
Bullous Pemphigoid Established by Direct Immunofluorescence: Case
Report
Vidyani Adiningtyas, Hasnikmah Mappamasing, Septiana Widyantari, Trisiswati Indranarum,
Sawitri, Evy Ervianti, Sunarko Martodiharjo, Dwi Murtiastutik
Department of Dermatology and Venereology, Faculty of Medicine, Universitas Airlangga / Dr. Soetomo General Hospital
Surabaya
Keywords: bullous pemphigoid, autoimmune, subepidermal blistering, direct immunofluorescence, anti-BP180/230.
Abstract: Bullous pemphigoid (BP) is the most common autoimmune subepidermal blistering disease of the skin and
mucous membranes. It is characterized by autoantibodies against hemidesmosomal proteins of the skin and
mucous membranes. Collagen XVII and dystonin-e have been identified as target antigens. BP is usually a
chronic disease, with spontaneous exacerbations and remissions.
The diagnosis of BP relies on
immunopathologic findings, especially based on both direct and indirect immunofluorescence microscopy
observations, as well as on anti-BP180/BP230 enzyme-linked immunosorbent assays (ELISAs). The
primary objectives are therefore to control both the skin eruption and itch, as well as to minimize any
serious side-effects of the treatment.
1 INTRODUCTION
Bullous pemphigoid affects mostly the elderly
(Wojnarowska et al., 2002; Di Zenzo et al., 2012).
The incidence of the disease is increasing gradually
and is associated with high morbidity and mortality.
Clinically, BP is characterized by an intensely
pruritic eruption with widespread bullous lesions.
The clinical diagnosis can be challenging in the
setting of atypical presentations. Both the morbidity
of bullous pemphigoid and its impact on quality of
life are significant (Bernard et al., 2017).
Treatment
is mainly based on topical and/or systemic
glucocorticoids, but anti-inflammatory antibiotics
and steroid sparing adjuvants are useful alternatives.
Localized and mild BP can be treated with topical
corticosteroids alone (Bastuji-Garin et al., 2011;
Culton et al., 2012). Specifically, the goals of the
management are: (i) to treat the skin eruption, reduce
itching and prevent/reduce the risk of recurrence; (ii)
to improve the quality of life of patients; and (iii) to
limit the side-effects related to the newly introduced
drugs, particularly in the elderly (Wojnarowska et
al., 2002; Schmidt et al., 2012).
2 CASE
This case involved a 74 year-old male, whose chief
complaint consisted of pain and erosions all over his
body. This had been occurring for two weeks.
Previously – one month earlier – there had been
tense blisters on his chest, containing clear fluid.
These were not easily broken but several of the
blisters became eroded and pain was present in the
eroded skin. Itchiness was minimal. There was no
odour and no wound on the lips or genitalia. The
patient also had a history of diabetes mellitus. His
routine activity was cycling in the morning until
noon. Dermatological examination revealed tense
seropurulent bullae on non-sharply marginated
erytematous macule. Bullae size varied from small
to 2cm. Despite some erosion and thin scales, there
was negative Nikolsky sign, no mousy odour and no
lesions on the genitalia or lips. Upon the patient’s
admission, blood, urinary, electrolyte, liver and renal
function tests were all performed and found to be
within normal limits. Albumin, eosinophil and
random blood glucose were abnormal. From the
histopathological examination, the result was
bullous pemphigoid and the direct
immunofluorescent test found linear IgG and C3 in
the basal membrane zone. The patient was treated
Adiningtyas, V., Mappamasing, H., Widyantari, S., Indranarum, T., Sawitri, ., Ervianti, E., Martodiharjo, S. and Murtiastutik, D.
Bullous Pemphigoid Established by Direct Immunofluorescence: Case Report.
DOI: 10.5220/0008157103470350
In Proceedings of the 23rd Regional Conference of Dermatology (RCD 2018), pages 347-350
ISBN: 978-989-758-494-7
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
347
with methylprednisolone 4mg 3x16mg and dapsone
1x100mg daily, tapering off the steroid after there
were signs of clinical improvement including no
new lesions.
