
The Effect of Macrophage Dose to Secretion Interleukin 6 (IL-6) on
Model of Tuberculosis Granuloma In Vitro
Imam Agus Faizal
1ab
, Agung Dwi Wahyu Widodo
2
Jusak Nugraha
3
1 a
Post Graduate Master of Immunology, Sekolah Pascasarjana, Universitas Airlangga, Surabaya, Indonesia
b
Department of Stem Cell Institute of Tropical Disease, Universitas Airlangga, Indonesia
2
Department of Microbiology Clinic, Faculty of Medicine, Dr. Soetomo Hospital, Indonesia
3
Department of Patology Clinic, Faculty of Medicine, Dr. Soetomo Hospital, Indonesia
Keywords: Granuloma, Macrophage, Interleukin 6.
Abstract: Granuloma is a pathological sign of host response as a system of defense against infection of
Mycobacterium tuberculosis (Mtb) causing Tuberculosis (TB) disease in humans estimated to be 8.7 million
new cases, 1.4 million deaths, and about 2 billion latent infections. Macrophages are responsible for
activating protective immune responses in innate and adaptive immune to control or eliminate infection. The
inner body protects against Mtb infection producing various secretions of cytokines interleukin 6 (IL-6) that
play the role of activating multinucleated giant cells, differentiation of macrophage T cells and thus
stimulating CD4
+
and CD8
+
T-cells to strengthen macrophage antimicrobial capacity as early response
reactions (early phase). The aim of this research is to see the effect of dose of macrophage on secretion and
IL-6 expression on a TB granuloma model in vitro. Human blood was made by Peripheral Blood
Mononuclear Cell (PBMC) and treated with the addition of a dose of macrophages of 1x10
5
cells/well,
2x10
5
cells/well and 3x10
5
cells/well and control (without macrophages). Then the bacterium
Mycobacterium tuberculosis was added and then observing the secretion The enzyme-linked
immunosorbent assay (ELISA) method of IL-6 cytokine during the 1st, 2nd, 3rd, 4th, and 5th days. The
results of IL-6 examination on ELISA obtained p value of 0.7520 (p> 0.05). The conclusion of the study
was that there was no effect of adding macrophage dose to cytokine secretion of IL-6 levels on granuloma
TB model in vitro.
1 INTRODUCTION
Bacteria Mycobacterium tuberculosis (Mtb) is the
cause of Tuberculosis (TB) disease in humans
causing death and one of the infectious agents in
humans worldwide. There are an estimated 8.7
million new cases, 1.4 million deaths and about 2
billion latent infections caused by Mtb (Fitzgerald et
al., 2014). Based on data from the World Health
Organization (WHO) in 2014, the cases of TB in
Indonesia reach 1 million and the number of deaths
due to TB is estimated at 110,000 each year
(Kesehatan and Indonesia, 2017).
Granulomas are a pathological sign of the host
response to Mycobacterium tuberculosis infection
that has innate immunity, inflammation, but has
developed into a complex and dynamic structure
capable of being mediated by T cell response (Orme
and Basaraba 2014). Granulomas are tissue
compounds consisting of infected macrophages and
multinucleated giant cells, surrounded by
aggregations of new monocytes or macrophages, and
neutrophils and lymphocytes (Parasa, 2014).
Macrophages are responsible for the activation of
protective immune responses, innate and adaptive
immune, thus playing an important role in ongoing
cross-cell communication that is needed to control or
eliminate host cell infection in the early phase
(Murugesan V.S., Rajaram et al., 2015).
Pathophysiology begins with internalization of Mtb
by host cell macrophages so that activated
macrophages secrete various cytokine markers such
as IL-8, IFN-γ, and TNF-α (Kapoor et al., 2013).
Mycobacterial infected macrophages also release
large amounts of IL-6 that play the role of active
macrophage differentiation into multinucleated giant
cells (Fitzgerald et al., 2014). IL-6 secreted by Mtb
infected macrophages suppresses an uninfected
306
Agus Faizal, I., Dwi Wahyu Widodo, A. and Nugraha, J.
