
Serological Antibody Profile IgM and IgG of Mycobacterium leprae
PGL-1 and L-ESAT-6 in Patients and Household Contact from
Leprosy Endemic Area in East Java Indonesia
A. Dinar Adriaty
1
, M. Irfan Hadi
1,3
, Irmadita Citrashanty
2
, Iswahyudi
1
, Indropo Agusni
1,2
, Shinzo
Izumi
1
, Cita Rosita S. P.
1
1
Leprosy Study Group-Institute of Tropical Disease, Universitas Airlangga
Kampus C, Mulyorejo Surabaya 60115 East Java Indonesia
2
Dermatology and Venereology, Faculty of Medicine Universitas Airlangga, Surabaya 60286 Indonesia
3
Faculty of Psychology and Health Science, Islamic State University of Sunan Ampel, Surabaya 60237 Indonesia
Keywords: L-ESAT-6, PGL-1, Mycobacterium leprae, leprosy, patients, household contact
Abstract: Leprosy patients in Indonesia is the third largest in the world. The problem of high transmission is the
difficulty of early detection of leprosy. At present, diagnosis leprosy still based on clinical sign. Some of
supporting diagnostic tools are developed, such as serological test. The most widely used antigen for
diagnostics is Phenolic-glycolipid-1(PGL-1), but some limitations of the antigen, provide a challenge to find
a potential candidate antigen representing specific Mycobacterium leprae. Purpose of this research is for
studying Mycobacterium leprae L-ESAT-6 (epitope AA11-36) compare to PGL-1 to leprosy patients and
household contacts in leprosy endemic region. Analysis have been conducted to MB and PB leprosy patients,
as well as their families with total 173 respondents by testing Indirect ELISA of the L-ESAT-6 and PGL-1. In
general, the profile of ELISA test anti PGL-1 vs L-ESAT-6 in all the patients don’t have significant difference,
but in household contacts, with the Pearson correlation test, it can be concluded that there are significant
difference between PGL-1 and L-ESAT-6 (p-value = 0.049; p <α = 0.05). Healthy individuals who are exposed
to M.leprae found high titers of antibody anti L-ESAT-6 and lower profile of antibody anti PGL-1. It is
assumed that the individual is relatively immune to M.leprae. So it can be concluded that L-ESAT-6 (AA11-
36) can be used as candidate diagnostic test which is a predictor marker for people living in endemic leprosy
areas, for it still need a further research.
1 INTRODUCTION
Leprosy in Indonesia still become a health problem
with the discovery of new cases is still increasing
from year to year (WHO, 2016). The most important
cause of the high incidence rate is the difficulty of
early detection which play an important role in the
transmission process so that subclinical leprosy stage
continues to be manifest (Agusni, 2001). Diagnostic
tools to detect leprosy is still limited, based on clinical
sign. Laboratory testing based on a serological by the
method of ELISA (Eenzyme Linked Immunosorbent
Assay) and MLPA method (Mycobacterium leprae
particle agglutination) is used mostly using PGL-1
(Phenolic-glycolipid-1), but the lack of this antigen,
provide a challenge for finding a potential biomarker
used for early detection of leprosy (Spencer et al,
2012). Inspired from the successful use of
Mycobacterium tuberculosis ESAT-6 (T-ESAT-6)
for the detection of M. tuberculosis associated
specific responses (Parkash et al, 2007), we have
previously found that the use of ESAT-6 of M. leprae
was limited. Mycobacterium leprae ESAT-6 (L-
ESAT-6) is a protein secreted by the extracellular
M.leprae molecular weight 6kDa and an antigen
peptide representing specific epitopes of M. leprae
potent. L-ESAT-6 encoded by the genes of
pathogenic RD1 consists of 95 amino acids that has
multiple epitopes of which are located on the N-
Terminus (Geluk et al, 2002). In previous studies
conducted by Kurdi (Kundi et al, 2010) on epitope
tracking with B cell epitope scanning techniques, has
proven that serum leprosy patients type MB, PB and
subclinical leprosy reactive against L-ESAT-6 (AA
Adriaty, A., Hadi, M., Citrashanty, I., , I., Agusni, I., Izumi, S. and S. P., C.
