
A Rare Case of Erythroderma in a Primary Cutaneous Anaplastic
Large Cell Lymphoma: A Diagnostic Challenge
Agung Mohamad Rheza
1
, Irene Dorthy Santoso
1
, Ika Anggraini
1
, Venessa Fikri
1
, Riesye Arisanty
2
,
Selviyanti Padma
1
, Sondang P. Sirait
1
1
Department of Dermatology and Venereology, Faculty of Medicine Universitas Indonesia / dr. Cipto Mangunkusumo
National General Hospital, Jakarta
2
Department of Anatomic Pathology, Faculty of Medicine Universitas Indonesia / dr. Cipto Mangunkusumo National
General Hospital, Jakarta
Keywords: erythroderma, anaplastic large cell lymphoma, Hodgkin lymphoma, non-Hodgkin.
Abstract: Anaplastic large cell lymphoma (ALCL) is a rare form of non-Hodgkin's lymphoma. It usually originates in
lymph nodes, although its origin in other tissues, including skin, has been reported. Identification of the
lymphoid activation antigen (CD30) has now clearly established the lymphoid origin of ALCL. Recent reports
suggest that in some cases cutaneous ALCL pre-existing skin disease may be a feature. This is usually mycosis
fungoides (MF), although psoriasis has also been reported. A diagnosis of ALCL is usually made by a
dermatologist following a series of diagnostic tests and procedures, including physical examination and
history, blood tests, skin biopsy and/or lymph node biopsy, immunophenotyping may also be done to identify
specific types of lymphoma. In selected cases, molecular tests may be helpful in establishing the diagnosis. A
fifty one years old man came with chief complaint of reddish, pruritic patch all over his body and nodules on
his right axilla and buttock. Two skin biopsies were collected, resulted a spongiotic psoriasiform hyperplasia
found in drug eruption and mixed cellularity Hodgkin lymphoma. Immunohistochemistry (IHC) shows
positive CD30 and CD43, Ki-67 on 80% of the cells and negative CD3, CD20, ALK, CD1a, CD15, PAX-5,
AE1/AE3 with conclusion of non-Hodgkin lymphoma - ALCL.
1 INTRODUCTION
Erythroderma is defined as a generalized redness and
scaling of the skin. However, it does not represent a
defined entity, as it is the clinical presentation of a
variety of diseases. Most commonly, erythroderma is
due to generalization of pre-existing dermatoses (such
as psoriasis or atopic dermatitis), drug reactions or
cutaneous T-cell lymphoma (CTCL). Attention
should also be focused on the potential systemic
complications of erythroderma including death. In
addition, long-lasting erythrodermas may be
accompanied by cachexia, diffuse alopecia,
palmoplantar keratoderma, nail dystrophy and
ectropion (Bolognia et al., 2012; Dento et al., 1992).
Primary cutaneous CD30
+
lymphoproliferative
disorders (PCLPD) are the second most common
group of CTCL, accounting for approximately 30% of
CTCLs after MF/Sézary syndrome (SS). This group
includes primary cutaneous anaplastic large cell
lymphoma (C-ALCL), lymphomatoid papulosis
(LyP), and borderline cases (Stein et al., 2000).CD30
+
ALCL itself is a rare form of non-Hodgkin lymphoma
(NHL), accounts for 2-3% of all NHLs and 10.2% of
all T/NK-cell lymphomas (Willemze et al., 2005;
Foss, 2013). ALCL may be classified according to the
location of the original tumor: primary nodal, primary
cutaneous, and secondary cutaneous. Primary nodal
ALCL describes disease arising in lymph nodes. It
typically occurs in children, runs an aggresive course,
and may spread to extranodal sites, including the skin,
lung, bone, and gastrointestinal tract. Primary C-
ALCL originates in the skin. In contrast to primary
nodal disease, it is most common in adults and is
indolent. It comprises one or more tumor nodules
exceeding 2 cm in diameter. Tumor nodules are often
purplish red and frequently become ulcerated. Most
common sites are trunk and extremities. Secondary
cutaneous ALCL arises in patients with underlying
CTCL, and like primary nodal disease is aggresive and
carries a poor prognosis. The cutaneous lesions of all
types of ALCL typically present as solitary or multiple
500
Rheza, A., Santoso, I., Anggraini, I., Fikri, V., Arisanty, R., Padma, S. and Sirait, S.