The Effect of Macrophage Dose to Secretion Interleukin 6 (IL-6) on Model of Tuberculosis Granuloma In Vitro.
DOI: 10.5220/0007541803060311
In Proceedings of the 2nd International Conference Postgraduate School (ICPS 2018), pages 306-311
ISBN: 978-989-758-348-3
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

macrophage response to IFN-γ. Increased levels of
IL-6 correlate with elevated levels of IL-1β and IL-
11 so that IL-6 can play many roles (pleotropic
cytokines) contributing positively or negatively to
host control cells against Mtb infection (Romero-
Adrian., 2015).
Based on the above phenomenon, this research
was conducted to analyze the role of the addition of
dose of macrophage to the formation of granuloma
TB and the role of cytokine levels of IL-6 in the
formation of granuloma TB in vitro so that a
granuloma model can be used in latent and active
stage with kedapannya done to develop effective
diagnosis and develop a vaccine.
2 MATERIALS AND METHODS
Research data is quantitative. The independent
variables were the addition of the dose of
macrophages as 1x10
5
(dose 1), 2x10
5
(dose 2), and
3x10
5
(dose 3) cells/well and duration of incubation
days 1, 2, 3, 4 and 5 on granuloma model in vitro.
While the dependent variable is the amount of IL-6
secretion level.
2.1 PBMC
PBMC is made from adult blood, a healthy criterion.
After the blood was taken it was immediately
isolated. Blood sentrifuge blood plasma was taken
1.5 ml conical tube and added 3 ml PBS and 5 ml
histopague and then centrifuge 30 minutes/ 1700
rpm taken buffycoat centrifuge plus PBS centrifuge
5 minutes/1300 rpm then transferred to tube 1.5 ml
save temperature -30°C.
2.2 Macrophages
Buffycoat is taken 60 ml. 50 ml conical tubes were
prepared with a histopague of 15 ml each. Prepared
one tube every 10 ml of buffycoat. Histopagues are
used at room temperature. Then sterile scissors and
tubes for buffycoat with ethanol 70%, cut the ends
of the tube and buffycoat poured into tissue culture
flask 75 cm
2
. Buffycoat was diluted with 2% FBS
and PBS volume and then stirred slowly then
centrifuge was taken ring cell on the surface
between histopag and plasma cell as much as 50 ml.
Buffycoat is taken 60 ml. 50 ml conical tubes
were prepared with a histopague of 15 ml each.
Prepared one tube every 10 ml of buffycoat.
Histopague are used at room temperature. Then
sterile scissors and tubes for buffycoat with ethanol
70%, cut the ends of the tube and buffycoat poured
into tissue culture flask 75 cm
2
. Buffycoat was
diluted with 2% FBS and PBS volume and then
stirred slowly then centrifuge was taken ring cell on
the surface between histopague and plasma cell as
much as 50 ml. Discarded supernatant and
centrifuge. Plate at 10 cm culture dishes (10 ml /
dish) incubated for 1-2 hours at 37ºC 5% CO
2
.
Observe macrophage cells under a microscope then
discard the supernatant and then add PBS and
homogenize. Let the cell differentiate for 7 days
observed macrophage cells daily.
2.3 Model Granuloma In Vitro
PBMC added media Roswell Park Memorial
Institute (RPMI). Plate 96 well (control, treatment
day 1, 2, 3, 4, 5 per/well). Bacterial strain
Mycobacterium tuberculosis strain H37Rv (obtained
from ITD department Tbc) inoculated as much as
1x10
5
CFU into all wells. Then macrophages were
added at doses 1, 2, 3. Plate was incubated at 37ºC
CO
2
5% to 5 days per day granuloma harvested and
50 μl for ELISA examination.
2.4 ELISA
Examination of ELISA content using reagent kit
Elabscience Biotechnology Inc. All Rights
Reserved, 2017 (Interleukin 6). Preparation of
reagent consists of wash buffer, standard working
solution, Biotinylated Detection Ab working
solution, Concentrated HRP Conjugate working
solution. Advanced examination can be seen in the
guide book and determine the optical density (OD
value) of each well with 450 nm.
