
MnO
2
Nanoflowers Array/ Graphene Composite on Carbon Cloth
as Flexible Electrode for Non-Enzymatic Hydrogen Peroxide
Sensing
Yanan Zhao, Shiying Zhou, Danqun Huo and Changjun Hou
*
Key Laboratory for Biorheological Science and Technology of Ministry of Education, State and Local Joint
Engineering Laboratory for Vascular Implants, Bioengineering College of Chongqing University, Chongqing
400044, PR China
Keywords: Flexible electrode; 3D MnO
2
nanoflowers array; carbon cloth; H
2
O
2
detection
Abstract: In this work, 3D MnO
2
nanoflowers (MnO
2
NFs) array supported on the graphene oxide (GO)
modified carbon cloth (CC) was successfully fabricated via a hydrothermal method. MnO
2
NFs act
as the catalysts for hydrogen peroxide (H
2
O
2
) electroreduction were directly grown on conductive CC
without use of polymer binders and additives for active materials immobilization. The experimental
results show that the flexible electrode demonstrates large linear range, excellent selectivity and a
satisfactory stability for H
2
O
2
detection, making that it is a promising electrochemical sensor in field
of food analysis, environment protection and medicine.
1 INTRODUCTION
Flexible electronics have attracted a great deal of
attention owing to their extraordinary potential
application in wearable devices and smart
electronics(Dong et al., 2016; Yousaf et al., 2016).
The design of flexible biosensors creates special
needs for freestanding substrates with superior
mechanical strength and flexibility. Among various
electronic devices, flexible electrochemical
biosensors have been considered to be one of the
most promising candidates for monitoring molecule
in clinical diagnostics and environmental
monitoring. In order to realize the high-performance
of flexible electrodes, the interfacial properties of
high conductive and surface area could be
developed. Carbon cloth (CC) is a new flexible free-
standing films with a three-dimensional structure,
high conductivity and good chemical stability,
which have been widely attracted attention in the
fields of flexible solid state supercapacitors(Yu et
al., 2015). The fabrication of such flexible electrode
by directly grow electroactive nanostructures on CC
surface is benefit for exposing more active site.
Moreover, the CC is interwoven by bundles of
carbon fibers that can provide multiple porous
channels for liquid diffusion, leading to the
enhanced activity.
In recent years, The CC have been research as a
promising supporting material for flexible
electrochemical biosensors. For example, Wang et
al. constructed nickel borate nanoarray on carbon
cloth (Ni@Bi/CC) for H
2
O
2
electro-reduction in
neutral media(Wang et al., 2017). Xu et al.
synthesized MnOOH nanorod arrays on CC
substrate by hydrothermal route(Xu et al., 2016).
The MnOOH nanorods are uniformly distributed on
the CC substrate with a 3D porous network
structure. In comparison with the rigid graphite
supported electrode, the MnOOH/CC electrode
exhibits a higher sensitivity and a wider linear range
for H
2
O
2
detection. Until now, due to the low cost,
relatively high stability, and excellent electrical-
activity behavior of transition metal oxides,
numerous non-enzymatic biosensors have been
constructed based on those transition metal
oxides(Wang et al., 2018; Xie et al., 2018).
Graphene and carbon nanotubes, which possess
excellent properties of high conductivity and high
surface area, are employed as supporting materials
combining with transition metal to improve the
sensitivity of the biosensor(Jeong et al., 2018).
However, In comparison with graphene or carbon
110
Zhao, Y., Zhou, S., Huo, D. and Hou, C.
MnO2 Nanoflowers Array/ Graphene Composite on Carbon Cloth as Flexible Electrode for Non-Enzymatic Hydrogen Peroxide Sensing.
