
Acral Lentiginous Melanoma Diagnosed using Combination of
Dermoscopic and Histopathological Examination
Calvin Santosa
1
, Ni Luh Putu Ratih Vibriyanti Karna
1
, A. A. Ari Agung Kayika Silayukti
2
, Herman
Saputra
3
1
Dermatovenereology Department Sanglah General Public Hospital, Denpasar, Indonesia
2
Dermatovenereology Department Mangunsada General Public Hospital, Badung, Indonesia
3
Pathology Department Sanglah General Public Hospital, Denpasar, Indonesia
Keywords: melanoma, acral lentiginous, dermoscopy, histopathology
Abstract: Melanoma is a malignant tumor, which arises from melanocyte, and most commonly appears initially on the
skin. Melanoma may arise also on mucosal surface even the leptomeningeal. Risk factors for melanoma
could come endogenously and exogenously. Melanoma is still one of the leading causes of death by cancer
in the world. Acral lentiginous melanoma (ALM) is an uncommon type of melanoma and usually diagnosed
in the elderly on the extremities or nails. Clinically acral melanoma may be diagnosed as fungal infection or
benign nevus lesion. Dermoscopy has helped clinician in differentiating between benign and malignant
nevus lesion, hence not all cases need histopathological examination. Pathognomonic findings in melanoma
lesion through dermoscopic examination may assist dermatologist in diagnosing melanoma. Main treatment
for melanoma is still wide excision of the lesion and periodic monitoring post excision necessary to evaluate
the risk of metastasis and mortality.
1 INTRODUCTION
Melanoma is a malignant tumor of melanocytes that
can occur in the skin, mucosa and leptomeningeal. A
clinical feature that may resemble ordinary nevus
often makes the patient unaware of the condition
(Garbe and Bauer, 2012). Epidemiologically
melanoma is more commonly found in Fitzpatrick
skin type I and II who received excessive sun
exposure. In Europe an incidence of 10-25 new
cases per 100,000 population is found per year,
whereas in the United States 20-30 cases per
100,000 people and the highest in Australia is 50-60
cases per 100,000 population (Garbe and Leiter,
2009; Ferlay et al., 2013). In Indonesia alone the
incidence vary but still very low. In RS Dr. M.
Djamil Padang found 9 cases of melanoma during
2002-2007 (Azamris, 2011).
Acral lentiginous melanoma (ALM) is a rare
type of cutaneous melanoma and is often diagnosed
at the later stage with lesions in the palms, soles or
inside or around the nails. This condition tends to be
found in African and Asian racial groups where
rarely found melanoma cases due to excessive sun
exposure (Bradford et al., 2009). The way to
diagnose melanoma still requires histopathological
examination, but with the discovery of dermoscopy
has reduced the number of nevus lesions that do not
show malignant features (Saida et al., 2011).
The main therapy for melanoma is surgical
excision. If there is a sign of metastasis, additional
chemotherapy and immunotherapy should be given
(Saiag et al., 2007).
The following case of acral
lentiginous melanoma is diagnosed using
combination of dermoscopy and histopathology
examination. This case is reported due to a relatively
low incidence in Indonesia and to increase
understanding of melanoma, diagnosis and
appropriate management.
2 CASE
A 53-year-old Balinese mancame to
Dermatovenereology polyclinic of Mangunsada
Badung Hospital with a chief complaint of dark spot
on the right sole of his foot.
Patient has a history oftrauma from a tree thorn 1
year ago and the patient felt that there was a thorn
that is embedded in his foot. Patients tried to remove
Santosa, C., Vibriyanti Karna, N., Silayukti, A. and Saputra, H.
Acral Lentiginous Melanoma Diagnosed using Combination of Dermoscopic and Histopathological Examination.
DOI: 10.5220/0008157503630367
In Proceedings of the 23rd Regional Conference of Dermatology (RCD 2018), pages 363-367
ISBN: 978-989-758-494-7
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
363
it without any help of medical personnel. About 10
months ago the patient claimed to have black spots
appear on the trauma area on his right sole. Patients
claimed no pain or itching on that area. The dark
spots slowly spread to the adjacent area. History of
previous mole on the area is denied. Patients
mentioned about awound which appear on the black
spots 2 weeks before admission. The patient does
not feel any pain on the when being touched.
Patients claimed to have never treated the
condition since 1 year ago. History of similar
condition in other location was not found. History of
malignancy is denied. History of systemic diseases
such as hypertension, diabetes mellitus, bleeding
disorders are denied by the patient. History of any
immunosuppressive conditions was denied. History
of similar disease in the patient's family is denied.
