
Establishing the Diagnosis Histopathology Findings of Papular
Pruritic Eruption in HIV-infected Patients: Preliminary Study
Hajar Imtihani
1
*, Mochammad Rifky Luthfiandi
1
, Qamariah
1
, Hanggoro Tri Rinonce
2
,
Nurwestu Rusetiyanti
1
, Devi Artami Susetiati
1
, Satiti Retno Pudjiati
1
and Sunardi Radiono
1
1
Departement of Dermatology and Venereology, Universitas Gadjah Mada/Sardjito General Hospital, Yogyakarta-
Indonesia
2
Department of Anatomical Pathology, Universitas Gadjah Mada/Sardjito General Hospital, Yogyakarta-Indonesia
Keywords: Papular pruritic eruption, PPE, HIV, histopathology
Abstract: Papular Pruritic Eruption (PPE) is a common clinical manifestation in patients infected with Human
Immunodeficiency Virus (HIV). The hallmark of PPE is a chronic pruritus with a symmetrically scattered
papular eruption on the body and extremities. The pathophysiology of PPE is still unknown. Clinically, PPE
is difficult to distinguish between prurigo nodularis, insect bite, or other dermatitis, thus requiring
histopathologic examination to exclude other diagnosis. Nevertheless, the consensus on the histopathology
picture of PPE is still limited. So the of this study was to found the histopathology spectrum of PPE in HIV-
infected patients. This is a descriptive analitycal study to characterize the histopathology pattern in some PPE
cases.
1 INTRODUCTION
The distinctive mark of Human Immunodeficiency
Virus (HIV) infection is progressive
immunosuppression leading to a diverse spectrum of
clinical manifestations such as opportunistic
infections and tumors, wasting syndrome, and failure
of various central nervous systems (Sued et al., 2016)
Skin disorders that are often associated with HIV
infection include seborrhoeic dermatitis, molluscum
contagiosum, tinea corporis, and Kaposi sarcoma, as
well as pruritus and dermatitis with unclear causes,
such as papular pruritic eruptions (PPE) (Chua et al.,
2014). The prevalence of PPE varies in different parts
of the world, ranging from 11% to 46%.(Chua et al.,
2014; Kaushik et al., 2014) A study in Surabaya
showed a 15.4% prevalence of PPE from all patients
with HIV (Arista et al., 2015).
Clinical features of PPE are very similar to
eosinophilic folliculitis in HIV patients, usually
occurs in HIV patients with low CD4 counts, and may
also be part of the Immune Reconstitution
Inflammatory Syndrome (IRIS) (Mostwaledi et al.,
2014). Also, the possibility of drug reaction should be
considered in any case of inflammatory dermatosis in
HIV infection. Therefore, skin biopsy has an
important diagnostic role when some form of this
distinct skin disorder arises as a similar clinical
manifestation, but the normative histopathology
criteria of PPE does not exist yet. This objective of
this study was to characterize the PPE
histopathological findings.
2 METHODS
This research is a descriptive analytical study that
conducted in the sexually transmitted disease clinic,
Department of Dermatology and Venereology in
Sardjito General Hospital, Yogyakarta, during
October to December 2017. The study subjects were
HIV-infected adult patients, who had pruritic,
symmetrically scattered multiple papule lesions with
a duration of at least 1 month. Skin biopsy was
performed on the latest skin lesion with a punch
technique, 5mm in size. The tissue is then delivered
and processed in Anatomical Pathology laboratory of
Sardjito General Hospital. The tissue preparation is
cut and stained with standard hematoxylin and eosin.
Interpretation is focused on the pattern of skin
reaction, exocytosis in the epidermis, and dermis
infiltrate, such as type, density, and location of the
Imtihani, H., Luthfiandi, M., Qmariah, ., Rinonce, H., Rusetiyanti, N., Susetiati, D., Pudjiati, S. and Radiono, S.
Establishing the Diagnosis Histopathology Findings of Papular Pruritic Eruption in HIV-infected Patients: Preliminary Study.
DOI: 10.5220/0008153501810185
In Proceedings of the 23rd Regional Conference of Dermatology (RCD 2018), pages 181-185
ISBN: 978-989-758-494-7
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
181

infiltrate. Inflammatory cell counts were performed
using a direct observation under microscopy in high
power field in 5 field of view, with a single observer
of anatomical pathologist.
