
Isolation and Detection of Escherichia Coli Trimethoprim Resistance
Gene from Layer Chickens in East Java Province, Indonesia by
Polymerase Chain Reaction
Emy Koestanti Sabdoningrum
1
, Sri Hidanah
1
, Sri Chusniati
2
, Wiwik Misaco
3
,
Retno Sri Wahyuni
4
,
Laras Retno Kinasih Harianto
5
1
Department of Animal Husbandry, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya Indonesia
2
Department of Veterinary Microbiology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya Indonesia
3
Department of Clinic, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya Indonesia
4
Department of Veterinary Basic Medicine, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya Indonesia
5
Student, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya Indonesia
Keywords: Escherichia coli, Trimethoprim resistance gene, Layer chicken, Polymerase Chain Reaction.
Abstract: Aim: The aim of this research was to detect the presence of a gene which is responsible for Escherichia coli
trimethoprim resistance (TEM gene) using Polymerase Chain Reaction from layer chickens in East Java
Province, Indonesia. Methods: Purposive sampling of 60 infundibulums has been done in laying chicken
farms in those indicating Colibacillosis from 6 districts in East Java Province, Indonesia including Sidoarjo,
Mojokerto, Jombang, Kediri, Bojonegoro and Blitar. Ten samples were collected from each district.
Samples were isolated using Eosin Methylene Blue Agar and five biochemical tests of Sulfide Indol
Motility (SIM), Simons Citrate Agar (SCA), Triple Sugar Iron Agar (TSIA), Urea Agar (UA) and Sugar
Test were used for bacteria identification. The DNA was then isolated from Escherichia coli-positive
samples prior to Polymerase Chain Reaction (PCR) amplification to investigate TEM gene that indicated
trimethoprim antibiotic resistance. Result: Bacteriological tests showed that 30 of 60 samples (50%) were E.
coli positive. Ten of those were from Blitar, 10 from Bojonegoro, 5 from Sidoarjo, and 5 from Jombang.
Two samples from Kediri and Mojokerto districts were negative. From PCR amplification showed that 28
of 30 samples were negative due to TEM gene, and there were 2 positive samples shown from Bojonegoro
and Blitar. This indicated that 6.66% of total samples were positive containing TEM gene. Conclusion:
Based on bacteriological examination 50% of samples from six districts in East Java, Indonesia were E. coli
positive, and 6.66% were trimethoprim resistant based on occurrence of TEM gen in PCR amplification.
1 INTRODUCTION
Avian colibacillosis is an infectious avian disease of
caused by Escherichia coli infection and is
responsible for significant economic losses in the
poultry industry (Matsuda et al., 2010). Avian
Pathogens Escherichia coli (APEC) are increasingly
encountered in the field and its relationship to
various conditions of the disease, either as a primary
pathogen or as a secondary pathogen and may be
infectious to humans or zoonotic. Chicken
colibacillosis is characterized in acute form by
septicemia resulting in death and subacute form by
pericarditis, air sacculitis and perihepatitis. Avian
colibacillosis has been recognized as a major
contagious disease of all ages. Avian Pathogenic
Escherichia coli (APEC), spreads to various internal
organs and causes colibacillosis characterized by
systemic fatal disease. The diseases that are infected
by E. coli enter the host through ingestion or
inhalation, after which it trans-locates across
mucosal layers, then colonizes in other tissue via
bloodstream, it can cause air sacculitis, enteritis,
arthritis, panoptalmitis, reproduction genital
infections, bursitis stenalis (Krisnaningsih, 2005),
and in layers can cause salphingitis, omphalitis,
misshapen, pedunculated eggs and egg peritonitis
(Anyanwu, 2014).
Treatment with many antibiotics causes
resistance. In the use of antibiotics in APEC it is
292
Koestanti Sabdoningrum, E., Hidanah, S., Chusniati, S., Misaco, W., Sri Wahyuni, R. and Retno Kinasih Harianto, L.
Isolation and Detection of Escherichia Coli Trimethoprim Resistance Gene from Layer Chickens in East Java Province, Indonesia by Polymerase Chain Reaction.
