
Effect of Tumor Necrosis Factor Alpha (Tnf-A) And Interleukin-10
(II-10) Levels of Aggressive Periodontitis In Rats (Rattus Norvegicus)
Induced by Agrregitibacter Actinomycetemcomitans
Dwi Leni Yuliana
1
, Yoes Prijatna Dachlan
2
and Heny Arwati
3
1
Post graduate school Universitas Airlangga, Surabaya, Indonesia
2
Faculty of Medicine Universitas Airlangga, Surabaya, Indonesia
3
Departement parasitology faculty of medicine Universitas Airlangga, Surabaya, Indonesia
Keywords: Aggregatibacter actinomycetemcomitans, Tumor Necrosis Factor Alpha (TNF-α), Interleukin 10 (IL-10),
Agressive Periodontitis.
Abstract: Aggregatibacter actinomycetemcomitans (A.actinomycetemcomitans) is a gram negative and a major
bacterial agent associated with aggressive periodontitis in young adults. These bacteria are an important
factor in pathogenesis of aggressive periodontitis. A. actinomycetemcomitans possesses fimbriae with an
adhesin protein that was is the first bacterial molecules to make physical contact with host. A complex
network of pro- and anti-inflammatory cytokines act in inflamed periodontal tissues. Among other
cytokines, interleukin-10 (IL-10) is an important multifunctional cytokine. An increase or decrease in IL-10
levels caused by bacterial infection is critical for the individual control of balance between inflammatory,
humoral and microbial challenges. Tumor necrosis factor-α (TNF-α) plays an important role in periodontal
inflammation as it has substantial potential to increase bone resorption and is involved in connective tissue
degradation by stimulating prostaglandin-E2 and colagenase. The purpose of this research was to analyze
TNF-α and IL-10 levels of aggressive periodontitis in rats (Rattus norvegicus) induced by A.
actinomycetemcomitans. This research was a true experimental study with Post Test Only Control Group
Design. Rats were divided into 4 groups for 0,25; 0.5 and 0.75 CFU/mL, and negative control. Each group
contained 5 rats. Aggressive periodontitis in rats was induced by injecting A.actinomycetemcomitans, at 48
hrs and 96 hrs post injection the inflammatory signs were observed, thee days later plasma were then
collected to measure TNF-α dan IL-10 level by ELISA. Analysis of Variance (ANOVA) showed
significantly increase levels of TNF-α in the infected group compared with that of the control group.
Aggressive periodontitis in rats showed by redness, abscess and tooth mobility. This condition indicated that
A.actinomycetemcomitans has the ability to adhere and invade the periodontal tissue further producing a
colony that caused periodontitis. High plasma level of TNF was seen in rats infected with 0.75 CFU/mL OF
ac, while IL-10 was low as seen in rats infected with 0.5 CFU/mL OF Ac.
1 INTRODUCTION
Periodontal disease and dental caries are the most
prevalent infections affecting the human dentition
(Brown et al., 1996). Periodontal disease is a chronic
bacterial infection characterized by persistent
inflammation, connective tissue breakdown and
alveolar bone destruction (Yamamoto et al., 2011).
Periodontitis, which is bacterially induced, can be
defined as a chronic inflammatory disease initiated
by dental plaque biofilm and perpetuated by a
deregulated immune response (Suvan et al., 2011)
usually accompanied by gingivitis resulting in
irreversible destruction of the connective tissues that
support the tooth, including the alveolar bone
(Yamamoto et al., 2011).
The gingiva, periodontium, alveolar bone and
cementum are structures that provide support to the
tooth. Any pathological process affecting
periodontium is defined as periodontitis. For a long
time, it was thought that gingivitis and periodontal
disease appeared as a result of aging of the
periodontal tissues that gave rise to inflammation
and recession of the gingival tissues bone and finally
Yuliana, D., Dachlan, Y. and Arwati, H.
Effect of Tumor Necrosis Factor Alpha (Tnf-A) And Interleukin-10 (II-10) Levels of Aggressive Periodontitis In Rats (Rattus Norvegicus) Induced by Agrregitibacter Actinomycetemcomitans.
DOI: 10.5220/0007542603430350
In Proceedings of the 2nd International Conference Postgraduate School (ICPS 2018), pages 343-350
ISBN: 978-989-758-348-3
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
343

tooth loss. However, several studies have indicated
that this is not just an adult disease, but also appears
frequently in children (Escudero et al., 2008).