3 RESULT
There were 8 patients included, consisting of 2 female
and 6 male (Table 1). The mean age of patient is 38.87
years old. Four patients received a combination of
drugs in the form of Tenovofir, Hiviral, and Efavirenz
(THE), while the rest received Duviral and Neviral.
Duration of the patient's complaint varies from 3
months to 2 years. Two patients complained of
itching after dropping out of the first drug regimen,
while the rest complained of itching before starting
ARV. The mean CD4 count in peripheral blood are
138,25 cells/mm
(Arista et al., 2015).
As shown in table 2, orthokeratosis basket weave type
is the most common feature of epidermis, with normal
stratum granulosum and stratum spinosum, however
one specimen showed mild spongiosis and one
specimen showed severe spongiosis with
intraepidermal cleft.
Table 1. Overview of the patients
Case
no.
Sex Age Blood CD4
count
1 Male 30 y.o 79 cells/mm
3
2 Female 48 y.o 300 cells/mm
3
3 Male 21 y.o 144 cells/mm
3
4 Male 30 y.o 245 cells/mm
3
5 Male 48 y.o 23 cells/mm
3
6 Male 50 y.o 247 cells/mm
3
7 Female 44 y.o 17 cells/mm
3
8 Male 32 y.o 51 cells/mm
3
Table 2. Histopathological findings in 8 cases of PPE
Epidermis
Orthokeratosis basket weave type 87,5%
Normal epidermis 12,5%
Acanthosis 12,5%
Eugranulosis 100%
Mild spongiosis 12,5%
Severe spongiosis with intraepidermal cleft 12,5%
Vacuolar degeneration of basal cell 62,5%
Hyperpigmentation 25%
Exocytosis 12,5%
Dermal-epidermal
interface
Clear 100%
Dermis
Type of infiltrate Lymphocyte 100%
Eosinophil 75%
Density Sparse 37,5%
Moderate 62,5%
Distribution Perivascular 100%
Periadnexal 50%
Wedge-shape 0%
Eosinophils count/ 0 12,5%
5 HPF 1-20 50%
21-50 25%
>50 12,5%
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
182
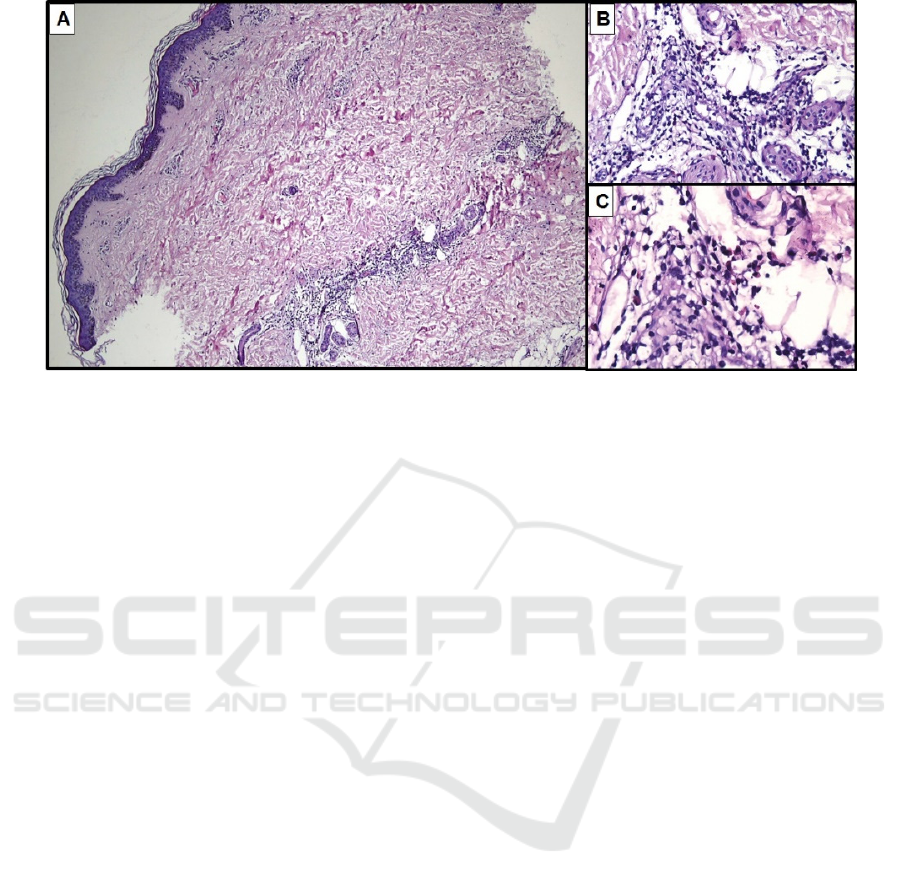
Figure 1. A. Epidermis showing orthokeratosis basket weave and normal layer of granulosum and spinosum. There is
perivascular and periadnexal distribution of inflammatory infiltrate in the dermis. B. Lymphocytes predominance of infiltrate
in periadnexal. C. Eosinophils are moderately scattered.
