
Oral Amoxicillin Clavulanic Acid as Systemic Therapy in a Patient
Suspected with Actinomycetoma
Laila Tsaqilah, Lies Marlysa,
Risa Miliawati.
Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran - Dr. Hasan Sadikin Hospital,
Bandung 40161 Indonesia
Keywords: actinomycetoma, amoxicillin clavulanic acid, Propionibacterium propionicum.
Abstract: Actinomycetoma is a local chronic granulomatous infectious disease that affects both cutaneous and
subcutaneous tissues, and is caused by anaerobic facultative Gram-positive filamentous bacteria of the
Actinomycetaceae and Propionibacteriaceae family. Propionibacterium propionicum (family
Propionibacteriaceae) and Actinomyces species are very sensitive to beta lactam antibiotics, especially
penicillin G or amoxicillin. A rare case of actinomycetoma in a 38-year-old woman was reported. The
working diagnosis was based on clinical features and anaerobic bacterial culture from the erythematous
papules and nodules of the left arm, left elbow, right hip, and buttocks, as well as shallow ulcers on the left
buttocks, and the growth of Propionibacterium propionicum. Based on antibiotic susceptibility test results,
the patient was treated with amoxicillin clavulanic acid for 7 days. The size of ulcerative lesion with
granulation tissues became smaller. Various antibiotics combination therapy may be given to
actinomycetoma, but the combination of amoxicillin and clavulanic acid has significant effect.
1 INTRODUCTION
Mycetoma known as Maduramycosis or Madura foot
is a chronic localized granulomatous infection that
affects cutaneous and subcutaneous tissues.
Infections caused by fungi are called eumycetoma,
and actinomycetoma are caused by filamentous
bacteria (Branscomb, 2003; Sobera & Elewski, 2008;
Hay & Asbee, 2010; Hay, 2012). The filamentous
bacteria that cause mycetoma are Actinomyces sp.,
Streptomyces sp., and Nocardia sp. (Welsh et al.,
2007; Bravo et al., 2012).
Actinomycetoma is a
bacterial infection of the skin, subcutaneous tissues,
muscles, and bones with a chronic and suppurative
joint gaps, due to endemic facultative anaerobic
bacteria (Sardana et al., 2001; Bravo et al., 2012). The
Gram-positive bacilli family were Actinomycetaceae
and Propionibacteriaceae (Bravo et al., 2012).
Actinomycetoma can be diagnosed when there is one
of three clinical features, which are the existence of
an inflammatory infiltration of the skin or
subcutaneous tissue, a sinus formation with drainage,
and responsiveness to short-term antibiotics
(Reichenbach, 2009; Bravo et al., 2012).
The most
common predilections of actinomycetoma are the
legs, lower limbs, hands, head and back (Hay, 2012).
In the case reported, the lesions on the buttocks
atypical mycetoma (Chaves et al., 2002).
Actinomyces
sp. a bacteria which sensitive to beta lactam
antibiotics especially penicillin G or amoxicillin.
Therefore, the appropriate treatment options were
penicillin G or amoxicillin (Valour et al., 2014).
There were two cases of actinomycetoma treated with
amoxicillin clavulanic acid. The first case was treated
for 5 months and the second case for 6 months. There
were a clinical improvements and bone
improvements as shown by the radiological
examination. Also the absence of grain was shown by
direct mycological microscopy examination (Gomez
et al., 1993).
To our knowledge, this is a rare case of
actinomycetoma which could be treated by oral
amoxicillin clavulanic acid.
2 CASE
A 38-year-old woman, came with the chief complaint
of multiple painful ulcers on the left buttock, and
nodules on the left arm, left elbow, right waist, and
buttock that sometimes feelts itchy. Since two months
before the consultation, there were nodules on the left
arm, left elbow, right hip, and buttock that sometimes
Tsaqilah, L., Marlysa, L. and Miliawati, R.
Oral Amoxicillin Clavulanic Acid as Systemic Therapy in a Patient Suspected with Actinomycetoma.