A Rare Case of Erythroderma in a Primary Cutaneous Anaplastic Large Cell Lymphoma: A Diagnostic Challenge.
DOI: 10.5220/0008160705000504
In Proceedings of the 23rd Regional Conference of Dermatology (RCD 2018), pages 500-504
ISBN: 978-989-758-494-7
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

ulcerated nodules, and sometimes progressed to
erythoderma. Primary C-ALCL is classified as
malignant tumor, normally affects adults. The
majority of primary C-ALCL cases published in the
literature do not show spontaneous regression (Stein
et al., 2000; Willemze et al., 2005; Foss, 2013). There
are two types of ALCL, ALK (anaplastic lymphoma
kinase)-positive ALCL and ALK-negative ALCL.
ALK-negative ALCL is a mature T-cell lymphoma
with CD30 expression, morphologically identical to
ALK-positive ALCL but lacks the expression of ALK.
It represents 40-50% of all ALCLs, but occurs in older
population (male predominance, median age 58 years)
(Foss et al., 2013). In ALK-negative cases, one study
showed the frequencies of the T-cell antigen
expression were CD2 (100%), CD3 (50%), CD4
(40%), CD7 (40%), CD5 (25%), and CD8 (20%)
(Muzzafar et al., 2009). Other study noted the
expression of CD15 is aberrant, and a negative CD20
and PAX-5 (both are rarely positive in ALCL)
indicated a NHL (Pletneva & Smith, 2014).
The affected individuals present with
lymphadenopathy, and extranodal involvement is very
rare. Because C-ALCL and systemic forms are
morphologically and immunophenotypically identical
(and C-ALCL cen extend locoregionally to lymph
nodes), the clinical information is imperative to
distinguish between the two (Stein et al,. 2000). When
skin lesions are presenting manifestation of systemic
ALCL (S-ALCL), the distinction of skin lesions in S-
ALCL from CD30
+
PCLPD is imperative. The
distinctions between these two can be difficult on
purely clinical grounds and may be difficult as well to
achieve by routine histopathology. ALK protein is
expressed in skin lesions of most patients with S-
ALCL but not in the large majority of patients with
CD30
+
PCLPD (Foss et al., 2013).
The cytology of the tumor cells is identical to
ALK-positive ALCL but, in general, the tumor cells
tend to be larger and pleomorphic than its ALK-
positive counterpart. Histologic variants are not
strictly defined, but some cases resemble the
lymphohistiocytic variant and others the Hodgkin-like
forms. The background cells can include histiocytes,
plasma cells, eosinophils, and small lymphocytes
(Stein et al., 2000). Tumor cells in both LyP and C-
ALCL are derived from activated T-cells which
express CD30 antigen. The CD30
+
cells are larger than
normal lymphocytes and have basophilic cytoplasm
and large nuclei with a prominent nucleolus
resembling immunoblasts. These cells often are bi- or
multinucleated giving the appearance of Reed-
Sternberg cells. Mitoses are frequent and often
atypical. In C-ALCL, tumor cells form large clusters
or sheets that generally extend from the dermal-
epidermal junction down into the subcutaneous fatty
tissue (Foss, 2013).
Although there is accumulating evidence that
ALCL and Hodgkin disease (HD) are biologically
distinct, the morphologic and immunophenotypic
border between these disease categories is not sharp in
all instances. These ambiguous cases contain
relatively dense nodules or sheets of tumor cells with
features of classic Hodgkin and Reed-Sternberg cells.