3 DISCUSSION
Figure 1: Gram staining reveals leukocyte only, without
any coccus
The name ‘bullous pemphigoid’ (BP) is a pleonasm,
as ‘pemphigoid’ is derived from Greek and means
‘form of a blister’ ( pemphix, blister, and eidos,
form) (Feliciani et al., 2015).
Bullous pemphigoid typically occurs in patients
over 60 years of age, with a peak incidence
occurring among those patients in their 70s. There
are several reports of bullous pemphigoid in infants
and children, although this is rare
(Wojnarowska et
al., 2002; Culton et al., 2012; Di Zenzo et al., 2012;
Bernand & Antonicelli, 2017).
This is consistent
with the patient in our case; he is 74 years old. Old
age is the major risk factor for the occurrence of BP.
Most cases of bullous pemphigoid occur
spontaneously without any obvious precipitating
factors. However, there are several reports in which
bullous pemphigoid appears to be triggered by
ultraviolet (UV) light, either UVB or following
PUVA therapy, and radiation therapy. Certain
medications have also been associated with the
development of bullous pemphigoid including
penicillamine, efalizumab, etanercept, and
furosemide (Batsuji-Garin et al., 2011; Culton et al.,
2012; Bernand & Antonicelli, 2017).
Since the patient stated that he routinely cycles
every morning until 10am and never wears a hat or
applies sun protection, we suggest that one possible
trigger might be UV light. In addition, various
autoimmune disorders, psoriasis, and neurologic
disorders have also been described in association
with BP (Lipsker & Borradori et al., 2010; Venning
et al., 2012).
The patient’s chief complaint consisted of
blisters, erosion and pain on his body. Upon
examination, tense bullae on erythematous macule
were discovered. These were non-sharply
marginated, contained clear fluid, were not easily
ruptured and were Nikolsky- and Asboe Hansen-
sign negative. Eroded skin was widespread on the
body from the ruptured blister; crust, scales and
xerosis were also discernible on the body. This is
consistent with the literature: clinical criteria BP
typically presents with tense, mostly clear skin
blisters, in conjunction with erythematous or
urticarial plaques that are associated with moderate
to severe pruritus.
4
Although the pruritus was
minimal in this patient. pruritus may be intense in
some patients, but minimal in others. These lesions
are most commonly found on flexural surfaces such
as the lower abdomen and thighs, although they may
occur anywhere (Culton et al., 2012). Predilection
sites in the patient include the limbs and abdomen.
The mucosae of eyes, nose, pharynx, oesophagus
and anogenital areas are rarely affected (Culton et
al., 2012; Venning et al., 2012).
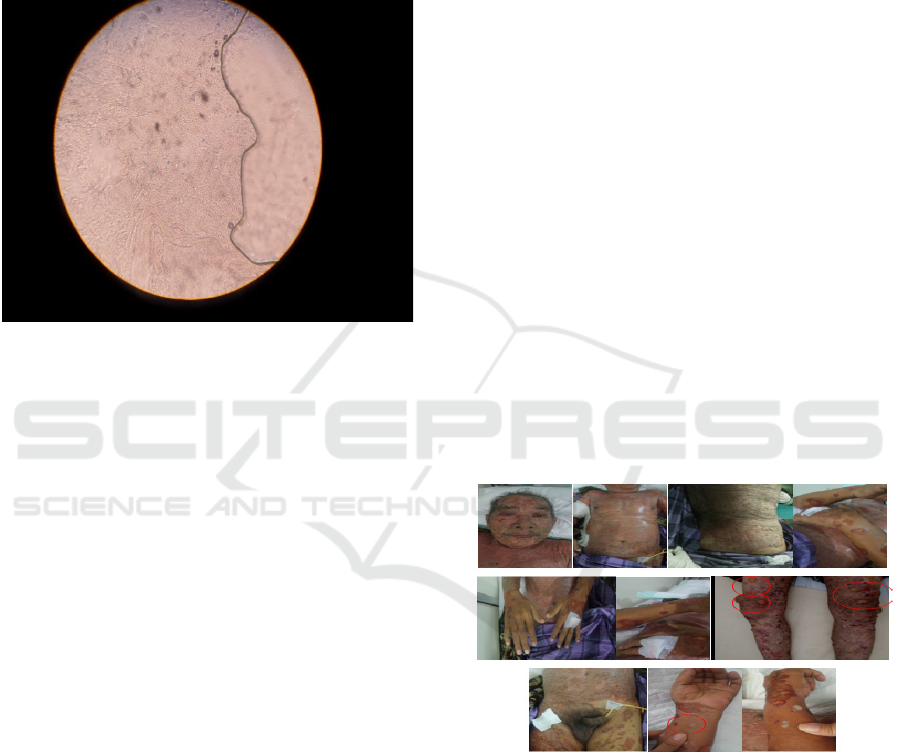
Figure 2: on regio cruris dextra et sinistra: tense blisters on
erythematous macule; these were non-sharply marginated,
contained clear fluid and were not easy to break.