History of malignancy, asthma, hypertension or
diabetes mellitus in patient families is denied.
Currentlypatient works as a farmer and often does
not use any footwear during work. Patients have no
habit of alcohol consumption or smoking.
Physical examination found the general
condition of the patient good. Blood pressure 120/80
mmHg, pulse 84x/minute, respiratory frequency
20x/minute and axillary temperature 36.4°C.
General status were within normal limit. No
enlarged lymph nodes were found. The
dermatological status onthe right plantar pedis there
are multiple hyperpigmentation macules, well-
defined margin, sized 2x3 cm to 5x7 cm with a
solitary ulcer above it with rising and regular edge,
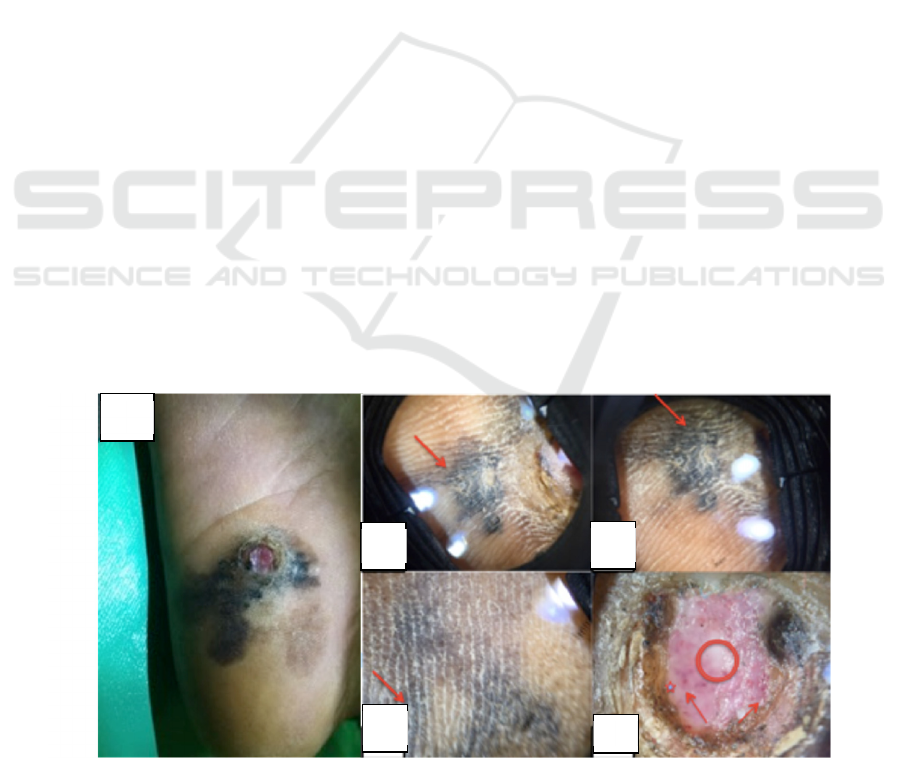
clean base, round, 1.5 cm in diameter. (Figure 1A)
On the investigation using dermoscopy, there are
found parallel ridge pattern on the hyperpigmented
lesion (red arrow Figure 1B-D) andon the ulcer
showed blood vessel in dots form (red arrow
Figure 1 E), bluish globule (asterisk Figure 1E) and
structureless area (circle Figure 1E). On the edge of
the ulcer there were mass of keratin with a
homogeneous black spot on some foci.
Histopathological examination found epidermal
layer with a picture of acanthosis accompanied by a
thick layer of keratin. There is a proliferation of
atypical melanocyte cells along the basal epidermis
with disturbed cohesion with relatively large sized
morphologies, vacuolized cytoplasm, bizarre
nucleus, hyperchromatic, partially with prominent
nucleus, and irregular nucleic membrane. These
cells contain brownish pigmented granules (melanin).
Some of these cells extend along the superficial
dermis. Mitosis can be found. The superficial dermis
layer looks degenerated and thin. At 1 focus contains
necrotic areas with necrotic debris. This fit with the
morphological picture of an acral-lentiginous
melanoma. (Figure 2).
Based on the dermoscopic and histopathological
examination, this patient was diagnosed with acral
lentiginous melanoma and was performed wide
excision of the lesion with a margin of 1 cm and as
deep as subcutaneous tissue. The tissue obtained
from the excision were examined again and there
were clear margin of the lesion. The patient later on
got a skin graft from calf area to close the surgical
area. The patient was also recommended to do
annual check up for the first 5 year.