DOI: 10.5220/0008186401100114
In The Second International Conference on Materials Chemistry and Environmental Protection (MEEP 2018), pages 110-114
ISBN: 978-989-758-360-5
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

nanotube hybrid nanocomposites modified common
rigid electrodes, use of CC as a flexible electrode
substrate can provide multiple porous channels and
numerous electroactive sites for rapid liquid
diffusion, which is benefit to the diffusion between
the electrolyte and electrode material.
Herein, 3D MnO
2
nanoflowers array, vertically
grown on the graphene oxide (GO) modified CC,
were synthesized via a facile hydrothermal method.
The CC films were treatment with GO to present
hydrophilic with amounts of reactive oxygen
functional groups. Moreover, the conductivity of
CC can be further improved after GO reduced at
high temperature. MnO
2
NFs array were directly
grown on conductive CC without use of polymer
binders and additives for active materials
immobilization, in which MnO
2
nanoflowers act as
the catalysts for hydrogen peroxide (H
2
O
2
)
electroreduction. The constructed MnO
2
NFs
/reduced graphene oxide/CC (MnO
2
NFs /rGO/CC)
binder-less electrode demonstrates high sensitivity,
large linear range and excellent selectivity for H
2
O
2
detection.
2 EXPERIMENTAL SECTION
2.1 Reagents and Materials
The CC (W0S1002) purchased from Taiwan
CeTech with the thickness of 360 μm and basis
weight of 125 g m
−2
. Graphene oxide (GO) was
obtained from Nanjing XFNANO Materials Tech
CO.Ltd(China).Ethanol, acetone, KMnO
4
, H
2
O
2
,
(30%) and glucose were purchased from Chongqing
Chuan Dong Chemical Group (China). Cystine
(Cys), Tryptophan (Trp) were purchased from
Cheng Du Ke Long (China). Glutataione (GSH) and
uric acid (UA) were purchased from Sigma-Aldrich
(Shanghai, China).
2.2 Synthesis of MnO
2
NFs /rGO/CC
CC was cleaned with 1M hydrochloric acid,
acetone, deionized (DI) water, and ethanol,
respectively, under sonifcation. Then the cleaned
CC was soaked in 1 mg mL
-1
GO solution for one
week and dried at 70 °C. MnO
2
NFs arrays were
synthesized by a facile hydrothermal method. In a
typical experiment, pieces of CC were immersed in
30 mL 5 mM KMnO
4
and the whole solution with
CC was further transferred into Teflon-lined
autoclave, then it maintained at 140 °C for 12 h.
During the hydrothermal process, the GO was
reduced at high temperature. After that, the obtained
MnO
2
NFs /rGO/CC was rinsed with DI water and
dried at 70 °C.
2.3 Characterization and
Electrochemical Measurements
The morphologies were analyzed by field-emission
scanning electron microscope (SEM JEOL-6300F).
The crystal structures were investigated by powder
X-ray diffraction (XRD, Maxima-X XRD-7000).
Electrochemical measurements were performed on a
CHI 660E electrochemical workstation (Shanghai
CH Instrument, China) with a three-electrode
system including MnO
2
NFs /rGO/CC (active area
0.5 cm × 1.0 cm) as working electrode, a
silver/silver chloride (Ag/AgCl) reference electrode
and a platinum wire counter electrode. 0.01 M
phosphate buffered saline (PBS, pH 7.4) solution as
electrolyte was ventilated with high-purity nitrogen
for 20 min to remove dissolved oxygen before all
electrochemical measurements.