Nikolsky- and Asboe Hansen-signs were negative.
Erosion, scales and crust were found.
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
348
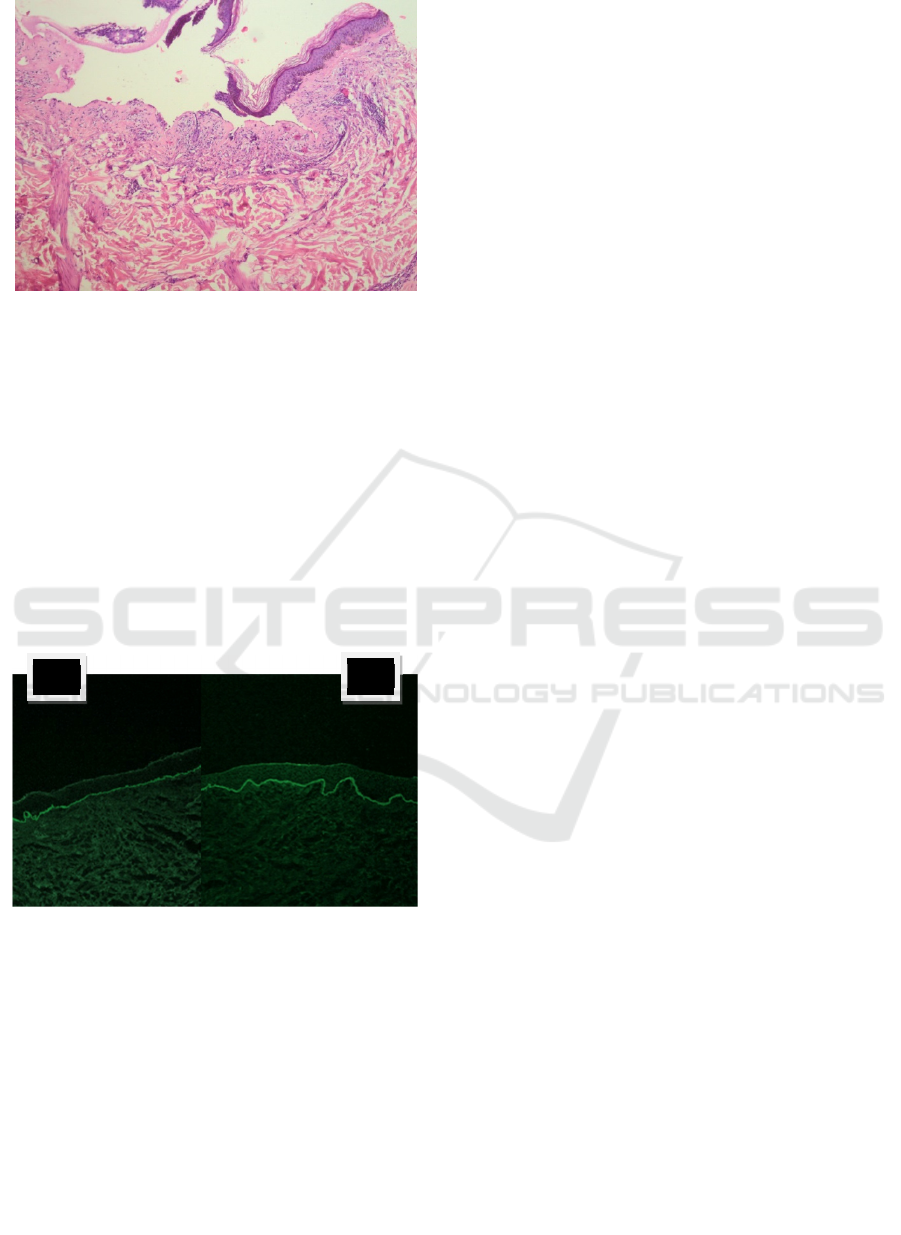
Figure 3. Histopathology examination with hematoeosin
staining, subepidermal blister with with inflammatory
infiltrate numerous eosinophil and several neutrophil,
100x magnification.