There is lymphocytic and eosinophilic infiltrate
inside this cleft, showing a feature of spongiotic
pustule. This specimen also have an exocytosis of
eosinophil. There was only one specimen showing
acanthosis of epidermis. All specimens showed clear
dermal-epidermal interface.
In the dermis, all specimens show lymphocyte
infiltrate predominance. They are scattered in the
dermis, and four of them showed sparse infiltrate in
perivascular distribution only, while the rest of them
have both periadnexal and perivascular infiltration
with moderate density. Other infiltrate found is
eosinophils in six cases (75%). In contrast to previous
studies, no single specimen with wedge shaped
infiltrate was found in this study.(7)
Eosinophils are inflammatory cells thought to
play an important role in the mechanism of PPE
before, so we calculated the amount of eosinophils in
high power field magnification (HPF) (400x) in 5
fields of view. Based on the number of eosinophils,
the samples are classified into three categories: 0 or
no eosinophil, 1-20, 21-50, and >50, to illustrate the
number of eosinophil scattered. Most of the spesimen
have eosinophil count 1-20 cells in 5 HPF or
moderately dense. The largest number of eosinophils
count is 133 cells/5 HPF.
4 DISCUSSION
Skin is the most frequent organ affected in HIV
infection and the lesion of skin disorders have a
higher clinical severity than the general population.
Some noninfectious dermatoses are important to note
because of their high prevalence, uncommon
manifestation, extensive or resistant clinical lesions.
In addition, atypical histopathologic features often
make it misdiagnose. This study was conducted to
observe the histopathologic characteristics of one of
the most common non-infectious dermatoses, papular
pruritic eruption (PPE) that is defined as a very itchy,
chronic, and symmetrically scattered papule,
especially on the extremities (Chua et al., 2014).
Because of its very itchy complaints, the papules will
become excoriated and susceptible to secondary
infections, or are characterized by prurigo-like
features (Mostwaledi et al., 2014)
The most commonly observed of histopathologic
finding from previous studies were insect bite feature.
A various cutaneus lesions (maculae, urticae, papules,
vesicle, and nodules) are associated with cutaneous
lesion of insect bite, will showed variation in
histological finding. Spongiosis was the predominant
feature charaterizing the epidermal change.
Sometimes there are erosion or ulceration, or scale
crust with neutrophils or eosinophils above the
keratin layer. The keratin layer will show a
orthokeratosis with some case showed mild
acanthosis. Dermis showed an edema in the papillary
dermis and wedge-shaped infiltrate with moderately
dense distribution in perivascular and periadnexal,
consisted mostly by lymphocytes, eosinophil, and
neutrophils, sometimes macrophages, with no plasma
cells. Most infiltration could involve the adnexal
structures such as sweat glands, hair follicle, and
sebaceous glands (Miteva et al., 2009).
Establishing the Diagnosis Histopathology Findings of Papular Pruritic Eruption in HIV-infected Patients: Preliminary Study
183

Prurigo simplex and eosinophilic folliculitis
sometimes shows similar lesion clinically.