Gingiva is part of the mucosa of the oral cavity
that covers the alveolar bone and serves to protect
the underlying tissue. Normal gingiva has a pink
color, a supple consistency and a stippling texture or
orange peel. Periodontal ligaments are the
connective tissues that surround the teeth and bind
them to the bone. Periodontal ligaments serve to
protect blood vessels and nerves, tooth attachment to
bone and hard impact resistance due to occlusal
stress. Alveolar bone is a hard tissue composed of
layers of bone that serves as a support for teeth. The
cementum is the part that envelops the tooth root, is
hard, has no vena and serves as a periodontal
ligament adhesion (Carranza et al., 2006).
Periodontitis comes from interactions between
certain sub-gingival microorganisms, inflammation
and immune responses. Aggressive periodontitis is
predominantly the bacterium
A.actinomycetemcomitans which is the cause of
periodontal disease with progressive damage.
Bacteria A.actinomycetemcomitans release several
virulence factors as endotoxins and leukotoxins, and
infection factors by localized and systemic humoral
immune response (Carranza et al., 2002).
Bacteria A.actinomycetemcomitans have a
number of virulence factors that help the progression
of disease. Virulence determines the strength of the
pathogenic potential and also means the relative
capacity (quantity and quality) of the bacteria that
cause damage to the host and its ability to control
the body's defenses. Such bacterial virulence
includes its capacity in tissue destruction, invasive
bacterial levels, and the ability to avoid host defense
responses (Carranza et al., 2006).
Bacteria present in the plaque, including
lipopolysaccharide (LPS) and lipoteichoic acid,
interact with toll-like receptors in epithelial cells,
macrophages, leucocytes and fibroblasts, stimulating
the production of cytokines such as TNF-α, IL-1β,
IL-6, IL-8 and prostaglandin E2 (PGE2). To
facilitate leukocyte infiltration, fibroblasts
stimulated by TNF-α and IL-1β secrete matrix
metalloproteinase (MMPs), which degrade
extracellular matrix molecules including collagen.
The inflammatory response of periodontal tissue can
lead to tissue destruction and alveolar bone
resorption (Suvan et al., 2011).
Tumor necrosis factor-α (TNF-α) plays an
important role in periodontal inflammation. TNF-α
is primarily produced by activated macrophages.
TNF-α has a strong potential factor to increase bone
resorption and is involved in degradation of
connective tissue by stimulating PGE2 and
collagenase (Moore et al., 1994).
The complex network of pro- and anti-
inflammatory cytokines works on inflammatory
periodontal tissues. Among other cytokines,
interleukin-10 (IL-10) is an important
multifunctional cytokine. Increased or decreased
levels of IL-10 host are essential for balance control
between inflamed individuals (Gray, 2000).
Interleukin-10 is an anti-inflammatory cytokine,
produced by T-helper2 (Th2) cells, macrophages and
B cells, which inhibit the synthesis of pro-
inflammatory cytokines such as TNF-α, interleukin-
1 (IL-1), interleukin-2 (IL-2), interleukin-6 (IL-6),
interleukin-8 (IL-8), and interferon-γ (IFNγ). IL-10
suppresses the production of metalloproteinase,
while increasing the synthesis of metalloproteinase
inhibitors in macrophages. In addition, it stimulates
the production of osteoprotegrin, which
consequently inhibits bone resorption by preventing
the involvement of the Receptor Activator of
Nuclear Factor Kappa-B Ligand (RANK-RANKL).
The IL-10 cytokine can be a protective cytokine in
periodontal disease and regulate pro-inflammatory
cytokines, including those involved in alveolar bone
loss. Individuals who are high IL-10 level producers
are more protected from periodontitis due to the
anti-inflammatory role of IL-10. Therefore, elevated
anti-inflammatory cytokine IL-10, will play a role in
regulating immune response against
periodontopatogenic bacteria (Bage, 2013).
The pathogenesis of periodontitis is initiated by
bacteria that release LPS. LPS then activates
inflammatory cells, resulting in the release of
cytokines and local factors. At the same time, the
bacterial components and inflammatory mediators
react directly to the osteoblast or progenitor,
resulting in a decrease in osteoblast function, and
then the loss of adhesions of periodontal and dental
tissue, including the alveolar bone and connective
tissue. Periodontitis is an inflammation that extends
through the gingiva and causes tissue damage
through tooth attachment. The dominant bacteria in
periodontitis are the gram negative ones that release
the LPS by activating the host mechanism primarily
that causes bone damage in periodontitis. Significant
periodontal damage is clinically known as
aggressive periodontitis (Carranza et al., 2006).
ICPS 2018 - 2nd International Conference Postgraduate School
344

2 BACKGROUND
Aggressive periodontitis generally affects
systemically healthy individuals less than 30 years
of age, though patients may be older. Aggressive
periodontitis is distinguished from chronic
periodontitis by the age of onset, the rapid rate of
destruction, composition of the subgingival
microflora, alteration in the host immune response,
familial aggregation of diseased individuals, and a
strong racial influence (Joshipura, 2015).