Serological Antibody Profile IgM and IgG of Mycobacterium leprae PGL-1 and L-ESAT-6 in Patients and Household Contact from Leprosy Endemic Area in East Java Indonesia.
DOI: 10.5220/0008156703230327
In Proceedings of the 23rd Regional Conference of Dermatology (RCD 2018), pages 323-327
ISBN: 978-989-758-494-7
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
323

11-36), while in healthy people in endemic areas no
such reactivity. Those studies also have found three
types of epitopes of the N-Terminus region of the L-
ESAT -6 as follows: epitope markers leprosy of type
LL and BB leprosy (MB) is LEQCQES (28-34),
epitope VNELQG (14-19) which is an epitope
markers of type TT leprosy (PB), epitope IDALLE
(24 -29) are only reactive with antibodies contained
in the sera of healthy household contact (HHC) group.
What is interesting in this case, that the three epitopes
found is not in the primary structure of the T-ESAT-
6 (Mycobacterium tuberculosis) so this epitopes are
highly specific to M.leprae (Spencer et al, 2002).
M.leprae is uncultivable mycobacteria, production of
synthetic proteins was conducted on L-ESAT-6
(AA11-36). The purpose of this study was to analyzed
the profile of specific antibodies reaction of L-ESAT-
6 (AA11-36) compare to PGL-1 against leprosy
patients and household contact. The development of
tools is important to facilitate diagnosis and provide
a more thorough understanding of transmission and
the incidence of M.leprae infection in high endemic
regions, even throughout the country.
2 METHODS
This study was approved by national ministry of
health and local ethic commission from Dr.Sutomo
Hospital Surabaya and paticipants were included only
after signing written informed consent forms. Patients
groups has the following inclusion criteria: newly
diagnosed and previously untreated or recently
diagnosed and within the first 3 months treatment
with WHO-MDT. Household contacts of both MB
and PB leprosy patients were recruited as a group at
elevated risk of subclinical leprosy. Samples
consisted of 3 groups, leprosy patients Multibacillary
(MB) type, Paucibacillary (PB) type and household
contact (HHC), each of the groups were 42, 36 and 95
respondents respectively and they were taken from a
district in Lamongan, one of the endemic areas in East
Java Indonesia. The number of samples is calculated
based on the formula stratified random sampling.
2.1 ELISA (Enzyme Linked
Immunosorbent Assay
A total of 3 mL of blood serum isolated to then
proceed with the analysis of indirect ELISA. The
antigen is a synthetic antigen : PGL-1 (NT-P-BSA)
and synthetic L-ESAT-6 (epitop AA 11-36) include 3
epitope markers for MB, PB and HHC, consists of
LEQCQES (28-34), VNELQG (14-19), IDALLE
(24-29)-N-terminus labeled with Biotin. Antigen
diluted 1 mg / ml with carbonate buffer, all
components coated into 96-wells microtiter plates
(Nunc, Maxisorp) for L-ESAT-6, microplate coated
with streptavidin. Blocking buffer consisting of 1%
skimmed milk / PBS and serum total diluted 1/300 in
0.1% skimmed milk / PBS / Tween-20. Samples were
analyzed in duplicate and incubated for 1 hour at
room temperature. The wells were washed with PBS-
Tween20, and then incubated with horseradish
peroxidase (- HRP-) conjugated antibodies (Dako,
Denmark) and then diluted to 0.1% skimmed
milk/PBS/Tween-20.
After washing, the plates stained with substrate
ortho-phenilen-diamine (OPD) and peroxidase 30%
(MERCK) in phosphate-citrate buffer and incubated
until developed a yellow color and stopped with
1.25M H
2
SO
4
. Both antibody IgM anti-NT-P-BSA
and antibody IgG anti-L-ESAT-6 were measured.