DOI: 10.5220/0008159504510455
In Proceedings of the 23rd Regional Conference of Dermatology (RCD 2018), pages 451-455
ISBN: 978-989-758-494-7
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
451
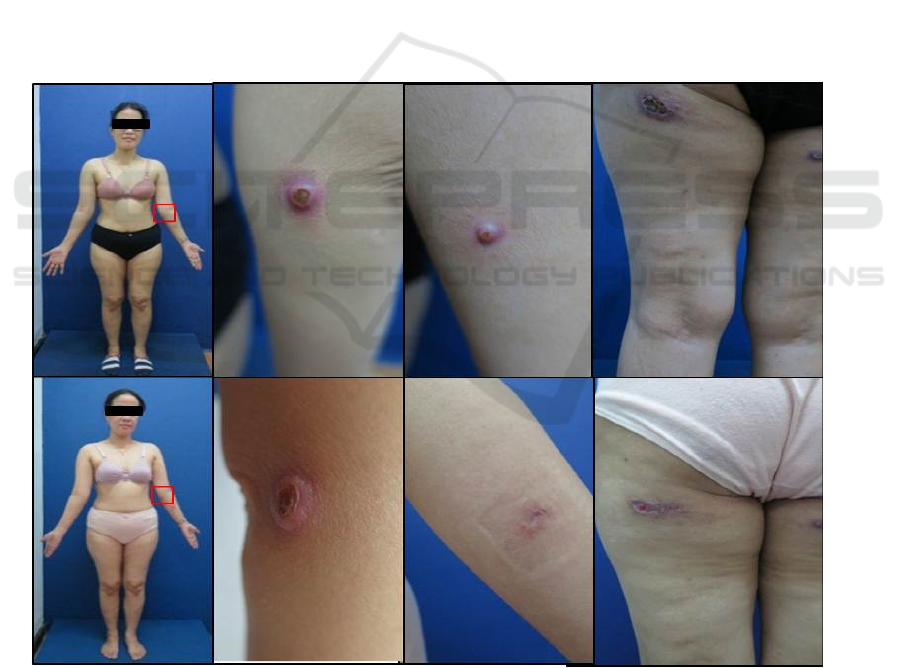
felts itchy. Since a month before consultation, the
nodules on the left buttock were getting bigger and
became ulcers which were accompanied by edema
and induration around the nodules. There were no
lymphadenitis. A history of black, red, or pale grain
in the fluid from an ulcer on the left buttock was
denied.
Three weeks before the consultation, the nodules
were getting bigger. The patient went to the
dermatologist and was given systemic and topical
therapy but there was no improvement, after which
the patient was referred to the RSHS Bandung. There
was no a history of insects bite, trauma, or skin
disorders, before the ulcers appeared. The patient did
not have diabetes mellitus, tuberculosis, hepatitis, in
her family history. She was a textile worker who did
not do any gardening, farming, or working in swamps
or fish ponds. She did not have pets at home. The
hygiene of the patient was quite good. The general
status shown by the physical examination was within
normal limits. Direct microscopic examination with
10% potassium hydroxide (KOH) solution showed
that there were no hyphae nor spores. There were no
Gram positive, Gram negative, or acid-fast from the
ulcer on the left buttock. Propionibacterium
propionicum was identified by anaerobic bacterial
culture from the tissue, and the antibiotic
susceptibility test showed those to be sensitive to
amikacin, amoxcillin clavulanic acid, clindamycin,
ceftriaxone, ceftazidime, ciprofloxacin, orbenin,
gentamicin, aztreonam, and vancomycin. There was
hyalinization of fibrocollageneous tissue with
inflammation cells (lymphocytes, histiocytes,
eosinofil, and polymorphonuclear cells). The
histopathology examination showed lymphocytes on
the perivascular tissue, no spores, no hyphae, no
epitelioid datia Langhans cells or caseous necrosis.
3 DISCUSSION
Figure 1: Clinical manifestation.
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
452

Figure 2: Histopathological results revealed lymphocytes on the perivascular tissue, no spores, no hyphae, no epitelioid datia
Langhans cells or caseous necrosis (hematoxylin and eosin, x400).