Disease of the periodontium occurring in an
otherwise healthy adolescent is characterized by
rapid loss of alveolar bone about more than one
tooth of the permanent dentition. The amount of
destruction is not commensurate with the amount of
local irritants (Albandar, 2014).
Key diagnostic criteria of this disease include an:
− Early age of onset, involvement of multiple teeth
with a distinctive pattern of clinical attachment
loss and radiographic bone loss.
− A relatively high rate of disease progression and
the absence of systemic diseases that
compromise the host's response to infection.
− Although in some patients the disease may start
before puberty, in most patients the age of onset
is during, or somewhat after, the circumpubertal
period. A typical patient shows disease onset at
an early age (i.e., before 25 years of age),
although identification of the affected patient
usually occurs after disease commencement.
− Initially, the periodontal lesions show a
distinctive pattern, depicted radiographically as
vertical bone loss at the proximal surfaces of
posterior teeth, and the bone loss usually occurs
bilaterally. In advanced cases of aggressive
periodontitis the periodontal lesions may be
depicted radiographically as a horizontal loss of
bone. The primary teeth may also be affected,
although early exfoliation of these teeth is not
common.
− Aggressive periodontitis may be localized or
generalized. In localized aggressive periodontitis
(LAP), tissue loss usually starts at the permanent
first molars and incisors, and with increasing
patient age the disease may progress to involve
the adjacent teeth. The generalized form of
aggressive periodontitis involves most or all of
the permanent teeth.
A.actinomycetemcomitans is a perio-pathogenic
bacteria that has long been associated with localized
aggressive periodontitis. The mechanisms of its
pathogenicity have been studied in humans and pre-
clinical experimental models. Although different
serotypes of A. actinomycetemcomitans have
differential virulence factor expression,
A.actinomycetemcomitans cytolethal distending
toxin (CDT), leukotoxin, and lipopolysaccharide
(LPS) have been most extensively studied in the
context of modulating the host immune response.
Following colonization and attachment in the oral
cavity, A.actinomycetemcomitans employs CDT,
leukotoxin, and LPS to evade host innate defense
mechanisms and drive a pathophysiologic
inflammatory response. This supra-physiologic
immune response state perturbs normal periodontal
tissue remodeling/turnover and ultimately has
catabolic effects on periodontal tissue homeostasis
(Herbert, 2016).
Figure 1 : A.actinomycetemcomitans colonizes the
gingival sulcus by attachment to the sulcular/junctional
epithelium cells (Herbert et al., 2016).
It subsequently invades through the epithelium
via pro-apoptotic virulence mechanisms and
penetrates into the subgingival connective tissue
where is stimulates epithelial cells and fibroblasts to
secrete pro-inflammatory cytokines (I). Neutrophils
and monocytes are thereby recruited to the local site
of infection and perpetuate the host inflammatory
response. Subsequently, B and T cells are recruited
to the diseased periodontium from the circulation
(II). T cells secrete pro-resorptive factors that drive
osteoclast (OC) formation and drive bone resorption.
A.actinomycetemcomitans simultaneously impairs
osteoblast (OB) function, perturbing bone
remodeling processes, which ultimately results in
catabolic alveolar bone loss (III).
A.actinomycetemcomitans virulence factors
interact with host cells to initiate an aberrant
inflammatory response in the periodontal gingival
tissues. While it has been reported that trans-
epithelial migration of polymorphonuclear
Effect of Tumor Necrosis Factor Alpha (Tnf-A) And Interleukin-10 (II-10) Levels of Aggressive Periodontitis In Rats (Rattus Norvegicus)
Induced by Agrregitibacter Actinomycetemcomitans
345

leukocytes (PMNs) into the gingival sulcus results in
a formed pseudo-barrier, which is several cell layers
thick between the plaque and junctional/sulcular
epithelium surface (Garant, 1976), this review
considers the gingival epithelium to be the initial
barrier to A.actinomycetemcomitans. First
responders are non-hematopoietic resident cells:
gingival fibroblasts and epithelium cells.
A.actinomycetemcomitans stimulates the host
responses via exotoxic and endotoxic virulence
factors, activating superficial epithelial cells and
underlying fibroblast cells.
A.actinomycetemcomitans can effectively migrate
through the gingival epithelium and once
A.actinomycetemcomitans bypasses these initial
barriers, a host inflammatory response is initiated.
Once A.actinomycetemcomitans penetrates deeper
in the subgingival tissues, a broader host immune
response is activated (Fives-Taylor et al., 1999).