Histopathological findings of prurigo simplex include
compact orthokeratosis, irregular acanthosis,
hypergranulosis, and sometimes focal parakeratosis
in the epidermis. Dermis will showed fibrosis in
papillary to reticular dermis, with main infiltrate are
lymphocytes and macrophages, sometimes
eosinophils (Miteva et al., 2009). Histological feature
of eosinophilic folliculitis include eusinophilic
spongiotic pustulosis involving infundibular region
of the hair follicle, and the infiltrate extends to
attached sebaceous glands. Sometimes there is
disruption or destruction of the follicular wall by
inflammatory infiltrate, including follicular necrosis
and folliculocentric necrotizing eosinophilic
vasculitis. Inflammatory infiltrate are moderately
dense, with perivascular and perifollicular
distribution composed of lymphocytes, mast cell,
neutrophils, and prominent eusinophils (Fujiyama et
al., 2013).
In this study, the pattern of epidermal reactions
and the characteristics of the infiltrate found in the
dermis were not too match to the findings of the insect
bite. Although there was 87,5% specimens showed
orthokeratosis of epidermis, only two specimens
showing spongiosis. Specimens with severe
spongiosis with moderate density of lymphocyte and
eosinophils might be resemble of vesicular stage of
insect bites. But we have no perfectly wedge-shaped
infiltrate found in all specimens. None of specimens
in this study also showed an adnexal structure
disruption, although there were infiltration around
adnexal structure. Our results are inconsistent with
previous research that stated the histopathologic
features of PPE are resemble the insect bite, instead it
showed variable feature of perivascular infiltrate of
lymphocyte and eusinophils.(Resneck et al., 2004;
Weedon, 2010) Although clinically the papules of
PPE closely similar, the histopathological feature of
prurigo simplex and eusinophilic folliculitis were not
found in this study.
Etiologic factors that suspected play a role in PPE
pathogenesis are: arthropod bites, hypersensitivity to
insect bites, generalized hypersensitivity to insect
saliva, host immune response to abnormal infection
(e.g scabies, demodecidosis, bacterial infection),
drugs, or direct effect of the virus through skin
immune disregulation. One of the study found that
there was an increase in local antibodies against
mosquito saliva antigens. It is thought that pruritus is
a form of chronic delayed type hypersensitivity
reaction that generalized to antigen based on non-
spesific B cell activation, common reaction that
involving a humoral reaction with Th2 predominant.
This phenomenon is also thought to be related to other
conditions in similar complaints that appear in
malignant diseases (Tokura et al., 2001). The variable
feature seen in this study that not so spesific does
made a estimation that PPE is only a part of HIV
immunosuppresion progression. Therefore, it may
further explain why PPE can improve with ARV
administration only.
As the HIV infection progression, there will be a
reduction of CD4 lymphocytes and increase of CD8
lymphocytes count.( Weedon, 2010; Rosatelli et al.,
2000) It is shown in this study by low mean CD4
count that are below 200 cells/mm
3
.(Samanta et al.,
2009). An increase in CD8 cells may exert functions
similar to the Th0/Th2 response, these cells could be
responsible for the production of cytokines,
explaining the increase of eosinophils in the
infiltrate.(Rosatelli et al., 2000) This normal response
also include an increase number of macrophage and
mast cells, that thought to be responsible to intense
itching complain of PPE patients. Observation of
mast cells count with toluidin blue staining might be
useful in future study, along with
immunohistochemistry staining such as CD4, CD8,
and CD20 to confirm the B cell role in this disease.
5 CONCLUSION
The histopathological findings of PPE in this study
are variable. The most observe feature are
orthokeratosis basket weave type, normal stratum
granulosum and spinosum, without dermal-epidermal
interface reaction, infiltration of inflammatory cells in
perivascular and periadnexa with lymphocyte
predominance and moderately dense eusinophils.
This findings was not spesifically resemble insect
bites feature like previous study stated. However,
owing to small number of patients in this study, it is
difficult to identify the true variability of PPE
histopathological feature. Therefore, studies with
larger numbers of patients and more
immunohistochemistry staining are required to
determine the complete characterization and compile
a histopathologic criteria of PPE.
ACKNOWLEDGEMENT
The research was funded by the Faculty of Medicine,
Universitas Gadjah Mada, Yogyakarta, Indonesia.