3 RESULTS
Granuloma was taken by BTA dye to confirm Mtb.
Then haematoxylin-eosin (HE) staining looked at
the morphology of the granuloma structure. Before
PBMC made media observed macrophage cells
under a microscope.
The Effect of Macrophage Dose to Secretion Interleukin 6 (IL-6) on Model of Tuberculosis Granuloma In Vitro
307
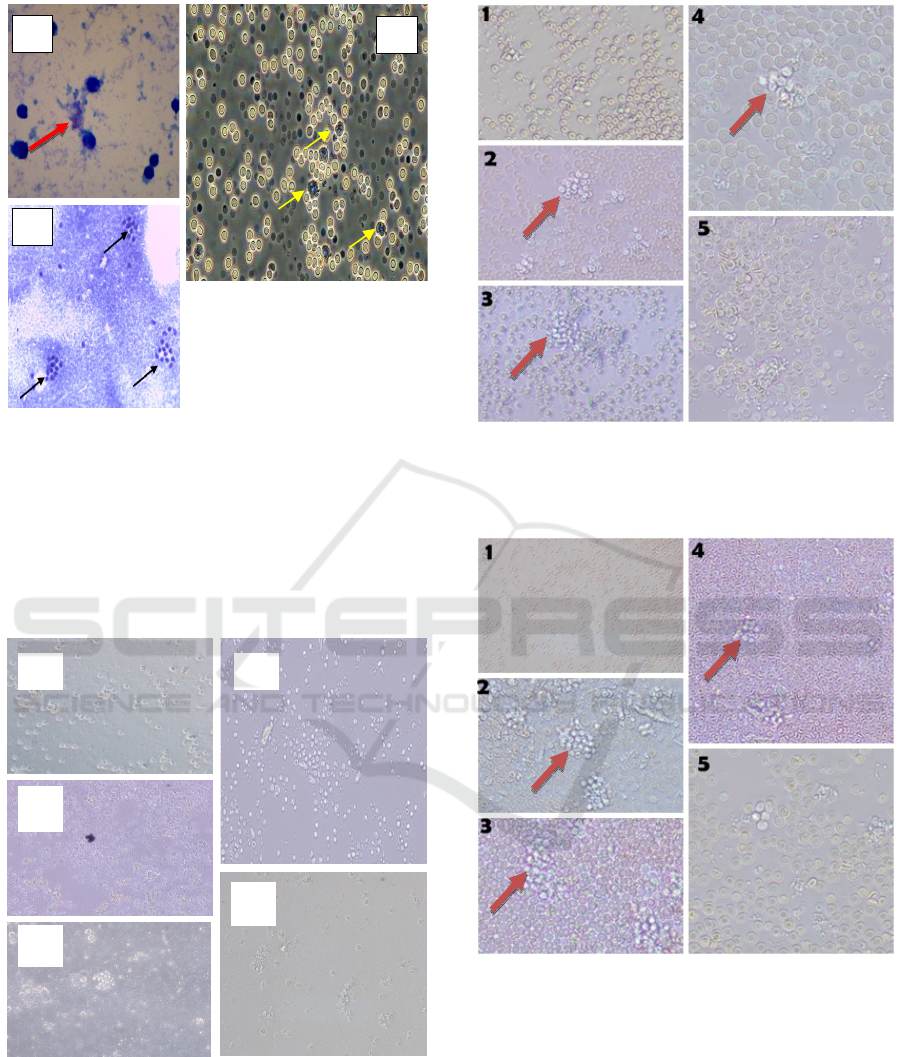
Figure 1: A: bacteria Mtb (red arrows) B: granuloma
morphology of clusters (black arrows) C:macrophage cells
(yellow arrows).
3.1 Direct Granuloma Observation
Granulomas are observed directly in an inverted
microscope per day. Seen granuloma morphology,
aggregation and cells form granuloma.