One of the most difficult differential diagnoses of
primary cutaneous ALCL is large cell transformation
of MF which carries a worse prognosis. The large MF
cells occur in sheets and are usually CD30
+
, both
features shared with C-ALCL. The diagnosis of large
cell transformation of MF is generally made clinically
when there are accompanying patches and plaques
typical of MF. MF tumors with large cell
transformation often contain a spectrum of
lymphocytes with convoluted nuclei generally lacking
in primary cutaneous ALCL (Foss, 2013; Muzzafar et
al., 2009). ALK-positive ALCL usually has a better
prognosis (5-year survival of 70%) compared with
ALK-negative ALCL (5-year survival of 49%) (Foss,
2013; Pletneva et al., 2014).
2 CASE
A 51-year-old man admitted to our outpatient clinic
with chief complaint of reddish and pruritic, scaly
patch throughout his body which he started to
recognize initially on both thighs three years ago
without hospital admission. Having no improvement
with herbal medicine (mangosteen extract) for about
two years, and widespread of the patch, he went to the
local hospital later on. He was diagnosed as psoriasis,
and the dermatologist gave him moisturizer and
cetirizine but still no improvement. He was then
referred to Cipto Mangunkusumo National Hospital
because two nodules appeared on right buttock and
armpit, with the latter accompanied with purulent
wound. From physical examination (Figure 1), a scaly
erythematous patch spread all over his body along
with alopecia of the scalp, eyebrows, and eyelashes.
Fissures of the palms and soles, ectropion from both
eyes, were also found. On his right axilla, a single
tumor-presenting nodule covered with granulated,
purulent, and necrotic tissue. The tumor was tender,
ouval with 5 cm in diameter. The other one on his
right buttock was 4 cm in diameter and no pain on
palpation.
A Rare Case of Erythroderma in a Primary Cutaneous Anaplastic Large Cell Lymphoma: A Diagnostic Challenge
501
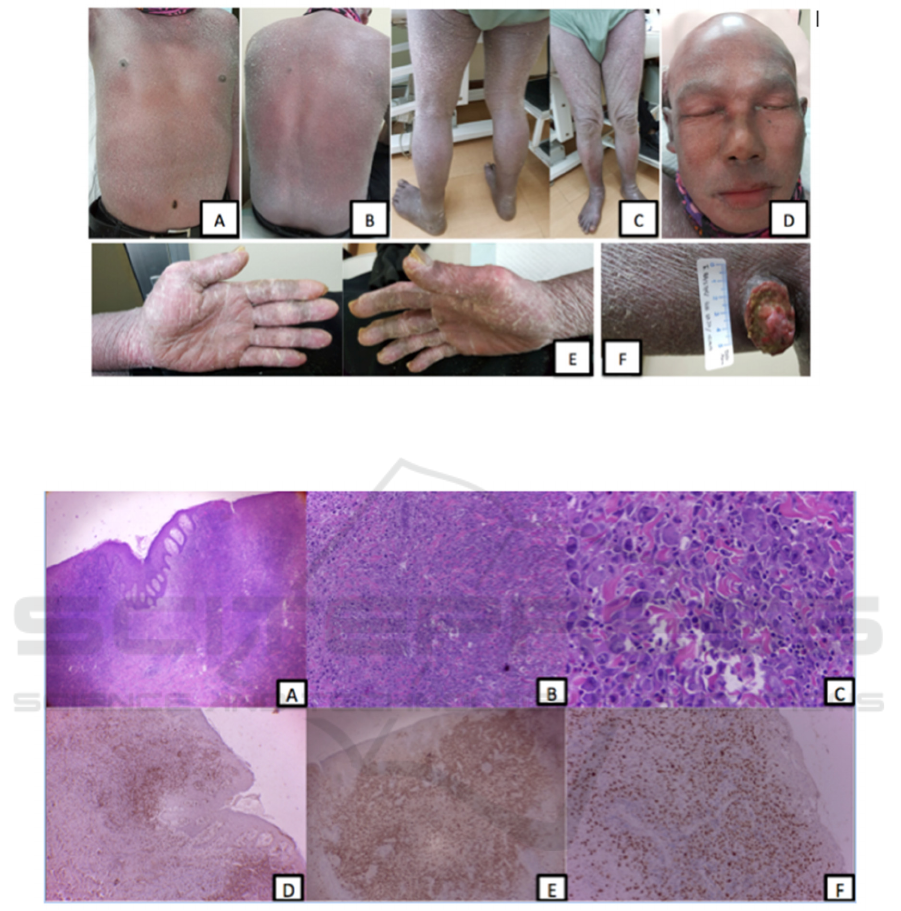
Figure 1. Patient clinically presenting with generalized erythroderma (A, B, C). Hair, eyebrows, eyelashes, mustache loss and
ectropion are also seen (D). Fissures on both palms (E). A tumor-presenting ulcerated nodule on right axilla (F)
Figure 2. (A) Skin biopsy showing infiltrate with large Hodgkin-like cells in a background of small lymphocytes. (B) Lymph
node from the same patient, showing thick bands of fibrosis mimicking classical Hodgkin lymphoma. (C) Section of the right