A.actinomycetemcomitans immune stimulation
in the periodontal microenvironment elicits a
pathophysiologic pro-inflammatory state, which
disrupts normal periodontal tissue remodeling
processes ultimately promoting collateral tissue
damage. In periodontal disease, the supra-
physiologic level of pro-inflammatory and pro-
resorptive cytokines favors alveolar bone resorption
by monocyte or defined osteoclast progenitor
(dOCP) derived osteoclasts, versus alveolar bone
formation by mesenchymal derived osteoblastic
cells. When bone resorption exceeds bone
formation, an unbalanced bone remodeling process
having catabolic effects on alveolar bone
homeostasis, ultimately results in net alveolar bone
loss (Baron et al, 1978). Bone-lining tartrate
resistant acid phosphatase (TRAP) positive
multinucleated osteoclasts secrete bone degradation
enzymes, including matrix metalloproteinases
(MMPs) and cathepsin K in the acidic sealing zone
microenvironment, via integrin protein adherence to
the bone surface (McCauley et al, 2002; Teitelbaum
et al, 1997).
A.actinomycetemcomitans has been shown to
induce osteoclast formation and bone loss in rodent
animal models. Pro-inflammatory cytokines with
potent pro-resorptive actions, including tumor
necrosis factor (TNF)-α, IL-1, and IL-6 are highly
upregulated by A.actinomycetemcomitans and thus
promote osteoclast formation and bone resorption
(Hotokezaka et al., 2007). In humans,
A.actinomycetemcomitans positive patients had
significantly greater periodontal bone loss than the
A.actinomycetemcomitans negative subjects,
supporting A.actinomycetemcomitans’s remarkable
impact on periodontal disease associated alveolar
bone loss (Fine et al., 2007).
Tumor necrosis factor-a (TNF-α) plays a role in
periodontal inflammation. TNF-α is mainly
produced by activated macrophages. TNF-α has
strong potential to increase bone resorption and be
involved in tissue degradation with prostaglandin-E2
and collagenase (Morimoto et al., 2008). Several
studies have reported that there is an increase in
TNF-α levels in crevicular gingival fluid (CGF) and
gingival tissue in patients with periodontitis (Peggie
et al., 2015). Pro- and anti-inflammatory cytokine
tissue works on periodontal tissues that experience
inflammation. Among other cytokines, interleukin-
10 (IL-10) is an important multifunctional cytokine.
Increased or decreased levels of IL-10 host are very
important for controlling balance between
individuals who experience inflammation (Gonzales
et al., 2002).
Interleukin-10 is an anti-inflammatory cytokine,
produced by T-helper 2 (Th2) cells, macrophages
and B cells, which inhibit the synthesis of pro-
inflammatory cytokines such as interleukin-1 (IL-1),
interleukin-2 (IL-2), interleukin-6 (IL-6),
interleukin-8 (IL-8), tumor necrosis factor-a (TNF-
α) and interferon-γ (IFNγ). IL-10 production of radio
metalloproteinase also increases the inhibitory of
metalloproteinase tissue in macrophages (Lacraz et
al., 1995). In addition, production of the
osteoprotegrin hormone is a result of bone resorption
by preventing the Kappa-B Ligan Factor Nuclear
Receiver Activator (RANK-RANKL). Cytokines IL-
10 can be protective cytokines in periodontal disease
and regulate pro-inflammatory cytokines, including
those involved in lost alveolar bone. Individuals who
are high IL-10 producers are more protected from
periodontitis due to IL-10 anti-inflammatory.
Therefore, an increase in IL-10 anti-inflammatory
cytokines will play an immune response to
periodontopathogenic bacteria.
3 METHODS
This research was a true experimental study with
Post Test Only Control Group Design. Rats were
divided into 4 groups for 0.25; 0.5 and 0.75
CFU/mL, and negative control. Each group
contained 5 rats. How to obtain
A.actinomycetemcomitans bacteria with diluted
culture using 700 μl PBS with 2% Sodium
Carboxymethyl cellulose. Aggressive periodontitis
in rats was induced by injecting
A.actinomycetemcomitans, at 48 hrs and 96 hrs, post
ICPS 2018 - 2nd International Conference Postgraduate School
346

injection the inflamatory signs were observed, three
days later. Blood was centrifuged at 3000 rpm for 15
minutes to separate blood cells and plasma. Plasma
is stored in a freezer of -80
o
C then examination of
TNF-α and IL-10 levels. Plasma were then collected
to measure TNF-α dan IL-10 level by ELISA.