3 RESULTS AND DISCUSSION
3.1 Morphology and Structure
The MnO
2
nanostructure was synthesized by the
hydrothermal method. KMnO
4
can decompose to
form MnO
2
nuclei at high reaction temperature as
the following reaction mechanism: 4KMnO
4
+
2H
2
O = 4MnO
2
+ 4KOH + 3O
2
, and the MnO
2
nuclei on the surface of carbon cloth further growth
by Ostwald ripening mechanism(Shinde et al.,
2017). Figure 1 shows the SEM of the GO/CC and
MnO
2
/rGO/CC films at different magnifications. It
can be seen in the Figure 1B that a few wrinkles
coated on the surface of carbon cloth after modifed
by GO. The SEM images of MnO
2
/rGO/CC (Figure
1 C-J) show that the vertically aligned MnO
2
nanoarray uniformly covered on the surface of
carbon fibers, and the surface morphology of MnO
2
changes from acicular structure (100°C) to dense
flower-like structure (140°C) with the rise of the
hydrothermal temperature. At low reaction
temperature, the low reaction rate leads to the
isotropic growth of crystals, while at high reaction
rate, MnO
2
crystals aggregated on the surface of
carbon cloth by the manner of anisotropic growth.
MnO2 Nanoflowers Array/ Graphene Composite on Carbon Cloth as Flexible Electrode for Non-Enzymatic Hydrogen Peroxide Sensing
111

Figure 1 : FESEM images of MnO
2
/rGO/CC films
deposited at different hydrothermal temperature.(A, B)
/rGO/CC; (C, D) 100°C; (E, F) 120°C; (G, H) 140°C, (I,J)
160°C.
Figure 2 shows the XRD patterns of GO/CC and
MnO
2
/rGO/CC, respectively. The diffraction peaks
of carbon cloth at 25.9° is corresponding to
reflection peak (002) of graphite 2H. The
characteristic diffraction peaks at 37.3°, 43.7° and
65.3° correspond to the (021), (230) and (002)
planes, which coincides well with the standard data
of the tetragonal phase of MnO
2
(JCPDS card no.
44-0141)(Wang et al., 2015). The XRD spectrum
illustrates that the successful synthesis of MnO
2
on
the surface of carbon cloth.
Figure 2 : XRD patterns of as-prepared rGO/CC (black
line) and MnO
2
NFs/rGO/CC (red line).
3.2 Electrochemical Properties of the
MnO
2
/rGO/CC Films
The electrochemical properties were investigated by
Cyclic voltammetry (CV) in 5 mM [Fe(CN)
6
]
3-/4-
solution containing 0.1mol L
-1
KCl at scan rate of
0.05 V s
-1
. As shown in Figure 3A, the rGO-CC
exhibited increased redox peaks, indicating the
enhanced conductivity of rGO-CC films compared
with the CC films. After further functionalization
with MnO
2
NFs,
a larger CV curve was displayed
due to the MnO
2
nanocrystal synergistic with rGO
contributes the high capacitance of MnO
2
NFs/rGO/CC films. In addition, the CV at different
scan rates from 10 to 310 mV·s
-1
of the MnO
2
NFs/rGO/CC films were recorded in Figure 3B. It
can be seen that the peak current and the scan rate
presents a good linear relationship, which illustrated
that the mass transfer process is mainly adsorption-
controlled process at the modified electrode surface.
Figure 3 : (A) Cyclic voltammogram of CC, rGO/CC and
MnO
2
NFs/rGO/CC at a scan rate of 0.05 V s
-1
. (B) CVs
of the MnO
2
NFs/rGO/CC at different scan rates (10‒310
mVs
‒1
) (inset: the plot of peak current against the scan
rate) in 5 mM [Fe(CN)
6
]
3-/4-
solution containing 0.1mol
L
-1
KCl .
3.3 Electrochemical Behavior of MnO
2
/rGO/CC Films towards H
2
O
2
To investigated the electrocatalytic behaviors
toward H
2
O
2
of MnO
2
/rGO/CC films, CV was
performed in the absence and presence of H
2
O
2
in
0.01 M PBS (pH 7.4). As shown in the Figure 4A,
the modified carbon cloth show an obvious
reduction peak around -0.4V in CV curve, and the
reduction current is dramatically enhanced with the
increased H
2
O
2
concentration. The result indicated
the MnO
2
/rGO/CC films has excellent catalytic
activity for hydrogen peroxide reduction.