Figure 1: (A) Hyperpigmented lesion on right sole with solitary ulcer on it. Dermoscopic pattern of the lesion (B,C,D,E).
A
B
C
D
E
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
364
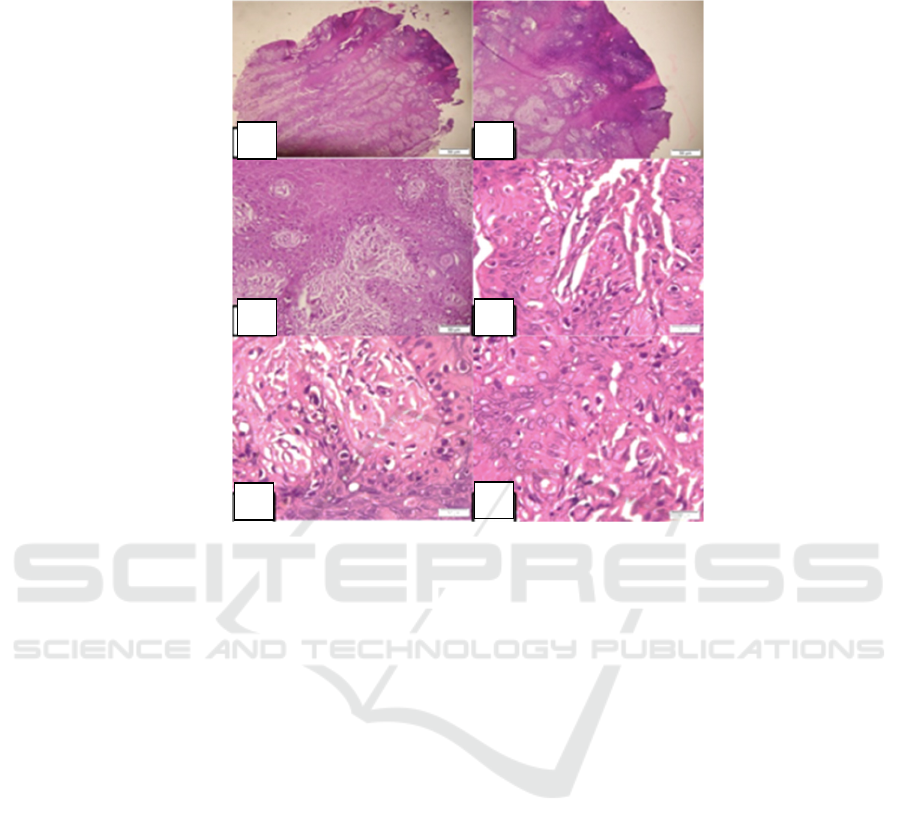
Figure 2: Histopathological image from the lesion suit with acral lentiginous melanoma.
3 DISCUSSION
Melanoma is a tumor derived from melanocytes and
can be foundon skin, mucosal, eyes and brain
meningeal. Acral melanoma itself is a subtype most
commonly found in non-white populations. This
subtype includes>70% melanoma in African races in
America as well as 50% in Asian racial groups
(Ishihara et al., 2008).
Risk factors from melanoma can be divided into
3 categories including genetic factors, phenotypic
manifestations of genetic and environmental
interactions also environmental factors. Genetic
mutations or polymorphism can increase one
person's tendency for the development of melanoma.
The focus of genes associated with familial
melanoma is CDKN2A, which plays a role in coding
proteins p16 and p14 that function in the course of
cell cycle. In addition, variants of the melanocortin-1
receptor gene (MC1R) were also found to increase
the risk of melanoma formation. Factors that are
phenotypic manifestations of genetic and
environmental interactions are melanocytic nevus,
atypical melanocytic nevus and ephelids and lentigo
solaris. Environmental factors that play a role in the
formation of cutaneous melanoma are ultraviolet
radiation; especially ultraviolet B. Chronic radiation
can cause mutations and DNA damage which disrupt
the cell cycle. In a study that assessed the role of
trauma in the occurrence of ALM in areas not
exposed to sunlight such as soles of the feet, hands
and nails found about 13-55% of cases of ALM has
a history of previous trauma, but its relation to the
development of melanoma is still unclear (Phan et
al., 2006).
Diagnosis of melanoma requires correlation of
clinical features, dermoscopy and histopathology.
Clinically lesions of melanoma appear as
asymmetrical lesions, having irregular edges, has
variety of colors, diameters greater than 5 mm and
found growth of nodules or regression components
in the lesions. The sensitivity of these clinical
features to diagnose melanoma can be as high as
70% if performed by an experienced dermatologist.