The antibody titer was measured by optical density
(OD) of all the wells that have been read at a
wavelength of 450 nm and a reference wavelength at
492nm. Real OD obtained from reduction of OD in
both wavelengths (delta-OD) and converted
automatically by the BIOLISE software to unit/mL.
2.2 Evaluation of ELISA Test Results
Interpretation of the ELISA test result is to see the
yellow signal above background values, it is
recommended to use the plot algorithm. The results
of diagnostic tests in the form of quantitative data and
for the statistical analysis used Pearson correlation
test by SPSS (Statistical Package for the Social
Sciences version 16.0).
3 RESULTS
3.1 Distribution of Samples
There were totally 173 inhabitants who participated
in this study, consisting of 69 respondents (39.88%)
were male and 104 (60.12%) were female. In terms of
age, respondents are divided into 3 groups: children
(0-21 years), adults (22-45 years) and elderly (more
than 45 years) and the adults group is the most
frequent 56.7% (98/173).
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
324
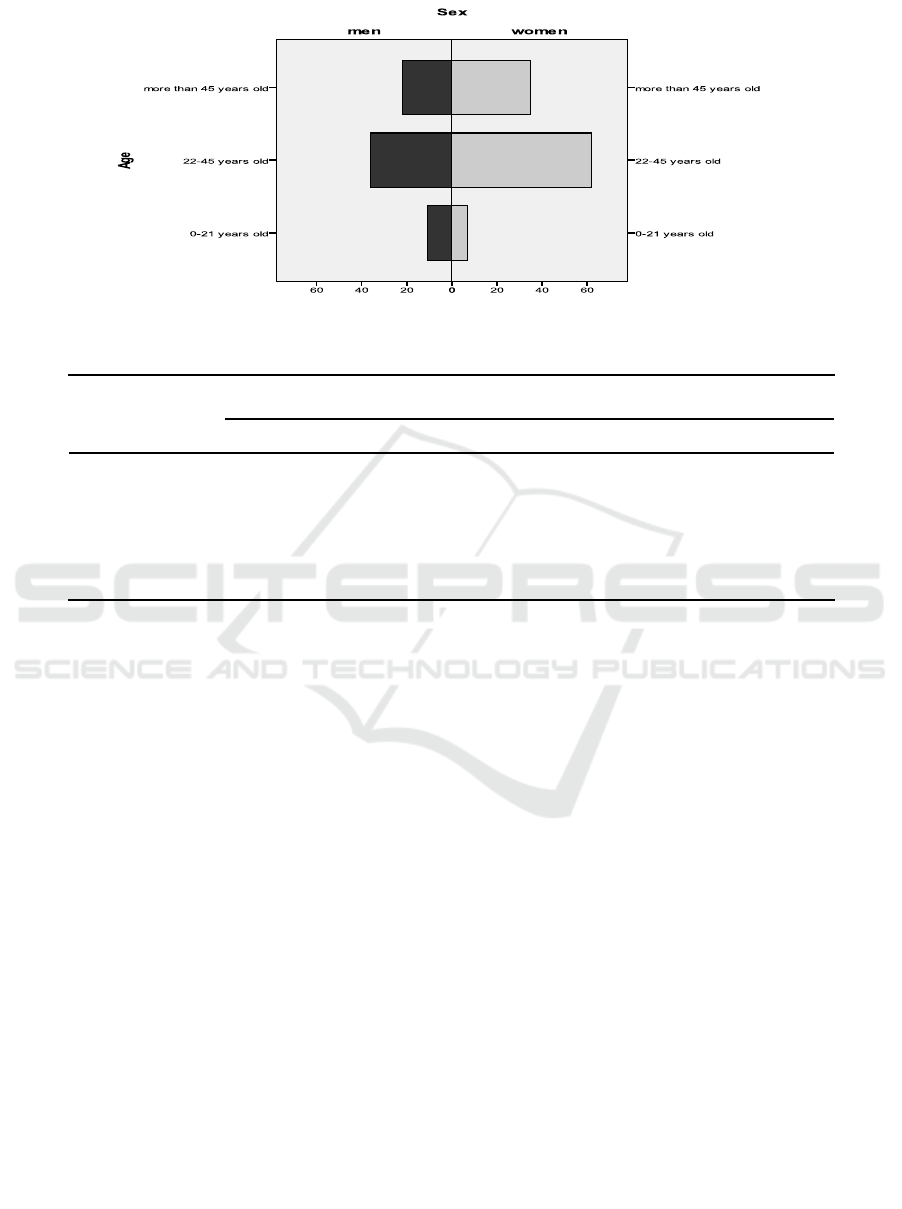
Table 1. Distribution of the respondents
Table 2. Antibody reaction of IgM anti PGL-1and IgG anti L-ESAT-6
AntibodyTiter
(unit/mL)
MB patients
PB patients
Household Contacts
(HHC)
Mean ± SD (range) Mean ± SD (range) Mean ± SD (range)
I
g
M anti PGL-1
32136.07±184706.60 (43-
126870
)
1508.36±1955.23 (219-
8240
)
774.93±752.79 (31-
4332
)
IgG anti L-
ESAT-6
990.28±1232.37
(
48-6300
)
575.5±349.38 (234-
1650
)
404.21±268.43 (108-
1512
)
3.2 Antibody-specific Responses in
PGL-1 and L-ESAT-6
Pearson correlation test, shows the levels of IgM anti
PGL-1 with IgG anti L-ESAT-6 epitope LEQCQES,