The typical current-time (i-t) curves were
recorded with the successive addition of 200 μM
H
2
O
2
into the stirred 0.01 M PBS solution at an
applied potential of -0.4V to compare the catalytic
activity of different modified carbon cloth (Figure
4B). It can be seen that the detection sensitivity of
H
2
O
2
reaches a maximum value at the hydrothermal
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
112
temperature risen to 140℃ and then the sensitivity
declines. The reason for the difference detection
sensitivity is probably due to the form of different
morphologies under difference hydrothermal
temperature, which provide different surface areas
result in different electrochemical properties.
Therefore, the MnO
2
/rGO/CC film, which is
prepared under hydrothermal temperature of 140℃,
was employed in the following experiments to
investigate the detection linear range, sensitivity and
detection limit of the flexible sensor.
Figure 4 : (A) CV curves of MnO
2
NFs/rGO/CC in 0.01
M PBS solutions (pH 7.0) containing 0-24 mM H
2
O
2
; (B)
the comparison of the electrochemical properties for H
2
O
2
reduction at different deposited hydrothermal temperature
of the MnO
2
/rGO/CC.
3.4 Amperometric Detection of H
2
O
2
the MnO
2
NFs/rGO/CC Films
Figure 5A displays the amperometric response of
the MnO
2
/rGO/CC film with successive additions
of varying concentrations H
2
O
2
in 0.01 M PBS
solution (pH=7.4) at −0.4 V. A stepwise current
response was observed after addition of H
2
O
2
. The
reduction current of the flexible electrode has two
segments linear relation with the H
2
O
2
concentrations in a range of 20μM-1mM and 1mM-
5mM with a correlation coefficient of 0.991 and
0.999, respectively (Figure 5B and C). The
sensitivity was calculated to be 68μA·M
−1
·cm
−2
and
the detection limit was 17.9μM (S/N=3). Therefore,
these results indicated that the excellent
performance of the MnO
2
/rGO/CC film for H
2
O
2
detection.
Figure 5 : (A) Amperometric response of MnO
2
NFs/rGO/CC to successive additions of H
2