After obtaining data from the examination
results, the data is processed in several stages. The
data obtained is collected and checked whether there
are writing errors, results mismatches, etc. so that it
must be corrected. Data that has passed the editing
process is coded so that it is easily displayed in the
results table and it is analyzed. The data that has
been coded is displayed in the table of the results of
the examination so that it is easy to analyze and
interpret its meaning. After tabulation, the data were
analyzed according to the research objectives. In this
study, the results of TNF-α and IL-10 ELISA test
data from the patient's plasma were associated with
the degree of blood of patients with normal control
of plasma TNF-α and IL-10 ELISA test results. The
relationship data for each observed group is
described as looking for its meaning. Then the
ELISA test result will be analyzed using ANOVA.
4 RESULT
Analysis of Variance (ANOVA) showed
significantly increased levels of TNF-α in the
infected group compared with that of the control
group. Aggressive periodontitis in rats showed by
redness, abscess and tooth mobility. This condition
indicated that A. actinomycetemcomitans has the
ability to adhere and invade the periodontal tissue
further producing a colony that caused periodontitis.
Table 1. The mean and standard deviation of TNF-α levels
in rats (Rattus norvegicus) with localised aggressive
periodontitis.
Sample Mean
± SD
Median Max-
Min
P
Control 777,4
±
164,6
784,7 961,1-
531
0,0075
P1 (Bakteri
Aa 0,25
CFU/ml)
8
90 ±
41,84
886,9 948,7-
832,7
P2 (Bakteri
Aa 0,5
CFU/ml)
1043
±
67,24
1032 1137-
976,9
P3 (Bakteri
Aa 0,75
CFU/ml)
1213
±
96,87
1178 1347-
1116
Table 2. The mean and standard deviation of IL-10 levels
in rats (Rattus norvegicus) with localised aggressive
periodontitis.
Sample Mean
± SD
Median Max-
Min
P
Control 576 ±
327
628,3 953,3-
224,2
0,0051
P1
(Bakteri
Aa 0,25
CFU/ml)
391,8
±
135,9
402,7 556,1-
178,8
P2
(Bakteri
Aa 0,5
CFU/ml)
125,4
±
55,8
137 187,5-
37,7
P3
(Bakteri
Aa 0,75
CFU/ml)
198,3
±
52,1
224,2 248,5-
137,1
Table 3. Ratio between TNF-α levels and IL-10
Mean Ratio
TNF-α IL-10
Control 777,4 576 1,3
0,25 CFU/ml 890 391,8 2,3
0,5 CFU/ml 1043 125,4 8,3
0,75 CFU/ml 1213 198,3 6,3
The highest ratio was found in the sample with
the treatment of injection of
A.actinomycetemcomitans bacteria with a
concentration of 0.5 CFU / ml because it had the
lowest IL-10 levels compared to the control group
(K), P1 and P3.
5 DISCUSSION
In periodontal health the oral cavity is colonized by
the oral commensal (non-pathogenic) flora. Under
physiological states the normal oral flora stimulates
the innate immune defense system in the
periodontium, which controls bacterial colonization
of periodontal tissues in close proximity to the
gingival sulcus. In this periodontal
microenvironment, A.actinomycetemcomitans
infection induces a supra-physiological immune
inflammatory response state, which disrupts normal
periodontal tissue homeostasis in the gingiva,
periodontal ligament (PDL), cementum, and alveolar
bone, ultimately promoting tooth loss.
A.actinomycetemcomitans virulence factors interact
with host cells to initiate an aberrant inflammatory
response in the periodontal gingival tissues. While it
Effect of Tumor Necrosis Factor Alpha (Tnf-A) And Interleukin-10 (II-10) Levels of Aggressive Periodontitis In Rats (Rattus Norvegicus)
Induced by Agrregitibacter Actinomycetemcomitans
347
has been reported that trans-epithelial migration of
polymorphonuclear leukocytes (PMNs) into the
gingival sulcus results in a formed pseudo-barrier,
which is several cell layers thick between the plaque
and junctional/sulcular epithelium surface (Garant,
1976). A.actinomycetemcomitans stimulates the host
responses via exotoxic and endotoxic virulence
factors, activating superficial epithelial cells and
underlying fibroblast cells.
A.actinomycetemcomitans can effectively migrate
through the gingival epithelium and once
A.actinomycetemcomitans bypasses these initial
barriers, a host inflammatory response is initiated.
Once A.actinomycetemcomitans penetrates deeper
in the subgingival tissues, a broader host immune
response is activated (Ahmed et al., 2001).