Mycetoma is a common disease in tropical and
subtropical developing countries (Linchon &
Khachemoune, 2006; Hay, 2012). Actinomycetoma
is more common in Central and South America. The
incidence rate of mycetoma in Indonesia has not been
clearly documented (Prasetyo & Suyoso; 2011).
Males are generally more affected than females, The
male to females rate in mycetoma patients is 3: 12
(Branscomb, 2003). Mycetoma affected mostly
adults (20 to 50 years) (Hay, 2012).
Mycetoma is
more common among people with livelihoods as
farmers, laborers, or shepherds (Bravo et al., 2012;
WHO, 2015). In this case, the patient was a 38 years
old woman that worked as a textile worker. The
commonest predilection of mycetoma are the feet
(68.7%), as in many cases their feet not protected by
footwear. In addition, mycetoma can occur on the
lower limbs (11.3%), the body (6.1%), the hands
(4%), arm (2.9%), head (2.1%), and buttocks (1,3 %).
There is one case report of mycetoma on the buttocks
which is a atypical mycetoma case in Senegal. The
lesions from that case were similar to this case report
were the affected area was the left buttock which is
an anusual predilection area.
The most common causes of actinomycetoma are
Nocardia brasiliensis, Actinomadura madurae,
Actinomadura pelletieri, and Streptomyces
somaliensis
5.12
which can be found in plants and
soils.
1
Whereas Actinomyces sp. and Nocardia sp. are
filamentous bacteria which have the same Class as
Actinobacteria and same Order as Actinomycetales
which can cause disease in the human skin. Aerobic
bacteria like Actinomyces sp. normally live at the
respiratory, digestive, and genitourinary systems and
can cause local suppurative disease by forming
fistulae. The aerobic environment of Nocardia sp. and
Actinomyces sp. can cause actinomycetoma (Bravo et
al., 2012). Propionibacterium propionicum known as
Actinomyces meyeri is a form of Actinomyces species
bacteria (Bravo et al., 2012). Actinomycetoma in this
case report was caused by Propionibacterium
propionicum that could be found by anaerobic
bacteria tissue culture.
The trias of mycetoma symptoms are tumefaction,
grains in the abscess, and the sinuses thorough which
that grains can emerge and reach the skin surface
(Sobera et al., 2008). At the early stages of mycetoma
the symptoms were, a small chronic solitary lesion of
painless subcutaneous nodules which have a hard or
soft consistency,
and no erythema lesion around the
nodules (Hay, 2012). The clinical forms of
actinomycetoma and eumycetoma are very similar,
but there are some differences between them. At the
early stage, the lesions of actinomycetoma are firm
nodules which tend to coalesce with the surrounding
tissues and progress rapidly (Branscomb, 2003).
Grains are the product of organisms that grow and
survive after inoculation. Grains are the components
of filamentous bacteria or fungi (Sobera & Elewski,
2008). Grain can be found in the abscess and contain
polymorphonuclear (PMN) cells, which will come
out onto the skin surface through the sinus tract (Hay,
2012). The size of the grains vary from 0.2 to 5 mm
and can be seen as sand grains attached to the sinuses
(Hay, 2012). Direct microscopic examination is
important to examine the grain because it can
determine the cause of mycetoma. Fungal grain is
black or brown, Actinomycetaceae grain is red or
pink, when the grain is white, this could be cause by
fungi and bacteria (Sobera & Elewski, 2008; Hay &
Ashbee, 2010; Hay, 2012).
A single case of
mycetoma was reported in a man who had no history
of grain. Histopathologic examination with
hematoxycillin-eosin-safran (HES) and Periodic
Acid-Schiff (PAS) staining diagnosed the patient
with mycetoma. Mycetoma can be diagnosed from
the culture of fungal and bacteria from the grains,
exudate or from tissue or aspiration (Branscomb,
2003). However, the culture often does not produce
satisfactory results due to various conditions such as,
bacterial contamination, or cultured tissue obtained
from late stage lesion containing fibrosis tissue rather
than purulent exudates. The negative cultured results
occur because the fungi did not grow as it has
received antibiotic therapy before. Direct
microscopic examination using Ziehl-Neelsen
Oral Amoxicillin Clavulanic Acid as Systemic Therapy in a Patient Suspected with Actinomycetoma
453

staining was done to detect the presence of acid fast
bacilii that could cause cutaneous tuberculosis or
infections of atypical Mycobacteria.