Figure 2: PBMC infected M.Tb without macrophage
(control). Figure 1, 2, 3, 4 and 5 have not formed
granuloma aggregation.
Figure 3: Direct observation of the group with the
addition of 1x10
5
macrophage (400x magnification). The
brown arrow indicates 2, 3 have formed granuloma
aggregations and 4 granulomas are more clustered.
Figure 4: Direct observation of the group with the addition
of 2x10
5
macrophage (400x magnification). The brown
arrow indicates 2, 4 have formed granuloma aggregation
and 3 perfect granulomas are more clustered and dense.
A
B
C
1
3
5
4
2
ICPS 2018 - 2nd International Conference Postgraduate School
308
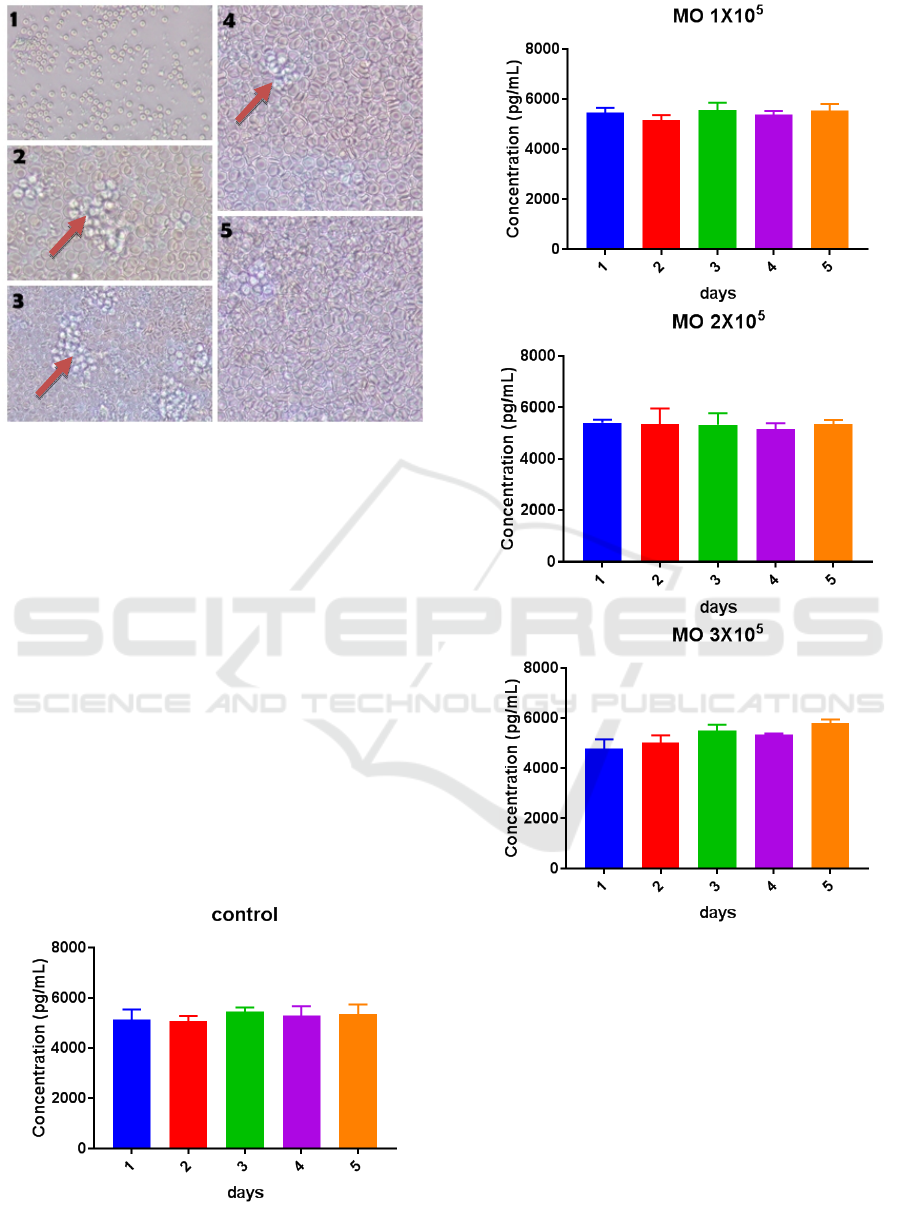
Figure 5: Direct observation of the group with the addition
of 3x10
5
macrophage (400x magnification).