DOI: 10.5220/0007541602920300
In Proceedings of the 2nd International Conference Postgraduate School (ICPS 2018), pages 292-300
ISBN: 978-989-758-348-3
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

important to note the different sensitivity of E. coli
serotype because some serotypes were resistant to
some antibiotics. The development of resistance
properties of E. coli bacteria is a serious problem
today especially related to the treatment and
handling of some diseases caused by E. coli, so it is
necessary to do a safe antibiotic replacement. The
use of inappropriate antibiotics with no benefit to
therapy is a feed stimulator or growth promoter for
livestock and poultry is one cause of loss of
antibiotic effectiveness. It can cause disruption of
normal microbial ecology balance and eliminate a
group of sensitive bacteria while a group of resistant
bacteria will grow and well developed becomes a
pathogenic population (Dibner & Richards, 2005).
Trimethoprim is a potent broad-spectrum
antibacterial agent with a slow bactericidal action
against susceptible bacterial infections (gram-
negative and gram-positive). It acts by inhibiting the
enzyme dihydrofolate reductase, which is
responsible for the conversion of folate to folinate
and reduces their pools of folinic acid that is
required as a co-factor (Papich, 2016). Nowadays,
there is widespread antibiotic resistance in the
poultry industry and can lead to reconsideration of
antibiotic use, especially trimethoprim which has
been widely used primarily by combining with the
sulfonamide group. Bacteria may become resistant
to trimethoprim by the following: reduced bacterial
uptake, alterations or mutations in dihydrofolate
reductase, overproduction of dihydrofolate reductase
(Kester, et al., 2012).
The aim of this research was to detect the
presence of trimethoprim antibiotic resistance gene
in isolated samples taken from layer chicken farms
from East Java Province, Indonesia using the latest
method of matching genes using PCR.
2 MATERIALS AND METHODS
The survey areas were the layer chicken farms
owned by breeder farmers in East Java Province,
Indonesia, at Sidoarjo, Mojokerto, Jombang, Kediri,
Bojonegoro and Blitar districts.
2.1 Sampling Areas
Ten samples of infundibulum were taken from each
district indicating colibacillosis and the total number
of samples was 60 samples.
2.2 Isolation of Samples
Samples were taken from layer chicken’s
infundibulum and then isolated using Eosin
Methylene Blue Agar medium and five biochemical
tests of Sulfide Indol Motility (SIM), Simons Citrate
Agar (SCA), Triple Sugar Iron Agar (TSIA), Urea
Agar (UA) and Sugar Test were used for bacteria
identification. Positive results of E. coli gained from
biochemical tests showed that SIM had a cloudy
streak area and a red ring on top of the medium,
TSIA medium showed A/A, H
2
S (-), and gas (+),
Urea Agar changed colour from pink to orange. On
Sugar Test almost all of the sugar was fermented
except mannitol, and for SCA Medium did not
change into any colour.
2.3 DNA Isolation
After biochemical test showed positive results, E.
coli was inoculated in Nutrient Agar to avoid any
disturbing colours as PCR processes take place from
EMBA medium. These were the following steps for
the extraction of DNA:
Took 20 μl QIAGEN Protease (or proteinase K)
using pipet into 1,5 ml micro-centrifuge tube. Add
180 μl ATL Buffer, then add 2-3 bacteria colony
from Nutrient Agar and 5 μl Lysozim. Incubation at
60°C for 30 minutes. Add 200 μl AL buffer into the
sample, then vortex for 15 seconds. Add 200 μl
ethanol 96% and mix up with vortex for 15 seconds,
then spin down. Put into QIAamp Mini spin column
(2 ml collection tube) compound from step before.