The hallmarks of bullous pemphigoid include the
presence of subepidermal blisters, lesional and
perilesional polymorphonuclear cell infiltrates in the
upper dermis and immunoglobulin (Ig) G
autoantibodies and C3 bound to the dermal
epidermal junction. Direct IF of perilesional skin
shows linear IgG (usually IgG1 and IgG4, although
all IgG subclasses and IgE have been reported) and
C3 along the basement membrane (Venning et al.,
2012; Zhao & Murrell, 2015).
Figure 4: Direct imunoflourescence examination. A. C3 B.
IgG
Diagnosis was made from anamnesis, physical
examination, laboratory examination, histopat
hology and direct immunofluorescence. The
diagnosis in this patient was bullous pemphigoid,
hypoalbuminemia and diabetes mellitus (DM) type
2.
Treatment was immediately started:
methylprednisolone 4mg in oral dosage 3 times 3
tablets daily (12mg-12mg-12mg) tapering off
depending on the progression of the lesion to find
maintenance dosage; dapsone 100mg once daily;
wound dressing using NaCl 0.9% on erosion lesion;
insulin injection as advised from internal medicine
department; insulin novorapid injection 3x6iu;
insulin levemir 1x8iu morning dosage only; backup
insulin 2iu with every 12mg methylprednisolone;
Vitamin D 300iu; calcium carbonat 600mg; diet B1
2100kkal/day; and high protein diet for the
hypoalbuminemia.
4 CONCLUSION
The diagnosis of bullous pemphigoid is made based
upon clinical, histologic, and immunofluorescence
(IF) features (Schmidt & Groves, 2016). The initial
evaluation of patients should encompass a complete
physical examination and, wherever possible, the
assessment of the initial damage (Bernard &
Antonicelli, 2017).
For decades, systemic corticosteroids have been
used and considered as the gold standard for the
treatment of this disease, especially for generalized
BP (Schmidt et al., 2016). Immunosuppressive
therapy with corticosteroid-sparing effects should be
considered a second-line therapy when
corticosteroids alone fail to control the disease, or in
cases of contraindications to oral corticosteroids and
comorbidities (such as diabetes, severe osteoporosis
and cardiovascular disorders) (Bower, 2010). Unless
glucose-6-phosphate dehydrogenase deficiency is
evident, the use of dapsone (up to 1.5 mg/kg/day
orally) may also be warranted, generally in
association with topical or systemic corticosteroids,
especially in the presence of mucosal involvement.
In elderly patients, the complications of systemic
glucocorticoid therapy (such as osteoporosis,
diabetes, and immunosuppression) may be
especially severe (Bouscarat et al, 2006; Bagci et
al.,2017). Therefore, it is important to try to
minimize the total dose and duration of therapy with
oral glucocorticoids (Culton et al., 2012; Bernand &
Antonicelli, 2017).
REFERENCES
Bağcı, I.S., Horváth, O.N., Ruzicka, T., Sárdy, M., 2017.