epitope marker for MB patients showed the value of
p = 0.598, p > 0.05. It concluded that there was no
correlation between them. Pearson correlation test
shows the levels of IgM anti PGL-1 with IgG anti L-
ESAT-6-epitope VNELQG, specific epitope for PB
patients is obtained p = 0.962, p> 0.05. It concluded
that there was no correlation between the levels of
IgM anti PGL-1.
The levels of IgM anti PGL-1 and IgG ESAT-6
epitope IDALLE, specific epitope for healthy
household contacts showed the value of p = 0.049
(p<0.05). It can be concluded that there is a
significant correlation between the levels of IgM anti
PGL-1 with IgG anti L-ESAT-6- epitope IDALLE.
The strength of the correlation is 0.584 it can be
seen from the Fig.2 that there is a pattern of negative
correlation between the levels of IgM anti PGL-1
with IgG antiL-ESAT-6-IDALLE.
4 DISCUSSION
The most extensively evaluated serologic test for
leprosy is still based on the detection of IgM anti-
PGL-1, because highly titer of anti-PGL-1 serology
reflects the bacillary load and has limited application
for diagnosis of all clinical forms of leprosy (8,9,10),
but according to the profile of IgM anti-PGL-1
compare to IgG ESAT-6, it seems this antigen still
recomended as an important adjunct tool for the
differentiation of PB and MB leprosy. For IgG anti L-
ESAT-6 profile, similar to anti-PGL-1 serology, the
presence of IgG antibodies that react to protein
antigens reflect to the bacillary load as well. Most MB
patients have high IgG titres but few PB patients are
responsive. The serological profile antibody anti- L-
ESAT-6 with specific epitope of MB and
PB patients
has not been able to support the lack of PGL-1.