Centrifuge at 8,000 rpm for 1 minute. Throw 2 ml
filtrate from collection tube and then replaced tube
with the new one (new 2 ml collection tube). Add
500 μl AW1 Buffer, then centrifuge at 8,000 rpm for
1 minute. Throw 2 ml filtrate from collection tube
and then replace tube with the new one (new 2 ml
collection tube). Add 500 μl AW2 Buffer, then
centrifuge at 13,000 rpm for 3 minutes. Throw 2 ml
filtrate from collection tube and then replace tube
with the new 2 ml collection tube. Centrifuge again
at 13,000 rpm for 1 minute. Move QIAamp Mini
spin column to 1.5 ml micro-centrifuge tube. Add 50
μl AE Buffer or distilled water. Incubation at room
temperature (15-25°C) for 1 minute, then centrifuge
at 8,000 rpm for 1 minute. Then obtain 50 μl DNA
template.
Isolation and Detection of Escherichia Coli Trimethoprim Resistance Gene from Layer Chickens in East Java Province, Indonesia by
Polymerase Chain Reaction
293
2.4 Polymerase Chain Reaction (PCR) and
Electrophoresis
Materials for Polymerase Chain Reaction running
were as follows: 20 μl QIAGEN Protease
(proteinase K), 180 μl Buffer ATL, 5 μl Lysozyme,
200 μl buffer AL, 200 μl ethanol 96%, 500 μl Buffer
AW1, 500 μl Buffer AW2, 50 μl Buffer AE, 2x PCR
Master mix (Intron), 1µl (50Pmol/µl) of TEM F3
(the sequence of primer pair is GTA TCC GCT CAT
GGA GAC AAT AAC CCT G) and TEM R3 (the
sequence of primer pair is CCA ATG CTT AAT
CAG TGG AGG CAC C) primer, and 5µl of DNA
template, agarose gel 1.5%.
Amplification of bacterial DNA was performed
with a volume of 20 µl as follows: 12.5 µl of 2X
master mix (Intron), 0.5µl of distilled water, 1 µl of
TEM F3 primer, 1 µl of TEM R3 primer, and 5 µl of
DNA template. Polymerase chain reaction steps are
Pre-Denaturation at 94
0
C for 5 minutes, then
Denaturation at 94
0
C for 1 minute; Annealing at
53
0
C for 30 seconds for 30 times; Extension at 72
0
C
for 1 minute, these 3 steps are repeated for 35 cycles
and last Final Extension at 72
0
C for 5 minutes.
PCR products are electrophoresed on a 1.5%
agarose gel then running for 30 minutes. A digital
image of the gel is captured in a computer, and the
amplification patterns were evaluated by visual
examination of inverted gel pictures.
3 RESULTS
The survey showed that layer chickens in Sidoarjo,
Mojokerto, Jombang, Kediri, Bojonegoro and Blitar
district indicating colibacillosis showed by number
of cases found in the field and from the checking of
post-mortem showed symptoms of colibacillosis.
There were 10 layer chickens obtained from each
district. The bacteriological test showed that 10
samples from Blitar, 10 samples from Bojonegoro, 5
samples from Sidoarjo, and 5 samples from
Jombang were E. coli positive. Samples from Kediri
and Mojokerto were negative.