O
2
at -0.4V in
0.01 M PBS. (B, C) Linear relation between the
amperometric response and H
2
O
2
concentration.
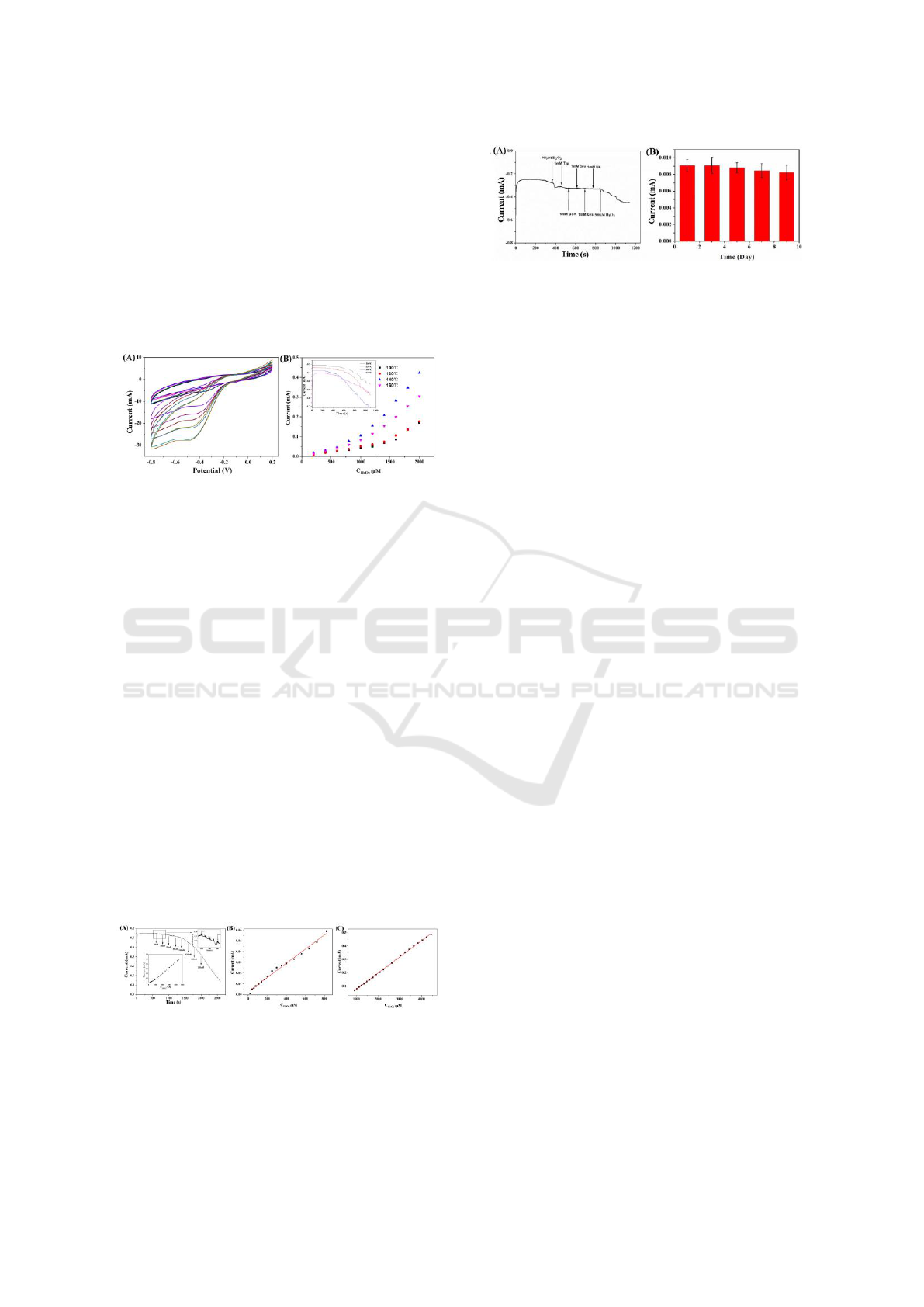
Figure 6 : The interference studies (A) and reproducibility
test of the MnO
2
NFs/rGO/CC (B).
The interference studies were performed to
evaluate the selectivity of MnO
2
/rGO/CC film
toward H
2
O
2
reduction (Figure 6). The addition of 1
mM tryptophan (Trp), 1 mM glutathione(GSH), 1
mM glucose(Glu), 1 mM cysteine (Cys) and 1 mM
uric acid (UA) result in negligible current
responses, and the 500μM H
2
O
2
produced an
obvious current change, demonstrating that the
flexible sensor have an excellent specificity for
H
2
O
2
detection. In addition, the stability of the
MnO
2
/rGO/CC film was also examined by
measuring the response to 500 μM H
2
O
2
for every
two days. It retained 90.47% of its initial response
after nine days when the sensors were stored at
room temperature, indicating the good stability of
the present sensor.
To further evaluate the applicability, real water
sample from Jia Ling River (Chongqing, China) is
collected and analyzed by our developed electrode.
The water sample was filtered, diluted and detected
by the standard addition method. In the case of
water sample spiked with 500 μM and 2 mM H
2
O
2
,
the results showed that the recoveries were 92.6 and
96.2% with relative standard deviations of 3.1% and
2.4%, indicating the appreciable practicality of the
non-enzymatic sensor for the determination of H
2
O
2
in real samples.
4 CONCLUSIONS
In summary, we developed a novel analytical device
for non-enzymatic detection of H
2
O
2
based on 3D
MnO
2
nanoflowers array on rGO modified carbon
cloth. The flexible carbon cloth as a freestanding
electrode plays a significant role in electrochemical
sensor because of its multiple porous channels for
liquid diffusion. The 3D MnO
2
nanoflowers array
growing on the surface of carbon cloth via one-step
hydrothermal method leads to the enhanced activity
because of the increased active surface areas.