Interesting result shows at the comparison
between IgM anti-PGL-1 and IgG anti-L-ESAT-6
with epitope IDALLE specific for healthy household
contacts. It has a negative significant correlation with
PGL-1 so that it can be said that healthy individuals
exposed to M.leprae if antibodies are found with high
Serological Antibody Profile IgM and IgG of Mycobacterium leprae PGL-1 and L-ESAT-6 in Patients and Household Contact from Leprosy
Endemic Area in East Java Indonesia
325
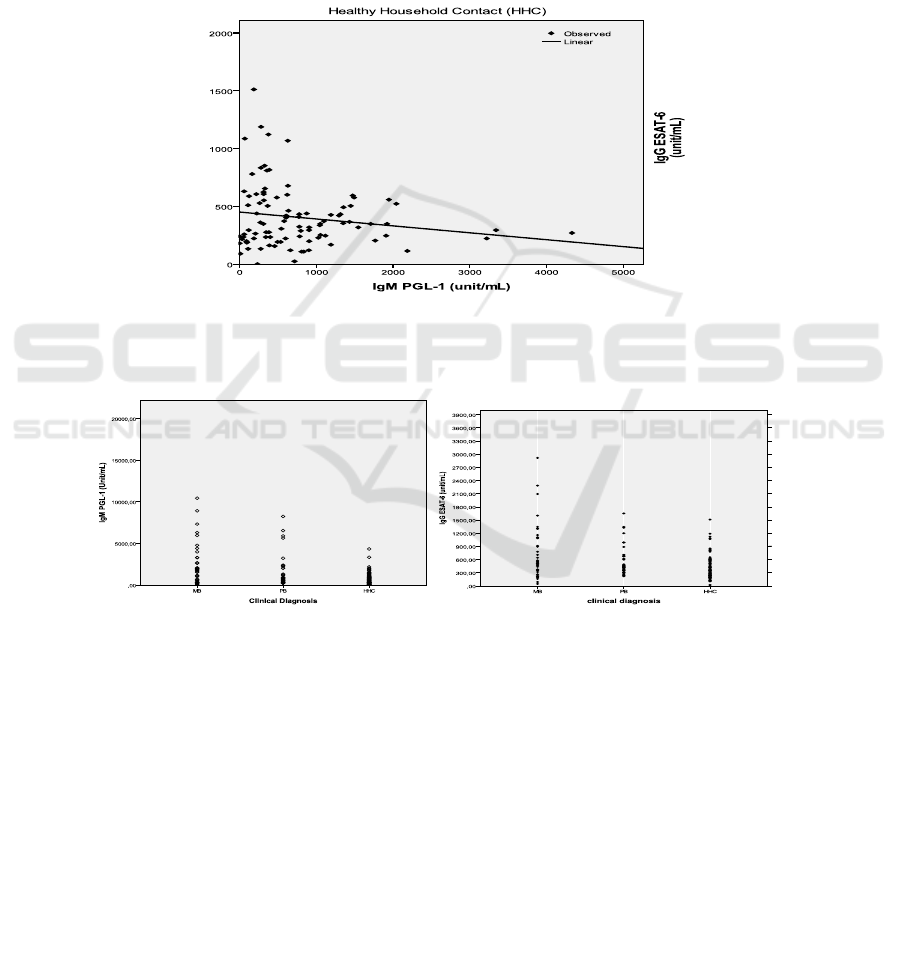
titers of L-ESAT-6 epitope IDALLE and could
suppressed the development of bacterial load in the
body which indicated from the titer of IgM anti-PGL-
1, then it is certain that the individual is relatively
immune against M.leprae. This correlation means
that household contacts with the greater risk of
becoming leprosy has the greater protective antibody
against leprosy.
5 CONCLUSION
In the future it might said that antigen L-ESAT-6 can
be a candidate for predictor marker in order to
develop an alternative tools for detecting leprosy in
the early stage. For these purpose still needed further
research. The result of IgG anti-L-ESAT-6 in leprosy
patients might have a different profile if possible
tested in L-ESAT-6 epitope IDALLE that specific for
healthy household contact. This can be also as a
suggestion for further research.
Figure 1. Antibody responses of leprosy patients and household contacts. Multibacillary (MB; n=42), Paucibacillary (PB;
n=36), healthy household contacts of confirmed patients (HHC; n=95) were assesed. Results from each individual serum
sample against the mean titer in units/mL both from antibodies anti PGL-1 (IgM) (A) and anti ESAT-6 (IgG) (B)
Figure 2. Antibody responses of IgM anti-PGL-1 and IgG anti- Mycobacterium leprae protein antigen ESAT-6 epitope
IDALLE from household contacts (HHC)
ACKNOWLEDGEMENT
The authors are extremely grateful to all the
volunteers for the cooperation and to all the field and
laboratory staff who made this work possible for their
assistance. Also for the leprosy patients in Puskesmas
Brondong, Lamongan, East Java Province and all
their family who willingly supported in this research.