The brown arrow 2, 3, 4 indicates the formation of
granuloma aggregation and formation. It is clearly visible
from the solid structure, with the high number of cells.
The granuloma on the second and third days,
while on the first day the granuloma was still in the
aggregation formation stage consisting of
macrophages containing lipids (foamy
macrophages), epitheloid cells and Langhans cells.
However, the fifth day of granuloma aggregation
observation began to rupture.
3.2 The Examination the levels of IL-6
Supernatant samples were then examined for levels
of IL-6 secretion using ELISA method and obtained
the average result from each treatment, either control
or sample.
Figure 6: Examination the levels of IL-6
Results of IL-6 examination by ELISA method
from 4 groups were control with the addition of dose
of macrophage 1x10
5
, 2x10
5
, 3x10
5
showed high
level. The highest levels of IL-6 secretion occurred
on day 5 with a dose of 3x10
5
. While the lowest
levels of IL-6 secretion occurred on day 2 with a
dose of 2x10
5
.
The Effect of Macrophage Dose to Secretion Interleukin 6 (IL-6) on Model of Tuberculosis Granuloma In Vitro
309
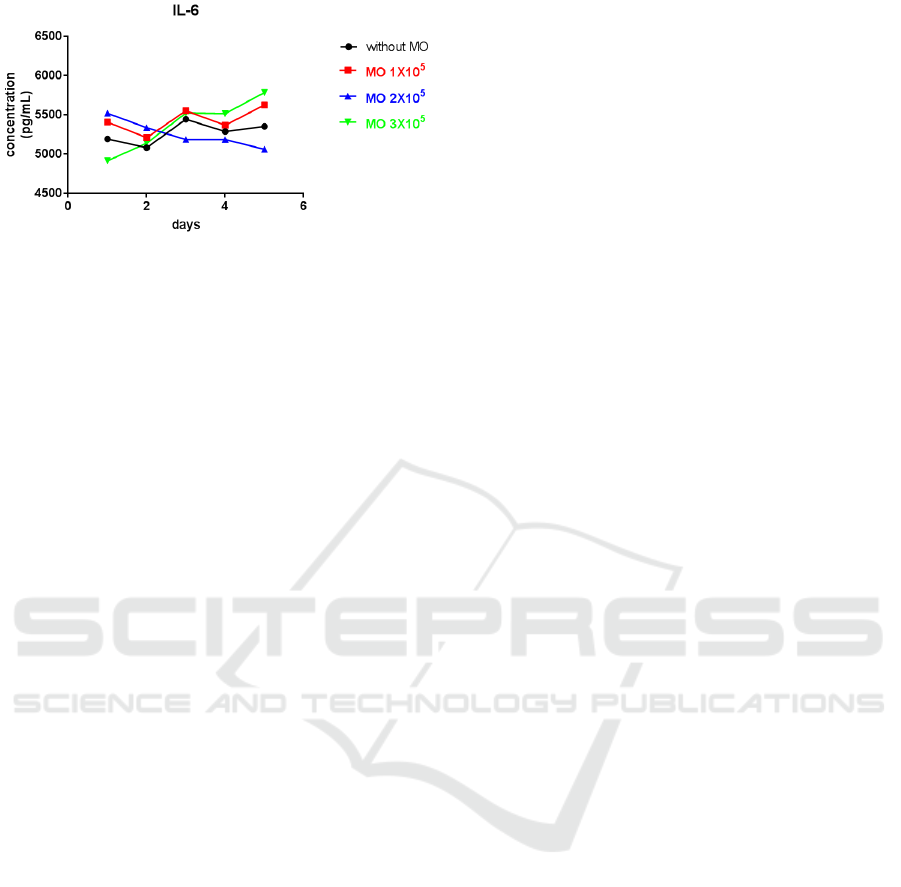
Figure 7: Test results using One-Way ANOVA
The test results using One-Way ANOVA which
aims to determine the significance of the price of the
proportion (p). In the control group with the addition
of dose of macrophage 1x10
5
, 2x10
5
, and 3x10
5
, p
value = 0.4624 was higher than 0.05 (p> 0,05) so it
showed no significant difference.