Polymerase chain reaction (PCR) examination
can detect the tuberculosis and nontuberculosbacteria
DNA from skin tissue. In this case report, the patient
first complained of painless nodules in the left arm,
left elbow, right hip, and buttocks since two months
before consultation. The nodules were getting bigger
and multiplied in the month before consultation and
the nodules on the left buttock became ulcers. There
was edema and indurations around the nodules. There
was no hystory of lymphadenitis or grain in the fluid
from the ulcer, no fungal growth in the fungal
cultures, no acid fast bacteria from the Ziehl-Neelsen
staining, and the results from the PCR was negative.
The results from the bacterial culture was anaerobic
bacteria (Propionibcaterium propionicum).
Histopathology examination showed hyalinisation of
the fibrocollageneous tissue with inflammation cells
(lymphocytes, histiocytes, eosinofil, and
polymorphonuclear cells), there were lymphocytes on
the perivascular tissue, no spores, no hyphae, and no
epitelioid datia Langhans cells or caseous necrosis.
The treatment of actinomycetoma could be done
with antimicrobial agents and surgery. Amputation as
a single therapy rarely gives good results. Surgery can
remove small lesions or reduce the size of the lesion.
The recommendations of drug regimens are based on
expert experience. There are no randomized
controlled trials for effective therapy regimens for
mycetoma. The treatment is given in combination
regimens to prevent drug resistance. The duration of
therapy is 3-24 months depending on the response of
the patient. The healing of the lesions can be assessed
based on subcutaneous nodules and sinus, or
indurations of the skin. Various antibiotic therapies
for actinomycetoma are aminoglycosides (amikacin
or netilmicin), rifampicin, amoxicillin-clavulanic
acid, fusidic acid, clindamycin, imipenem-silastin,
(Hay, 2012) moxifloxacin, or the tetracycline group
(oxytetracycline, minoxycline, or doxycycline)
(Bravo et al., 2012).
There is a case reporting actinomycetoma in a 32-
year-old woman treated with benzylpenicillin
8,000,000 IU injections four times daily and
cotrimoxazole tablet twice daily for twenty days,
followed by twice daily cotrimoxazole and 500 mg
amoxicillin four times daily. Two months after the
therapy there was significant improvement of sinus
fibrosis, no induration, no secretions, no new lesions,
and no systemic symptoms. The mechanism of action
of beta lactam antibiotics is bactericidal by binding to
specific proteins of penicillin-binding, inhibit
peptidoglycan synthesis and inhibit the autolithic
enzymes of bacterial cell walls. Clavulanic acid
obtained from isolated metabolite Streptomyces
clavuligerus,
11
can inhibit beta lactamase.
11
Amoxicillin-clavulanic acid has a major influence for
Gram positive and Gram negative bacteria.
11
In this
case the patients was given amoxicillin clavulanic
acid based on the results of antibiotic susceptibility
test against Propionibacterium propionicum and the
recommended therapy for actinomycetoma.
4 CONCLUSION
Our case demonstrates actinomycetoma with oral
amoxicillin clavulanic acid for therapy. Actinomyces
sp. can cause actinomycetoma. Propionibacterium
propionicum or known as Actinomyces meyeri is a
form of bacteria incorporated in the species
Actinomyces microorganisms cause actinomycetoma
(Bravo et al., 2012). Various regimens of antibiotics
can be given for actinomycetoma, such as
aminoglycoside group, rifampicin, amoxicillin-
clavulanic acid, fusidic acid, clindamycin, imipenem-
silastin, moxifloxacin, or tetracycline group
(oxytetracycline, minoxycline, or doxycycline).
Amoxicillin is often combined with clavulanic acid
which has a major effect on disease therapy infections
caused by Gram positive and Gram negative bacteria.