The most common and typical dermoscopic features
for ALM are the presence of parallel-ridge patterns
that have a specificity of 99%, sensitivity of 86%
and a positive predictive value of 84%. The next
most common pattern is the brownish-colored
pigment found in ALM in situ and invasive ALM.
Another feature that can be found is a serrated
pattern consisting of a streak image at the edge of
A
B
C
D
E
F
Acral Lentiginous Melanoma Diagnosed using Combination of Dermoscopic and Histopathological Examination
365

the tumor. Not infrequently also found ALM with
ridge pattern accompanied by diffuse pigmentation
area. In the case of an invasive ALM it tends to
destroy the furrow and ridges images so that it can
be seen only structureless pattern and pigmentation
spots. In addition it will show more color and
structure such as firm edges, irregular dots and
globules, atypical streaks, atypical blotches, blue
white veil, regression structure and atypical blood
vessels (Malvehy and Puig, 2012).
Histopathological examination remains a gold
standard in diagnosing melanoma. In ALM,
proliferation of atypical melanocytes in the
hyperplastic epidermal basal layer can be seen.
Atypical melanocytes are arranged one by one in
irregular nests in all layers of the epidermis. In the
stratum corneum layer melanocytes and melanin
granules are spread evenly. In difficult cases
immunohistochemical staining may help diagnosis.
Dyes that can detect antigens in melanocytes such as
HMB45, tyrosinase, Melan-A / MART-1 and S100
are useful in differentiating melanocyte cells with
other cells so as to visualize the extent of primary
melanoma as well as to help see the focus of
melanoma on lymph node biopsy (Garbe and Bauer,
2012; Merkel and Gerami, 2017). The patient in this
case shows the dermoscopic appearance which
suggesting to be a melanoma lesion, which later on
be confirmed from the histopathological
examination.
Management of melanoma can be divided into 3
in primary melanoma (stage 1 and 2), melanoma
with regional metastasis (stage 3) and melanoma
with distant metastasis (stage 4). In primary
melanoma therapy is still with surgical excision with
the aim of preventing local recurrence or persistent
disease. The last recommended guideline for the
management of primary melanoma is for in situ
melanoma cases an excision surgery of 0.5 cm from
the edge of the tumor should be performed, for
melanoma with a depth of ≤ 1 mm requires a 1 cm
margin from the edge of the tumor, for melanoma
depth of ≥ 2 mm minimum 2 cm margin from the
edge of the tumor. For cases in difficult locations
such as acral, mucous membranes or face Mohs
surgery may be a more appropriate choice. Local
recurrence is defined as a recurrence of the lesion
within 2 cm of the post-excision site. This results
from the spread of primary tumor or intralymphatic
spread. In such cases it is necessary to re-execute
and check on the regional lymph nodes to see if
there are any signs of metastasis or not (Garbe and
Bauer, 2012; Garbe et al., 2016). The patient is
diagnosed with primary acral lentiginous melanoma
and was treated with margin of 1 cm and as deep as
subcutaneous tissue, with histopathological
examination there are no melanoma cells on the
edges of the lesion.
The prognosis for melanoma stage 1 is still quite
good with proper and rapid management. The
survival rate in stage 1 of 93-97% decreased in stage
2 to 53-82%, in stage 3 it was found that 40-78%
and got 9-27% for stage 4. Monitoring of patients
with melanoma especially the first 5 years is very
important, where 90% of metastases occur at that
time period. Regular monitoring aims to identify
recurrence or early disease progression, can
diagnose early primary melanoma and skin cancer in
addition to melanoma, provide psychosocial
assistance, provide education to prevent patients and
families, provide education for patients and families
about a self-examination method for early detection
of melanoma, as well as for the administration of
adjuvant therapy if necessary. The recommended
timeframe according to the guidelines in Europe is 2
to 4 times per year for 5 to 10 years (Garbe and
Bauer, 2012; Garbe et al., 2016).
4 CONCLUSION
Reported a case of acral lentiginous melanoma in a
man with a clinical picture of a black spot on the
sole of the foot since 1 year ago without any
complaints. The dermoscopy examination, parallel
ridge pattern is seen that gives a suggestion of a
melanoma lesion. The patient then performed a
biopsy and presented atypical melanocytes with
bizzare nuclei and also found mitosis so that the
diagnosis tends to lead to acral lentiginous
melanoma. Patients were consulted to the surgical
department for further treatment and performed
excision surgery with 1 cm margin. The prognosis of
the patients is still need to be established by deciding
in the tumor stage, however periodic monitoring is
necessary to prevent and diagnose both primary and
metastatic melanoma lesions in order to improve
survival rates, especially the first 5 years.