4 DISCUSSION
Granulomas create a balance between both
preventing the spread of infection in the host and the
protection of Mycobacterium tuberculosis from
immune reactions (Majeed, Mir, and Sharma, 2015).
The advantage of this study is to know the effect of
dose of macrophage on the level of IL-6 secretion so
as to explain the early phase macrophage mechanism
on host cells infected by Mtb. It was also observed
that granulomas from the early stages of formation,
clustered from granuloma aggregation to rupture,
were seen based on the amount of secretion of IL-6
cytokine. The addition of the expected macrophage
dose suggests adding to the availability of
macrophages as a therapeutic strategy for
tuberculosis (Randolph 2015). The ability of Mtb
through the early secreted antigenic target of 6 kDa
(ESAT-6) is capable of replicating active Mtb to
modulate the number of cells secreted through the
mycobacterial type VII secretion system (ESX-1)
(V. R. Parasa et al., 2014).
The ability of Mtb to secrete ESAT-6 plays an
important role in inducing aggregate aggregation of
monocyte and macrophage cells from early stage
granuloma formation. This is in line with
observations made in the in vivo zebrafish embryo
that transparency permits a picture of the life of
neutrophil cells against granuloma formation (Je et
al., 2016). IL-6 is involved in the differentiation of
macrophage and cytotoxic T cells. IL-12 induces
IFN-γ to differentiate CD4 + T cells on Th1
effectors. Cytokines can also direct neutrophils,
monocytes, lymphocytes to CD4 + and CD8 + T-
cell-inducing infections to strengthen macrophage
antimicrobial capacity. ESAT-6 and culture filtrate
protein 10 (CFP-10) in Mycobacterium tuberculosis
are among the candidates of the Tb vaccine because
they induce immune-strong T cells in animal models
so that some previous studies raised extraordinary
problems related to ESAT-6/ CFP-10 candidates as
an effective vaccine against Tb (Abebe et al., 2017).
Using a model for studying human-like human
papillomavirus granulomas is important because: (1)
it is difficult to directly study human lung biopsy
specimens due to access limitations; (2) biopsy
specimens only show static images and should
foresee various possibilities for understanding
dynamic process in granuloma and (3) M.
tuberculosis has no natural host other than humans
so it takes an in vitro granuloma model to study the
initial steps of granuloma formation and treatment,
and has the potential to address more of the
translational aspects of human M. tuberculosis
infection. These in vitro models are less similar in
structures such as the lung and micro-tissue
environment (Guirado and Schlesinger, 2013). This
granuloma model has the potential to provide insight
into host-pathogen interactions at different stages of
human granuloma Tb formation (Kapoor et al., 2013
granuloma Tb manusia (Kapoor et al., 2013).
ACKNOWLEDGEMENTS
The authors would like to thank the technicians of
the Stem Cell Research Centre and Tuberculosis and
Leprosy Laboratory of Tropical Diseases (ITD) of
Airlangga University and all those who have assisted
in the completion of this research.
REFERENCES
Abebe, F., M. Belay, M. Legesse, A. Mihret, and K. S.
Franken. 2017. “Association of ESAT-6/CFP-10-
Induced IFN-Γ, TNF-α and IL-10 with Clinical
Tuberculosis: Evidence from Cohorts of Pulmonary
Tuberculosis Patients, Household Contacts and
Community Controls in an Endemic Setting.” Clinical
and Experimental Immunology 189 (2): 241–49.
doi:10.1111/cei.12972.