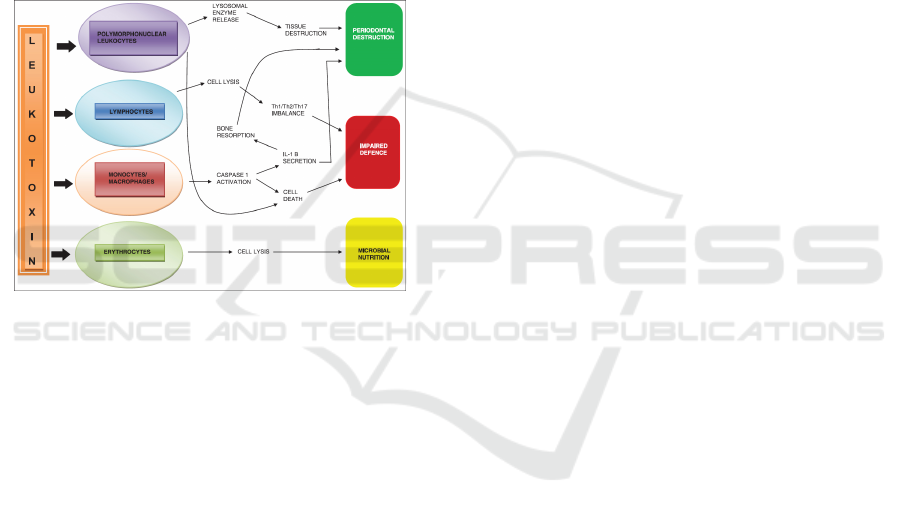
Figure 2. Effect of A.actinomycetemcomitans on human
blood cells causing periodontal inflammation and tissue
destruction (Malik et al., 2015).
One of the most studied virulence factors of
A.actinomycetemcomitans is leukotoxin. This toxin
is a 116 kDa protein produced by 56% of strains
isolated from LJP patients. The mechanism of
virulence leukotoxin is not only species specific but
also cell specific. The toxin binds to neutrophils,
monocytes, and a subset of lymphocytes; and forms
pores in the membranes of these target cells
overwhelming their ability to sustain osmotic
homeostasis, resulting in cell death. Interaction is
with polymorphonuclear leukocytes (PMNs).
Leukotoxin has shown to efficiently cause death of
human PMNs through extra-cellular release of
proteolytic enzymes from both primary and
secondary granules, along with activation and
release of matrix metalloproteinase-8, which can
contribute to periodontal tissue destruction (Malik et
al., 2015).
The ability of leukotoxin to induce apoptosis in
lymphocytes might impair the acquired immune
response of periodontal infections. A shift in the
balance between Th-1 and Th-2 subsets of T-cells is
found in periodontal inflammation, with the Th-2
cells to associate with chronic periodontitis. Its
ability to affect the lymphocytes also indicates a
possible role of this molecule in Th-1/Th-2/Th-17
differentiation, important in inflammatory
pathogenesis. Leukotoxin causes the activation of
caspase-1, which is a cytosolic cysteine proteinase
that specifically induces activation and secretion of
the pro-inflammatory cytokines interleukin-1 and
18, which result in monocyte/macrophage lysis by
incorporation in a cytosolic multimer complex
named the inflammasome (Malik et al., 2015).
TNF-α is a strong pro-inflammatory cytokine,
secreted by mononuclear leukocytes or macrophages
in the early stages of the inflammatory response.
TNF-α can stimulate osteoclasts, which lead to
alveolar bone resorption, and can increase the
release of matrix metalloproteinases (MMPs), which
leads to the destruction of the extracellular matrix of
periodontal tissue (Alexander et al., 1994). TNF-α is
strongly associated with the pathogenesis and
severity of periodontitis (Graves et al., 2003).
Compared with healthy periodontal, there was an
increase in TNF-α levels in Gingival crevicular fluid
(GCF) periodontitis and in inflammatory periodontal
tissues (Kennedy et al., 1990).
TNF-α affects cell migration by inducing and
regulating adhesion molecules to encourage
neutrophil turnover and adhesion to the vessel wall,
which can cause extravasation. It also stimulates
chemokine production, which is involved in the
migration of infected cells and inflammation
(Wajant et al., 2003). TNF-α also correlates with
extracellular matrix degradation and bone resorption
through the secretion of MMPs and RANKL
(Graves et al., 2008).
Periodontitis is a disease that involves bone
damage that can cause tooth loss. Therefore, anti-
inflammatory cytokines are needed to inhibit bone
resorption and increase alveolar bone regeneration
which is also associated with the role of IL-10 in
bone remodeling inhibiting bone resorption as well
as reducing inflammation (Cochran, 2008). IL-10 is
an anti-inflammatory cytokine that suppresses the
immunoproliferative and inflammatory responses.
As a factor produced by T helper2 cells (Th2), IL-10
inhibits cytokine production by Th1 cells. It is
known that IL-10 is also produced by many other
cell types, including B cells, mast cells, eosinophils,
macrophages, and dendritic cells (DC), and a large
number of T cell subsets such as CD8
+
T cells and
CD4
+
T-regulation cells (Petska, 2004).
