 72 (3) 205.
Ding, L., Wang, L., Georgios, K., Lü, Y., Zhou, W., 2017.
Thermal characterization of lauric acid and stearic acid
binary eutectic mixture in latent heat thermal storage
systems with tube and fins. Journal of Wuhan
University of Technology-Materials Science Edition 32
(4) 753.
Gandolfo, F.G., Bot, A., Flöter, E., 2003. Phase diagram of
mixtures of stearic acid and stearyl alcohol.
Thermochimica Acta 404 (1) 9.
Hasl, T., Jiricek, I., 2014. The prediction of heat storage
properties by the study of structural effect on organic
phase change materials. Energy Procedia 46 301.
Jeong, J., Kumar, R.S., Naveen, M., Son, Y.A., 2018.
Synthesis, thermochromic, solvatochromic and axial
ligation studies of Zn-porphyrin complex. Inorganica
Chimica Acta 469 453.
Jin, Y., Bai, Y., Zhu, Y., Li, X., Ge, M., 2017.
Thermosensitive luminous fiber based on reversible
thermochromic crystal violet lactone pigment. Dyes &
Pigments 146 567.
Kant, K., Shukla, A., Sharma, A., 2016. Performance
evaluation of fatty acids as phase change material for
thermal energy storage. Journal of Energy Storage 6 (C)
153.
Keleş, S., Kaygusuz, K., Sarı, A., 2005. Lauric and myristic
acids eutectic mixture as phase change material for
low‐ temperature heating applications. International
Journal of Energy Research 29 (9) 857.
Kim, D., Kim, J.H., Kwon, S.H., Lee, S.O., Seo, B., Lim,
C.S., 2017. The studies of physical properties of
dimeric fatty acid-modified thiodiphenyl epoxy resins.
Polymer Bulletin 74 (11) 4595.
Kumar, R.S., Jeong, J., Mergu, N., Oh, W., Son, Y.A., 2017.
Solvent effect on the thermochromism of new betaine
dyes. Dyes & Pigments 136 458.
Li, Z., Wu, X.-W., Wu, N., Fan, Y.-Y., Sun, X.-C., Song,
T.-T., Zhong, Q., 2018. Shape-stabilized
thermochromic phase-change materials. Journal of
Thermophysics and Heat Transfer 32(1) 269.
Liu, H., Yuan, L., Qi, H., Wang, S., Du, Y., 2017. In-situ
optical and structural insight of reversible
thermochromic materials of Sm
3
-xBi
x
Fe
5
O
12
(x= 0, 0.1,
0.3, 0.5). Dyes & Pigments 145 418.
Ma, Y., Zhang, X., Zhu, B., Zhu, B., Wu, K., 2002.
Research on reversible effects and mechanism between
the energy-absorbing and energy-reflecting states of
chameleon-type building coatings. Solar Energy 72 (6)
511.
Ma, Y., Zhu, B., Wu, K., 2001. Preparation and solar
reflectance spectra of chameleon-type building
coatings. Solar Energy 70 (5) 417.
Malherbe, I., Sanderson, R.D., Smit, E., 2010. Reversibly
thermochromic micro-fibres by coaxial electrospinning.
Polymer 51 (22) 5037.
Mapazi, O., Matabola, P.K., Moutloali, R.M., Ngila, C.J.,
2017. A urea-modified polydiacetylene-based high
Reversible Thermochromic and Phase Change Composites Based on Dicarboxylic Acid
121

temperature reversible thermochromic sensor:
Characterisation and evaluation of properties as a
function of temperature. Sensors & Actuators B
Chemical 252 671.
Oh, W., Angupillai, S., Muthukumar, P., So, H.S., Son, Y.,
2016. Synthesis of novel tert-butyl substituted fluorans
and an investigation of their thermochromic behavior.
Dyes & Pigments 128 235.
Oswald, H., Lachmann, A., Roy, S., Chandratre, S., 2014.
Color changing cleansing composition. Clariant
Finance.
Pospı
́
Šil, J., Nešpurek, S., 2000. Photostabilization of
coatings. Mechanisms and performance Progress in
Polymer Science 25 (9) 1261.
Raditoiu, A., Raditoiu, V., Nicolae, C.A., Raduly, M.F.,
Amariutei, V., Wagner, L.E., 2016. Optical and
structural dynamical behavior of Crystal Violet Lactone
– Phenolphthalein binary thermochromic systems. Dyes
& Pigments 134 69.
Sarı, A., 2005. Eutectic mixtures of some fatty acids for low
temperature solar heating applications: Thermal
properties and thermal reliability. Applied Thermal
Engineering 25 (14) 2100.
Sarı, A., 2006. Eutectic mixtures of some fatty acids for
latent heat storage: Thermal properties and thermal
reliability with respect to thermal cycling. Energy
Conversion & Management 47 (9) 1207.
Sarı, A., Alkan, C., Özcan, A.N., 2015 Synthesis and
characterization of micro/nano capsules of
PMMA/capric–stearic acid eutectic mixture for low
temperature-thermal energy storage in buildings.
Energy & Buildings 90 (2) 106.
Sarı, A., Sarı, H., Önal, A., 2004. Thermal properties and
thermal reliability of eutectic mixtures of some fatty
acids as latent heat storage materials. Energy
Conversion & Management 45 (3) 365.
Seyfouri, M.M., Binions, R., 2017. Sol-gel approaches to
thermochromic vanadium dioxide coating for smart
glazing application. Solar Energy Materials & Solar
Cells 159 52.
Sharma, A., Buddhi, D., 2005. Effect of thermophysical
properties of the PCM and heat exchanger material on
the performance of a latent heat storage system.
International Journal of Sustainable Energy 24 (2) 99.
Sharma, A., Shukla, A., Chen, C.R., Wu, T.N., 2014.
Development of phase change materials (PCMs) for
low temperature energy storage applications.
Sustainable Energy Technologies & Assessments 7 17.
Sharma, A., Tyagi, V.V., Chen, C.R., Buddhi, D., 2009.
Review on thermal energy storage with phase change
materials and applications. Renewable & Sustainable
Energy Reviews 13 (2) 318.
Shobo, A.B., Mawire, A., 2017. Experimental comparison
of the dynamic operations of a sensible heat thermal
energy storage and a latent heat thermal energy storage
system. International Conference on Domestic Use of
Energy. IEEE 240.
Wang, L., Meng, D., 2010. Fatty acid eutectic/polymethyl
methacrylate composite as form-stable phase change
material for thermal energy storage. Applied Energy
87(8) 2660.
Wu, N., 2016. Preparation and properties of stearic acid
composite phase change materials [D]. Beijing: China
University of Geosciences 36.
Wu, Z., Ma, X., Zheng, X., Yang, W., Meng, Q., 2014.
Synthesis and characterization of thermochromic
energy-storage microcapsule and application to fabric.
Journal of the Textile Institute Proceedings &
Abstracts 105 (4) 398.
Yu, H., Wei, Z.H., Hao, Y.H., Liang, Z.W., Fu, Z.J., Cai,
H., 2017. Reversible solid-state thermochromism of a
2D organic–inorganic hybrid perovskite structure
based on iodoplumbate and 2-aminomethyl-pyridine.
New Journal of Chemistry 41 9586.
Zalba, B., José, M., Cabeza, L.F., Mehling, H., 2003.
Review on thermal energy storage with phase change:
materials, heat transfer analysis and applications.
Applied Thermal Engineering 23 (3) 251.
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
122