This work was funded by a grant RISBIN
IPTEKDOK from National Ministry of Health.
REFERENCES
Agusni I, 2001. Kusta Stadium Subklinis dan
kedudukannya dalam epidemiologi penyakit kusta.
Majalah Kedokteran Indonesia 51(1): 22-25.
Buhrer-Sekula, S., Visschedijk, J., Grossi, MA., Dhakal,
KP., Namadi, AU., Klatser, PR., Oskam, L., 2007. The
ML flow test as a point of care test for leprosy control
programmes : potential effects on classification of
leprosy patients. Leprosy Review 78:70-79.
Geluk, A., van Meijgaarden, K. E., Franken, K. L. M. C.,
Subronto, Y. W., Wieles, B., Arend, S. M., …
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
326

Ottenhoff, T. H. M. (2002). Identification and
Characterization of the ESAT-6 Homologue
of Mycobacterium leprae and T-Cell Cross-Reactivity
with Mycobacterium tuberculosis . Infection and
Immunity, 70(5), 2544–2548.
http://doi.org/10.1128/IAI.70.5.2544-2548.2002
Geluk, A., van der Ploeg-van Schip, J. J., van Meijgaarden,
K. E., Commandeur, S., Drijfhout, J. W., Benckhuijsen,
W. E., … Ottenhoff, T. H. M. (2010). Enhancing
Sensitivity of Detection of Immune Responses
to Mycobacterium leprae Peptides in Whole-Blood
Assays . Clinical and Vaccine Immunology :
CVI, 17(6), 993–1004.
http://doi.org/10.1128/CVI.00046-10
Geluk A., van der Ploeg J., Teles, R., Franken, KL., Prins,
C., Drijfhout, JW., Sarno, EN., Sampaio, EP.,
Ottenhoff, TH., 2008. Rational combination of peptides
derived from different Mycobacterium leprae proteins
improves sensitivity for immunodiagnosis of M.leprae
infection. Clinical Vaccine Immunology 15:522-533
Kurdi, FN., Handojo, I., Agoesni, I., and Dahlan, YP.,
2010. Amino acid sequence of B-cell epitope of N-
terminal region of ESAT-6 Mycobacterium leprae role
as specific antigen for diagnosis of leprosy. Southeast
Asian Journal of Tropical Medicine and Public Health.
41(5):1158-63
Parkash, O., Pandey, R., Kumar, A., 2007. Performance of
recombinant ESAT-6 antigen (ML0049) for detection
of leprosy patients. Letter of Applied Microbiology
44(5): 524-530.
Spencer, JS., Duthie, MS., Geluk, A., Balagon, MF., Kim,
HJ., Wheat, WH., Chatterjee, D., Jackson, M., Li,
W., Kurihara, JN., Maghanoy, A., Mallari,
I., Saunderson, P., Brennan, PJ., Dockrell, HM., 2012.
Identification of serological biomarkers of Infection,
disease progression and treatment efficacy for leprosy.
Memorias Do Instuto Oswaldo Cruz 107:79-89.
Spencer, JS., Marques, MA., Lima, MCBS., Kipnis, APJ.,
Gregory, BC., Truman, RW., Brennan, PJ., 2002.
Antigenic Specificity of the Mycobacterium leprae
homologue of ESAT-6. Infection and Immunology
70(2): 1010-1013.
WHO, 2016. Weekly Epidemiological Record. No 35,
2016, 91, 405–420. http://www.who.int/wer.
Serological Antibody Profile IgM and IgG of Mycobacterium leprae PGL-1 and L-ESAT-6 in Patients and Household Contact from Leprosy
Endemic Area in East Java Indonesia
327