axilla lymph node showed mixed cellularity Hodgkin's disease. There was effacement of the normal architecture by a mixed
infiltrate comprising mononuclear Hodgkin's and bi-nuclear Reed-Sternberg cells, lymphocytes, plasma cells, eosinophils and
histiocytes. In some uninvolved lymphoid tissue, a marked histiocytic response was noted. Immunohistochemistry assay
shows: (D) diffuse CD3 in dermis, (E) CD30 highlights the tumor cells forming nodular aggregates divided by dense fibrosis
and variability in cell size of the tumor cells, (F) a more scattered image of CD20
The patient was diagnosed as erythroderma et
causa CTCL and tumor stage mycosis fungoides with
secondary infection, while waiting for the
confirmation from histologic findings and
immunohistochemistry assay. Ultrasonography test
was perfomed, with multiple lymphadenopathy were
found on both inguinal and axilla, confirming
systemic involvement, no intraabdominal
involvement, and a cystic mass at right gluteus. The
skin biopsies from two areas showed a spectrum of
drug eruption from erythrodermic area of upper right
arm, while the one from right axilla tumor showed
mixed cellularity Hodgkin's lymphoma. The
immunohistochemistry assay from right axilla tumor
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
502

resulted the following: positive CD30, CD43, Ki-67
on 80% of the atypical cells, and negative CD20,
CD3, ALK, CD1a, CD15, PAX-5, and AE1/AE3
(Figure 2). These findings came to a conclusion of
non-Hodgkin lymphoma, ALK-negative ALCL.
3 DISCUSSION
Anaplastic large cell lymphoma is an uncommon
disease which comprises a number of heteregenous
conditions. The disease usually arises in lymph nodes
where these tumors morphologically resemble
histiocytic lymphoma. Lymphonodal ALCL is an
aggresive lymphoma with rapid extranodal spread
and poor prognosis and skin involvement estimated
occurs in 15% of cases. The clinical spectrum of
primary C-ALCL includes plaques, nodules, and
ulcerated tumours and inflammatory lesions.
The patient reported here had an unusual clinical
course with a 1-year prodrome of non-specific
erythroderma preceded by reddish, pruritic scaly
patch on both thighs two years before. Most cases of
primary C-ALCL arise in normal skin, though pre-
existing mycosis fungoides is well recognized. At
first we diagnosed the patient with MF because florid
erythroderma with marked ectropion clinically
suggestive of MF/SS, however, we still underwent
further investigations for confirmation of diagnosis
since a painful, ulcerated nodule appeared on his right
axilla.