Electrochemical measurement results show that the
as-prepared carbon cloth-supported 3D MnO
2
nanoflowers array exhibit excellent catalytic activity
MnO2 Nanoflowers Array/ Graphene Composite on Carbon Cloth as Flexible Electrode for Non-Enzymatic Hydrogen Peroxide Sensing
113

toward H
2
O
2
with high selectivity and sensitivity,
which is a promising candidate for the design of
flexible non-enzymatic sensors for H
2
O
2
detection
in food analysis, environment protection and
medicine.
ACKNOWLEDGEMENTS
This work was supported by Chongqing Graduate Student
Research Innovation Project (No. CYB16038).
REFERENCES
Dong, L., Xu, C., Li, Y., Huang, Z. H., Kang, F., Yang,
Q. H., & Zhao, X, 2016. Flexible electrodes and
supercapacitors for wearable energy storage: a review
by category. Journal of Materials Chemistry A, 4(13),
4659-4685.
Jeong, H., Nguyen, D. M., Lee, M. S., Kim, H. G., Ko, S.
C., & Kwac, L. K, 2018. N-doped graphene-carbon
nanotube hybrid networks attaching with gold
nanoparticles for glucose non-enzymatic sensor.
Materials Science & Engineering C, 90, 38-45.
Shinde, P. A., Lokhande, V. C., Ji, T., & Lokhande, C. D,
2017. Facile synthesis of hierarchical mesoporous
weirds-like morphological MnO2 thin films on carbon
cloth for high performance supercapacitor application.
Journal of Colloid & Interface Science, 498, 202-209.
Wang, R., Wang, Z., Xiang, X., Zhang, R., Shi, X., &
Sun, X, 2018. MnO2 nanoarrays: an efficient catalyst
electrode for nitrite electroreduction toward sensing
and NH3 synthesis applications. Chemical
Communications, 54(73), 10340-10342.
Wang, Y., Sun, H., Ang, H. M., Tadé, M. O., & Wang, S,
2015. 3D-hierarchically structured MnO2 for catalytic
oxidation of phenol solutions by activation of
peroxymonosulfate: Structure dependence and
mechanism. Applied Catalysis B: Environmental,
164, 159-167.
Wang, Z., Xie, F., Liu, Z., Du, G., Asiri, A. M., & Sun, X,
2017. High-Performance Non-Enzyme Hydrogen
Peroxide Detection in Neutral Solution: Using a
Nickel Borate Nanoarray as a 3D Electrochemical
Sensor. Chemistry – A European Journal, 23(64),
16179-16183.
Xie, F., Cao, X., Qu, F., Asiri, A. M., & Sun, X, 2018.
Cobalt nitride nanowire array as an efficient
electrochemical sensor for glucose and H2O2
detection. Sensors and Actuators B: Chemical, 255,
1254-1261.
Xu, W., Liu, J., Wang, M., Chen, L., Wang, X., & Hu, C,
2016. Direct growth of MnOOH nanorod arrays on a
carbon cloth for high-performance non-enzymatic
hydrogen peroxide sensing. Analytica Chimica Acta,
913, 128-136.
Yousaf, M., Shi, H. T. H., Wang, Y., Chen, Y., Ma, Z.,
Cao, A., Naguib, H. E., & Han, R. P. S, 2016. Novel
Pliable Electrodes for Flexible Electrochemical
Energy Storage Devices: Recent Progress and
Challenges. Advanced Energy Materials, 6(17),
1600490.
Yu, N., Zhu, M. Q., & Chen, D, 2015. Flexible all-solid-
state asymmetric supercapacitors with three-
dimensional CoSe2/carbon cloth electrodes. Journal
of Materials Chemistry A, 3(15), 7910-7918.
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
114