Bullous pemphigoid. Autoimmunity Reviews.
doi:10.1016/j.autrev.2017.03.010
Bastuji-Garin, S., Joly, P., Lemordant, P., Sparsa, A.,
Bedane, C., Delaporte, E., Roujeau, J.C., Bernard, P.,
Guillaume, J.C., Ingen-Housz-Oro, S., Maillard, H.,
Pauwels, C., Picard-Dahan, C., Dutronc, Y., Richard,
M.A., French Study Grp Bullous, D., 2011. Risk
A
B
Bullous Pemphigoid Established by Direct Immunofluorescence: Case Report
349

Factors for Bullous Pemphigoid in the Elderly: A
Prospective Case-Control Study. Journal of
Investigative Dermatology 131, 637–643.
doi:10.1038/jid.2010.301
Bernard, P., Antonicelli, F., 2017. Bullous Pemphigoid: A
Review of its Diagnosis, Associations and Treatment.
American Journal of Clinical Dermatology.
doi:10.1007/s40257-017-0264-2
Bouscarat, F., Chosidow, O., Picard-Dahan, C., Sakiz, V.,
Crickx, B., Prost, C., Roujeau, J.C., Revuz, J., Belaich,
S., 1996. Treatment of bullous pemphigoid with
dapsone: Retrospective study of thirty-six cases.
Journal of the American Academy of Dermatology 34,
683–684. doi:10.1016/S0190-9622(96)80085-5
Bower, C. 2010. Bullous pemphigoid: guide to diagnosis
and treatment. Prescriber. 17(12):44-50.
Culton, D.A., Diaz, L.A., Liu, Z. 2012. Bullous
pemphigoid. In: Goldsmith L. Fitzpatrick T(ed).
Fitzpatrick's dermatology in general medicine. 1st ed.
New York: McGraw-Hill Medical.
doi:10.1017/CBO9781107415324.004 P 608-16
Di Zenzo, G., della Torre, R., Zambruno, G., Borradori,
L., 2012. Bullous pemphigoid: From the clinic to the
bench. Clinics in Dermatology 30, 3–16.
doi:10.1016/j.clindermatol.2011.03.005
Feliciani, C., Joly, P., Jonkman, M.F., Zambruno, G.,
Zillikens, D., Ioannides, D., Kowalewski, C.,
Jedlickova, H., Kárpáti, S., Marinovic, B., Mimouni,
D., Uzun, S., Yayli, S., Hertl, M., Borradori, L., 2015.
Management of bullous pemphigoid: The European
Dermatology Forum consensus in collaboration with
the European Academy of Dermatology and
Venereology. British Journal of Dermatology 172, 867–
877. doi:10.1111/bjd.13717
Lipsker, D., Borradori, L. 2010. ‘Bullous’ Pemphigoid:
What Are You? Urgent Need of Definitions and
Diagnostic Criteria. Dermatology. 221(2):131-134.
Schmidt, E., della Torre, R., Borradori, L., 2012. Clinical
Features and Practical Diagnosis of Bullous
Pemphigoid. Immunology and Allergy Clinics of North
America. doi:10.1016/j.iac.2012.04.002
Schmidt, E., Groves, R. Bullous pemphigoid. In: Griffith,
C., Barker, J., Bleiker, T., Chlamers, R., Creamer D.
2016. Rook’s Textbook of Dermatology.9
th
ed.Chichester, West Sussex: John Wiley & Sons Inc.
50.11-20
Schmidt, E., Zillikens, D., 2013. Pemphigoid diseases. The
Lancet 381, 320–332. doi:10.1016/S0140-
6736(12)61140-4
Venning, V.A., Taghipour, K., Mohd Mustapa, M.F.,
Highet, A.S., Kirtschig, G., 2012. British Association
of Dermatologists’ guidelines for the management of
bullous pemphigoid 2012. British Journal of
Dermatology. doi:10.1111/bjd.12072
Wojnarowska, F., Kirtschig, G., Highet, a S., Venning, V.
a, Khumalo, N.P., 2002. Guidelines for the
management of bullous pemphigoid. The British
journal of dermatology 147, 214–21.
Zhao, C.Y., Murrell, D.F., 2015. Advances in
understanding and managing bullous pemphigoid.
F1000Research. doi:10.12688/f1000research.6896.1
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
350