Fitzgerald, Liam E, Naiara Abendaño, Ramon A Juste, and
Marta Alonso-hearn. 2014. “Three-Dimensional In
Vitro Models of Granuloma to and Resuscitation of
Dormant Mycobacteria” 2014.
Guirado, Evelyn, and Larry S. Schlesinger. 2013.
“Modeling the Mycobacterium Tuberculosis
ICPS 2018 - 2nd International Conference Postgraduate School
310

Granuloma - the Critical Battlefield in Host Immunity
and Disease.” Frontiers in Immunology 4 (APR): 1–7.
doi:10.3389/fimmu.2013.00098.
Je, Sungmo, Hailian Quan, Yirang Na, Sang-nae Cho,
Bum-joon Kim, and Seung Hyeok Seok. 2016. “An in
Vitro Model of Granuloma-like Cell Aggregates
Substantiates Early Host Immune Responses against
Mycobacterium Massiliense Infection,” 1118–27.
doi:10.1242/bio.019315.
Kapoor, Nidhi, Santosh Pawar, Tatiana D Sirakova,
Chirajyoti Deb, William L Warren, and Pappachan E
Kolattukudy. 2013. “Human Granuloma In Vitro
Model , for TB Dormancy and Resuscitation” 8 (1).
doi:10.1371/journal.pone.0053657.
Kesehatan, Kementerian, and Republik Indonesia. 2017.
“Penanggulangan Tb Kini Lebih Baik,” no. 1990: 7–8.
Majeed, Shahnawaz, Shabir Ahmad Mir, and Sadhna
Sharma. 2015. “Dual Role of Inflammation in
Prognosis and Prevention of Tuberculosis” 6 (1): 1–9.
doi:10.4172/2155-9899.1000298.
Murugesan V.S. Rajaram, Bin Ni, Claire E. Dodd, and
Larry S. Schlesinger. 2015. “Macrophage
Immunoregulatory Pathways In Tuberculosis” 26 (6):
471–85.
doi:10.1016/j.smim.2014.09.010.Macrophage.
Orme, Ian M, and Randall J Basaraba. 2014. “Seminars in
Immunology The Formation of the Granuloma in
Tuberculosis Infection.” Seminars in Immunology 26
(6). Elsevier Ltd: 601–9.
doi:10.1016/j.smim.2014.09.009.
Parasa, V. R., M. J. Rahman, A. T. Ngyuen Hoang, M.
Svensson, S. Brighenti, and M. Lerm. 2014.
“Modeling Mycobacterium Tuberculosis Early
Granuloma Formation in Experimental Human Lung
Tissue.” Disease Models & Mechanisms 7 (2): 281–
88. doi:10.1242/dmm.013854.
Parasa, Venkata Ramanarao, Muhammad Jubayer
Rahman, Anh Thu, Ngyuen Hoang, Susanna
Brighenti, Maria Lerm, Venkata Ramanarao Parasa, et
al. 2014. “Modeling Mycobacterium Tuberculosis
Early Granuloma Formation in Experimental Human
Lung Tissue Modeling Mycobacterium Tuberculosis
Early Granuloma Formation in Experimental Human
Lung Tissue,” no. 7: 281–88.
doi:10.1242/dmm.013854.
Randolph, Gwendalyn J. 2015. “Previews Macrophage
Supply and Demand at the Core of the Necrotic
Granuloma.” Cell Host and Microbe 18 (1). Elsevier
Inc.: 3–4. doi:10.1016/j.chom.2015.06.014.
Romero-Adrian, Tania Beatriz. 2015. “Role of Cytokines
and Other Factors Involved in the Mycobacterium
Tuberculosis Infection.” World Journal of
Immunology 5 (1): 16. doi:10.5411/wji.v5.i1.16.
The Effect of Macrophage Dose to Secretion Interleukin 6 (IL-6) on Model of Tuberculosis Granuloma In Vitro
311