Two biopsies were performed during the course
of the erythrodermic phase of the illness. The one
from the right arm showed a hyperkeratotic
epidermis, half parakeratotic, spongiotic psoriasiform
hyperplasia, exocytosis of lymphocytes, and basal
cells vacuolisation. Lymphoid inflammatory cells
were accumulated at dermal papila. At superficial
part of the dermis there were sparse infiltrate of
lymphocytes. These features concluded as a drug
eruption. Histological examination from the right
axilla nodule showed infiltration of the dermis by
atypical cells which were arranged in strands and
sheets, rough chromatin, noted pleomorphic, and
eosinophilic cytoplasm. Epidermis part were
hyperkeratotic, parakeratotic, achantotic with
granulated tissue. Reed-Sternberg cells were also
found. Mitotic cells easily found. These concluded as
a mixed cellularity Hodgkin lymphoma. We cannot
concluded drug eruption as the diagnosis since it did
not match with the patient's history and clinical
features. A result of Hodgkin lymphoma was
sometimes deceiving as it could also mimicking non-
Hodgkin lymphoma. Thus an immunohistochemistry
assay is needed to confirm the diagnosis.
Immunohistochemistry assay of this patient
showed positive findings of CD30, CD43, and Ki-67
at about 80% of the anaplastic cells. CD20, CD3,
ALK, CD1a, CD15, PAX-5, AE1/AE3 were negative.
A positive CD30 and negative ALK strongly suggest
that this patient suffer from ALCL, ALK-negative
type. Based on one study, the frequency of CD3 in
ALK-negative ALCL was 50%. Another study
showed expression of CD15 is aberrant, and a
negative CD20 and PAX-5 indicated a NHL. Thus we
concluded this patient is still well-accepted in an
ALCL ALK-negative spectrum, regarding negative
results of CD3, CD20, and PAX-5.
4 CONCLUSIONS
We report a very rare case of erythroderma originated
from C-ALCL. It is very challenging for us to
establish a diagnosis regarding confusing relation of
history, clinical, histopathologic, and
immunohistochemistry findings of the disease,
including a Hodgkin disease-mimicking feature of
NHL and various immunohistochemistry assay
results. ALCL itself is already a rare case in a
population and an erythroderma with a painful,
ulcerated nodule make it more laborious for
dermatologists and pathologists to establish a
diagnosis. This unusual case both expands the
spectrum of cutaneous disease associated with
erythrodermic ALCL and highlight the importance of
early biopsy and immunochemistry in patients with
erythroderma presentation.
REFERENCES
Bolognia, JL., Jorizzo, JL., Schaffer, JV., 2012.
Dermatology. 3rd ed. China: Elsevier. Chapter 10,
Erythroderma; p. 171-82
Denton, K., Wilson, C. L., & Venning, V. A., 1992.
Primary cutaneous anaplastic large‐cell
lymphoma with a prolonged erythrodermic
prodrome. British Journal of Dermatology, 126(3),
pp. 297-300.
Foss, F., 2013. T-cell lymphomas. New York: Humana
Press. Chapter 2. Epidemiology and prognosis of T-cell
lymphoma; p. 25-40.
Foss, F., 2013. T-cell lymphomas. New York: Humana
Press. Chapter 5. Primary cutaneous and systemic
CD30
+
T-cell lymphoproliferative disorders; p. 71-86.
Muzzafar, T., Wei, E. X., Lin, P., Medeiros, L. J., &
Jorgensen, J. L., 2009. Flow cytometric
immunophenotyping of anaplastic large cell
lymphoma. Archives of pathology & laboratory
medicine, 133(1), pp. 49-56.
Pletneva, M. A., & Smith, L. B., 2014. Anaplastic large cell
lymphoma: features presenting diagnostic
A Rare Case of Erythroderma in a Primary Cutaneous Anaplastic Large Cell Lymphoma: A Diagnostic Challenge
503

challenges. Archives of Pathology and Laboratory
Medicine, 138(10),pp. 1290-1294.
Stein, H., Foss, H. D., Dürkop, H., Marafioti, T., Delsol, G.,
Pulford, K., ... & Falini, B., 2000. CD30+ anaplastic
large cell lymphoma: a review of its histopathologic,
genetic, and clinical features. Blood, 96(12), pp. 3681-
3695.
Willemze, R., Jaffe, E. S., Burg, G., Cerroni, L., Berti, E.,
Swerdlow, S. H., ... & Grange, F., 2005. WHO-EORTC
classification for cutaneous
lymphomas. Blood, 105(10), pp. 3768-3785.
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
504