IL-10 can reduce the synthesis of pro-
inflammatory cytokines and chemokines, such as IL-
ICPS 2018 - 2nd International Conference Postgraduate School
348

1, IL-6, and TNF-α. This can also reduce the
synthesis of nitric oxide, gelatinase, and collagenase.
IL-10 succeeded in increasing the neutralization of
synthesis of IL-1 and TNF-α. Therefore, IL-10 is
also considered an important regulator of
periodontal tissue homeostasis, in homeostatic and
inflammatory conditions (Lee et al., 2009). IL-10
can directly inhibit osteoclast formation. The
inhibitory effect of IL-10 on osteoclast formation is
by direct action on osteoclast precursors. The
molecular mechanism of this inhibition shows that
IL-10 increases the expression of osteoprotegerin
(OPG) but decreases the expression of NF-κB ligand
receptor activator (RANKL) and colony stimulating
factor-1 (CSF-1) (Liu et al., 2006).
Bone resorption is largely induced by the
production of pro-inflammatory cytokines, such as
TNF-a and IL-1. These cytokines can act by directly
increasing the proliferation and activity of cells in
osteoclasts or indirectly affecting the production of
osteoclast differentiation factors such as RANKL
and OPG through osteoblasts or stroma cells. IL-10
has been recognized to have strong anti-
inflammatory activity for a long time, and this has
proven to be an important endogenous suppressor of
bone resorption which means IL-10 can suppress
osteoclastic differentiation through the above aspects
(Boyle et al., 2003).
Gingival epithelial invasion and apoptosis. In the
in vitro model, A.actinomycetemcomitans was able
to migrate through the gingival layer and epithelial
cells by increasing the production of pro-
inflammatory cytokines TNF-a, IL-1β, IL-6, IL-8
and increasing cell apoptosis (Dickinson et al.,
2011). A study using A.actinomycetemcomitans in
model mice found that apoptosis of
A.actinomycetemcomitans was mediated by gingival
epithelial cells via the caspase3 and caspase7
pathways (Kang et al., 2012). At the subcellular
level, A.actinomycetemcomitans stimulates
phosphorylation via TGFβR1 which signals gingival
epithelial cells causing caspase3 activity to divide
and subsequent cell apoptosis (Yoshimoto et al.,
2014). This finding shows that A.
actinomycetemcomitans bacteria have the potential
to cross the gingival epithelium through the
mechanism of pro-apoptosis. Disruption of gingival
epithelial results in the induction of a periodontal
pathological inflammatory microenvironment that
supports recruitment of hematopoietic immune
response cells derived to subgingival tissues
(Yoshimoto et al., 2014).
Macrophages have been shown to play a role in
the pathogenesis of periodontitis caused by
A.actinomycetemcomitans. Monocytes enter the
tissue which results in local infection with
diapedesis from circulation where it can differentiate
into activated macrophages or osteoclasts. Llike
receptor toll receptors (TLRs) recognize pathogenic
constituents and have been extensively studied in
macrophage function. A.actinomycetemcomitans has
strong endotoxic LPS received by TLR2, TLR4, and
TLR5, with TLR4 currently being considered as the
main receptor for LPS, which clearly illustrates the
function of TLR2 and TLR4 signaling in
macrophage interactions with
A.actinomycetemcomitans (Park et al., 2014).
When bone marrow macrophages and TLR2 and
TLR4 were stimulated with
A.actinomycetemcomitans, TLR2 and TLR4
macrophages were attenuated and showed pro-
inflammatory production of TNF-α and IL-6
cytokines. MyD88 is an adapter protein for all TLRs
(except for TLR3), IL-1R, and IL-18R. MyD88
deficiency further reduces cytokine production by
A.actinomycetemcomitans, suggesting that while
TLR2 and TLR4 are critical regulators of
A.actinomycetemcomitans, which induce the
production of inflammatory cytokines, other
receptors also spread TNF-α and IL-6 production.
A.actinomycetemcomitans also stimulates the
production of IL-12p40 (IL-12B) in macrophages,
which mainly depend on MyD88. This finding
clearly shows that TLR signaling is very important
for the formation of inflammatory cytokines, but it
has not been explained how TLR4 and TLR2
interact with A.actinomycetemcomitans to modulate
their periopathogenesis (Park et al., 2014).
6 CONCLUSION
The induction of A.actinomycetemcomitans bacteria
on periodontal tissues can cause periodontitis by
being characterized when one of them is at high
levels of TNF-α and low levels of IL-10 in rats
(Rattus Norvegicus). In this case if TNF-α levels
rise and IL-10 levels fall, the severity of infection
and the prognosis of periodontitis in rats (Rattus
Norvegicus) is getting worse.