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
184

REFERENCES
Arista, A., Murtiastutik, D., 2015. Studi Retrospektif :
Karakteristik Papular Pruritic Eruption ( PPE ) pada
Pasien HIV / AIDS ( Retrospective Study :
Characteristic of Papular Pruritic Eruption in HIV /
AIDS Patients ). Berk. Ilmu Kesehat. Kulit dan
Kelamin 27, 204–210.
Chua, S.., Amerson, E.., Leslie, K.., McCalmont, T..,
Leboit, P.., Martin, J.., Bangsberg, D., Maurer, T..,
2014. Factors associated with pruritic papular eruption
of human immunodeficiency virus infection in the
antiretroviral therapy era. Br. J. Dermatol. 170, 832–
839. https://doi.org/10.1111/bjd.12721.Factors
Eisman, S., 2006. Pruritic Papular Eruption in HIV.
Dermatol. Clin. 24, 449–457.
https://doi.org/10.1016/j.det.2006.06.005
Fujiyama, T., Tokura, Y., 2013. Clinical and
histopathological differential diagnosis of eosinophilic
pustular folliculitis. J. Dermatol. 40, 419–23.
https://doi.org/10.1111/1346-8138.12125
Kaushik, S.B., Miracle, J., Pokhrael, A., Chen, S.C., Chan,
Y.H., Wilkin, A., Yosipovitch, G., 2014. Chronic
pruritus in HIV-positive patients in the southeastern
United States: Its prevalence and effect on quality of
life. J. Am. Dermatology 70, 659–664.
https://doi.org/10.1016/j.jaad.2013.12.015
Miteva, M., Eisner, P., Ziemer, M., 2009. A histopathologic
study of arthropod bite reactions in 20 patients
highlights relevant adnexal involvement. J. Cutan.
Pathol. 8, 26–33. https://doi.org/10.1111/j.1600-
0560.2008.00992.x
Motswaledi, M.H., Visser, W., 2014. The Spectrum of
HIV-Associated Infective and Inflammatory
Dermatoses in Pigmented Skin Aids HIV
Immunosuppression Infections Inflammatory
dermatoses Treatment. Dermatol. Clin. 32, 211–225.
https://doi.org/10.1016/j.det.2013.12.006
Resneck, J.S., Beek, M. Van, Furmanski, L., Oyugi, J.,
Leboit, P.E., Katabira, E., Kambugu, F., Maurer, T.,
Berger, T., Pletcher, M.J., Machtinger, E.L., 2004.
Etiology of Pruritic Papular Eruption With HIV
Infection in Uganda. J. Am. Med. Assoc. 292, 2614–21.
Rosatelli, J.., Soares, F.., Roselino, A.M.., 2000. Pruritic
papular eruption of the acquired immunodeficiency
syndrome: predominance of CD8 þ cells. Int. J.
Dermatol. 3, 872–80.
Samanta, M., Kundu, C., Sarkar, M., Bhattacharyya, S.,
Chatterjee, S., 2009. Papular pruritic eruptions: A
marker of progressive HIV disease in children:
Experience from eastern India. Indian J. Sex. Transm.
Dis. AIDS 30, 79–83. https://doi.org/10.4103/0253-
7184.62762
Sued, O., Figueroa, M.I., Cahn, P., 2016. Clinical
challenges in HIV/AIDS: Hints for advancing
prevention and patient management strategies. Adv.
Drug Deliv. Revies 103, 5–19.
https://doi.org/10.1016/j.addr.2016.04.016
Tokura, Y., Ishihara, S., Tagawa, S., Seo, N., Oshima, K.,
Takigawa, M., 2001. Hypersensitivity to mosquito bites
as the primary clinical manifestation of a juvenile type
of Epstein-Barr virus–associated natural killer cell
leukemia/lymphoma. J. Am. Acad. Dermatology 45,
569–78. https://doi.org/10.1067/mjd.2001.114751
Weedon, D., 2010. Section 6: Infections and Infestations,
in: Weedon’s Skin Pathology. Elsevier, p. 630.
Weigelt, N., Metze, D., St, S., 2010. Prurigo nodularis:
systematic analysis of 58 histological criteria in 136
patients. J. Cutan. Pathol. 37, 578–586.
https://doi.org/10.1111/j.1600-0560.2009.01484.x
Establishing the Diagnosis Histopathology Findings of Papular Pruritic Eruption in HIV-infected Patients: Preliminary Study
185