In this case patients are given amoxicillin clavulanic
acid therapy in accordance with the results of
antibiotic susceptibility test against
Propionibacterium propionicum and recommended
therapy for actinomycetoma.
REFERENCES
Branscomb, R., 2003. Mycetoma: an overview. Laboratory
Medicine. 11, pp. 803-808.
Bravo, F.G., Arenas, R., Sigall, D.A. 2012. Actinomycosis,
nocardiosis, and actinomycetoma. In: Goldsmith LA,
Katz SI, Gilchrest BA, Paller AS, Leffel DA, Wolff K,
editor. Fitzpatrick’s dermatology in general medicine.
8
th
edition. New York: McGraw-Hill. Page:2241-52.
Chávez, G., Estrada, R., Bonifaz, A., 2002. Perianal
actinomycetoma experience of 20 cases, in:
International Journal of Dermatology. pp. 491–493.
doi:10.1046/j.1365-4362.2002.01550.x
Executive Board. Mycetoma., 2015. World Health
Organization (WHO).
Gomez, A., Saul, A., Bonifaz, A., Lopez, M. 1993.
Amoxicillin and Clavulanic Acid in The Treatment Of
Actinomycetoma. International Journal of
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
454

Dermatology 32, 218–220. doi:10.1111/j.1365-
4362.1993.tb02800.x
Hay, R.J., 2012. Deep fungal infection. In: Goldsmith, L.A.,
Katz, S.I., Gilchrest, B.A., Paller, A.S., Leffel, D.A.,
Wolff, K., editor. Fitzpatrick’s dermatology in general
medicine. 8
th
edition. New York: McGraw-Hill.
Page:2312-28.
Hay, R.J., Ashbee, H.R., 2010. Mycology. In: Burns, T.,
Breathnatch, S., Cox, N., Griffiths, C., penyunting.
Rook’s textbook of dermatology. 8
th
edition. Oxford:
Blackwell. Page:36.5-93.
Lichon, V., Khachemoune, A., 2006. Mycetoma: a review.
American Journal of Clinical Dermatology.
doi:10.2165/00128071-200607050-00005
Prasetyo, A.D., Suyoso, S. 2011. Retrospective study:
subcutaneous mycoses treated in the ward of
Dermatology and Venereology Department of Dr.
Soetomo General Hospital, year 2000–2009 (10 years
period). Berkala Ilmu Kesehatan Kulit dan Kelamin.
2011;23(1):17-24.
Reichenbach, J., Lopatin, U., Mahlaoui, N., Beovic, B.,
Siler, U., Zbinden, R., Seger, R.A., Galmiche, L.,
Brousse, N., Kayal, S., Güngör, T., Blanche, S.,
Holland, S.M., 2009. Actinomyces in Chronic
Granulomatous Disease: An Emerging and
Unanticipated Pathogen. Clinical Infectious Diseases
49, 1703–1710. doi:10.1086/647945
Sardana, K., Mendiratta, V., Sharma, R.C., 2001. A
suspected case of primary cutaneous actinomycosis on
the buttock. Journal of Dermatology 28, 276–278.
doi:10.1111/j.1346-8138.2001.tb00132.x
Sobera, J.O., Elewski, B.E. 2008. Fungal disease. In:
Bolognia, J.L., Jorizzo, J.L., Rapini, R.P., editor.
Dermatology. 2
nd
edition. Edinburgh: Mosby Elsevier.
Page: 1135-57.
Valour, F., Sénéchal, A., Dupieux, C., Karsenty, J., Lustig,
S., Breton, P., Gleizal, A., Boussel, L., Laurent, F.,
Braun, E., Chidiac, C., Ader, F., Ferry, T., 2014.
Actinomycosis: Etiology, clinical features, diagnosis,
treatment, and management. Infection and Drug
Resistance. doi:10.2147/IDR.S39601
Welsh, O., Vera-Cabrera, L., Salinas-Carmona, M.C.,
2007. Mycetoma. Clinics in Dermatology 25, 195–202.
doi:10.1016/j.clindermatol.2006.05.011
Oral Amoxicillin Clavulanic Acid as Systemic Therapy in a Patient Suspected with Actinomycetoma
455