High plasma level of TNF was seen in rats
infected with 0.75 CFU/mL OF ac, while IL-10 was
low as seen in rats infected with 0.5 CFU/mL OF
Ac.
Effect of Tumor Necrosis Factor Alpha (Tnf-A) And Interleukin-10 (II-10) Levels of Aggressive Periodontitis In Rats (Rattus Norvegicus)
Induced by Agrregitibacter Actinomycetemcomitans
349

REFERENCES
Albandar, J., DeNardin, A., Adesanya, M., Diehl S., Winn
D. 2001. Associations between serum antibody levels
to periodontal pathogens and early-onset
periodontitis. J Periodontol ;72:1463-9.
Baron, R., Saffar, J. 1978. A quantitative study of bone
remodeling during experimental periodontal disease
in the golden hamster. J Periodontal Res ;13:309–315
Brown, L., Brunelle, J, Kingman, A., 1996. Periodontal
status in the United States, 1988-1991: prevalence,
extent, and demographic variation. J. Dental Res.
75:672-683.
Escudero, N., Perea, M., Bascones, A., 2008. Revisión de
la periodontitis cronica: Evolución y su aplicacion.
Avances en Periodoncia e Implantología Oral
20(1):27-37.
Fine, D., Markowitz, K., Furgang, D. 2007.
Aggregatibacter actinomycetemcomitans and its
relationship to initiation of localized aggressive
periodontitis: longitudinal cohort study of initially
healthy adolescents. J Clin Microbiol ;45:3859–3869.
Fives-Taylor, P., Meyer, D., Mintz, K., Brissette, C. 1999.
Virulence factors of Actinobacillus
actinomycetemcomitans. Periodontology. ;20:136–
167.
Graves, D. 2008. Cytokines that promote periodontal
tissue destruction. J Periodontol 79: 1585–1591 .
Gray, J., 2000. Parameter on Aggressive Periodontitis.
Journal of Periodontology, 71(5).
Herbert, B., Novince, C., Kirkwood, K. 2016.
Aggregatibacter actinomycetemcomitans, a potent
immunoregulator of the periodontal host defense
system and alveolar bone homeostasis. NCBI Journal.
Joshipura, V., Yadalam, U., Brahmavar, B., 2015.
Aggresive Periodontitis. India.
Lindberg TY, Bage T., 2013. Inflammatory mediators in
the pathogenesis of periodontitis. Expert Rev Mol
Med 15: e7
Liu, S. Yao, and G. E.Wise, 2006. Effect of interleukin-10
on gene expression of osteoclastogenic regulatory
molecules in the rat dental follicle. European Journal
of Oral Sciences, vol. 114, no. 1, pp. 42–49
Malik, R., Changela, R., Krishan, P., Gugnani, S and Bali,
D. 2015. Virulence factors of Aggregatibacter
actinomycetemcomitans. Journal of International
Clinical Dental Research Organization. Vol 7
McCauley, L., Nohutcu, R., 2002. Mediators of
periodontal osseous destruction and remodeling:
principles and implications for diagnosis and therapy.
J Periodontol ;73:1377–1391.
Moore WE, Moore LV., 1994. The bacteria of periodontal
diseases. Periodontology 2000 5:66-77.
Newman MG, Takei HH, Carranza FA, 2002. Clinical
Periodontology. 9th ed. Philadelphia: WB Saunders.
Newman M.G, Takei H.H, Klokkevoid P.R and Carranza
F.A., 2006. Carranza’s Clinical Periodontology, 10th.
St.Louis Missouri: Saunders Elsevier,: p 46-7, 68, 72-
75, 116-120.
Park, S., Kim, D., Han, S. 2014. Diverse Toll-like
receptors mediate cytokine production by
Fusobacterium nucleatum and Aggregatibacter
actinomycetemcomitans in macrophages. Infect
Immun ;82:1914–1920.
Pestka, C., Krause., Sarkar, D. 2004. Interleukin-10 and
related cytokines and receptors, Annual Reviewof
Immunology, vol. 22, pp. 929–979.
Suvan J, D’Aiuto F, Moles DR, Petrie A, Donos N., 2011.
Association between overweight/obesity and
periodontitis in adults. A systematic review. Obes.
Rev. 12(5):e381-404.
Teitelbaum, S., Tondravi, M., Ross, F. 1997. Osteoclasts,
macrophages, and the molecular mechanisms of bone
resorption. J Leukoc Biol ;61:381–388.
Yamamoto M, Kobayashi R, Kono T, Bolerjack B, Gilbert
R., 2011. Induction of IL-10- producing CD4 T-cells
in Chronic Periodontitis. J. Dent. Res. 90(5):653-658.
ICPS 2018 - 2nd International Conference Postgraduate School
350