REFERENCES
Azamris., 2011. Kanker kulit di bangsal bedah RS Dr. M.
Djamil Padang Januari 2002-Maret 2007. CDK 38(2):
109-10
Bailey, E.C., Sober, A.J., Tsao, H., Mihm Jr., M.C.,
Johnson, T.M., 2012. Cutaneous melanoma. In:
Goldsmith, A.L., Katz, I.S., Gilchrest, A.B.
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
366

Fitzpatrick’s Dermatology in General Medicine 8
th
ed.
New York: McGraw Hill p. 2668-80.
Bradford, P.T., Goldstein, A.M., McMaster, M.L., Tucker,
M.A., 2009. Acral lentiginous melanoma: Incidence
and survival patterns in the United States, 1986-2005.
Archives of Dermatology 145, 427–434.
doi:10.1001/archdermatol.2008.609
Bristow, I.R., Acland, K., 2008. Acral lentiginous
melanoma of the foot and ankle: A case series and
review of the literature. Journal of Foot and Ankle
Research 1. doi:10.1186/1757-1146-1-11
Ferlay, J., Steliarova-Foucher, E., Lortet-Tieulent, J.,
Rosso, S., Coebergh, J.W.W., Comber, H., Forman, D.,
Bray, F., 2013. Cancer incidence and mortality
patterns in Europe: Estimates for 40 countries in 2012.
European Journal of Cancer 49, 1374–1403.
doi:10.1016/j.ejca.2012.12.027
Garbe, C., Bauer, J. 2012. Melanoma. In: Bolognia, J.L.,
Jorizzo, J.L., Schaffer, J.V. Dermatology. Elsevier
Saunders p.1885-1912.
Garbe, C., Leiter, U., 2009. Melanoma epidemiology and
trends. Clinics in Dermatology 27, 3–9.
doi:10.1016/j.clindermatol.2008.09.001
Garbe, C., Peris, K., Hauschild, A., Saiag, P., Middleton,
M., Bastholt, L., Grob, J.J., Malvehy, J., Newton-
Bishop, J., Stratigos, A.J., Pehamberger, H.,
Eggermont, A.M., 2016. Diagnosis and treatment of
melanoma. European consensus-based
interdisciplinary guideline - Update 2016. European
Journal of Cancer 63, 201–217.
doi:10.1016/j.ejca.2016.05.005
Ishihara, K., Saida, T., Otsuka, F., Yamazaki, N. 2008.
The prognosis and statistical investigation committee
of the Japanese Skin Cancer Society: statistical
profiles of malignant melanoma and other skin cancers
in Japan: 2007 update. Int J ClinOncol 13:33-41.
Malvehy, J., Puig, S. 2012. Acrolentiginous melanoma. In:
Marghoob AA, Malvehy J, Braun RP. Atlas of
Dermoscopy 2ed. UK: Informa healthcare p. 210-219.
Merkel, E.A., Gerami, P., 2017. Malignant melanoma of
sun-protected sites: A review of clinical, histological,
and molecular features. Laboratory Investigation 97,
630–635. doi:10.1038/labinvest.2016.147
Phan, A., Touzet, S., Dalle, S., Ronger-Savlé, S., Balme,
B., Thomas, L., 2006. Acral lentiginous melanoma: A
clinicoprognostic study of 126 cases. British Journal
of Dermatology 155, 561–569. doi:10.1111/j.1365-
2133.2006.07368.x
Saiag, P., Bosquet, L., Guillot, B., Verola, O., Avril, M.F.,
Bailly, C., Cupissol, D., Dalac, S., Danino, A., Dréno,
B., Grob, J.J., Leccia, M.T., Renaud-Vilmer, C.,
Négrier, S., 2007. Management of adult patients with
cutaneous melanoma without distant metastasis. 2005
Update of the French Standards, Options and
Recommendations guidelines. Summary report.
European Journal of Dermatology 17, 325–331.
doi:10.1684/ejd.2007.0209
Saida, T., Koga, H., Uhara, H., 2011. Key points in
dermoscopic differentiation between early acral
melanoma and acral nevus. Journal of Dermatology
38, 25–34. doi:10.1111/j.1346-8138.2010.01174.x
Acral Lentiginous Melanoma Diagnosed using Combination of Dermoscopic and Histopathological Examination
367