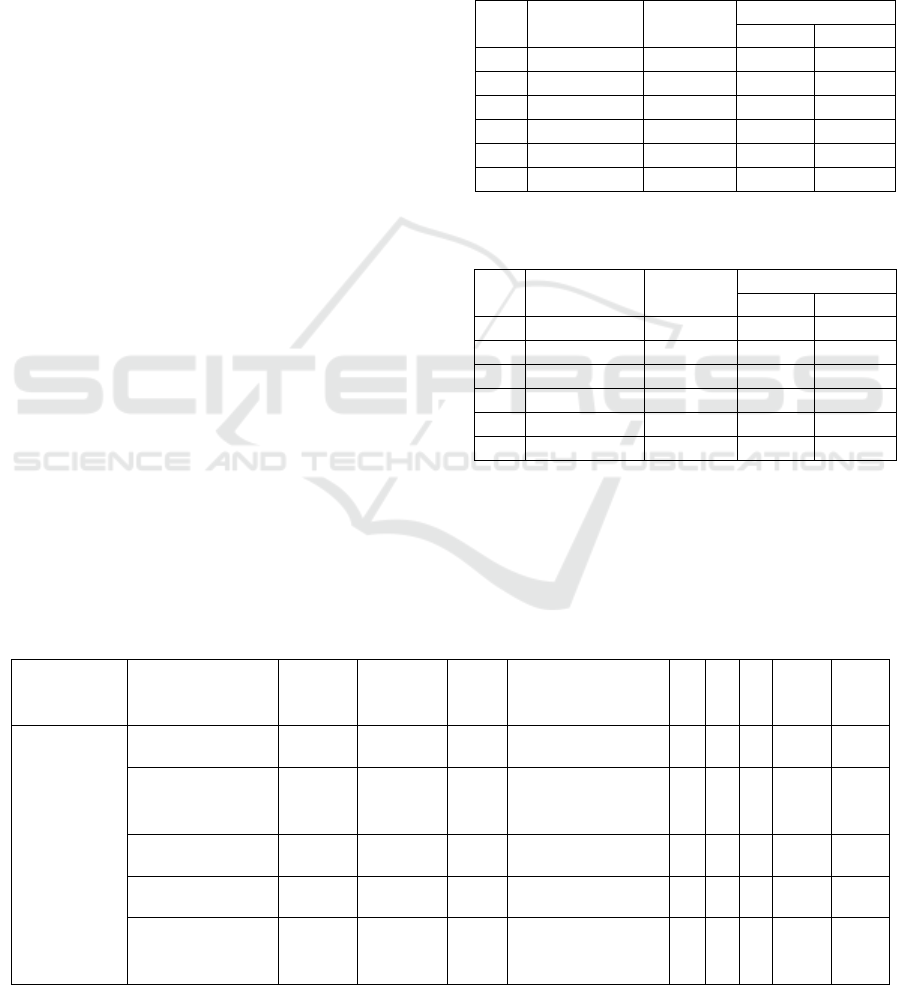
Table 1 : Result of biochemical test for E. coli
No. District
Samples
Amount
Result
Positive Negative
1. Sidoar
j
o 10 5 5
2. Mo
j
okerto 10 0 10
3. Jomban
g
10 5 5
4. Kediri 10 0 10
5. Bo
j
one
g
oro 10 10 0
6. Blitar 10 10 0
Table 2. Detection of TEM gene in samples from East
Java Province, Indonesia
No. District
Samples
Amount
Result
Positive Negative
1. Sidoar
j
o5 0 5
2. Mo
j
okerto 0 0 0
3. Jomban
g
50 5
4. Kediri 0 0 0
5. Bo
j
one
g
oro 10 1 9
6. Blitar 10 1 9
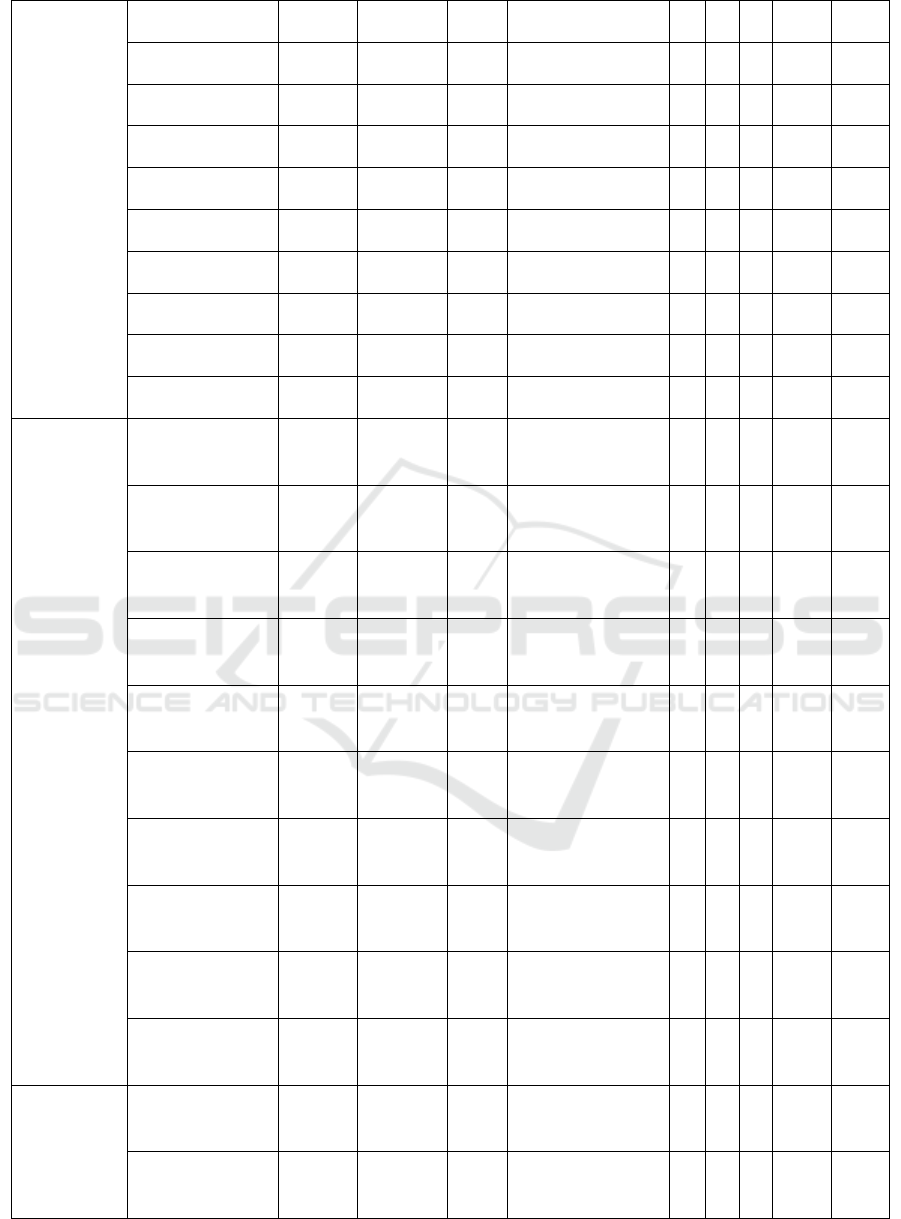
Table 3 : Results of isolation and identification for E. coli using bacteriological tests
Area
Number of
Sample
EMBA SIM SCA TSIA G L S Malt Man
Sidoarjo
1 - - - - - - - - -
2 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
3 - - - - - - - - -
4 - - - - - - - - -
5 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
ICPS 2018 - 2nd International Conference Postgraduate School
294

6 - - - - - - - - -
7 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
8 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
9 - - - - - - - - -
10 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
Mojokerto
1 - - - - - - - - -
2 - - - - - - - - -
3 - - - - - - - - -
4 - - - - - - - - -
5 - - - - - - - - -
6 - - - - - - - - -
7 - - - - - - - - -
8 - - - - - - - - -
9 - - - - - - - - -
10 - - - - - - - - -
Jombang
1 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
2 - - - - - - - - -
3 - - - - - - - - -
4 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
5 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
6 - - - - - - - - -
7 - - - - - - - - -
8 - - - - - - - - -
9 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
10 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
Isolation and Detection of Escherichia Coli Trimethoprim Resistance Gene from Layer Chickens in East Java Province, Indonesia by
Polymerase Chain Reaction
295
Kediri
1 - - - - - - - - -
2 - - - - - - - - -
3 - - - - - - - - -
4 - - - - - - - - -
5 - - - - - - - - -
6 - - - - - - - - -
7 - - - - - - - - -
8 - - - - - - - - -
9 - - - - - - - - -
10 - - - - - - - - -
Bojonegoro
1 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
2 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
3 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
4 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
5 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
6 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
7 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
8 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
9 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
10 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
Blitar
1 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
2 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
ICPS 2018 - 2nd International Conference Postgraduate School
296
3 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
4 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
5 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
6 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
7 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
8 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
9 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
10 + +/motile -
A/A, H
2
S (-) gas
(+)
+ + + + -
4 DISCUSSION
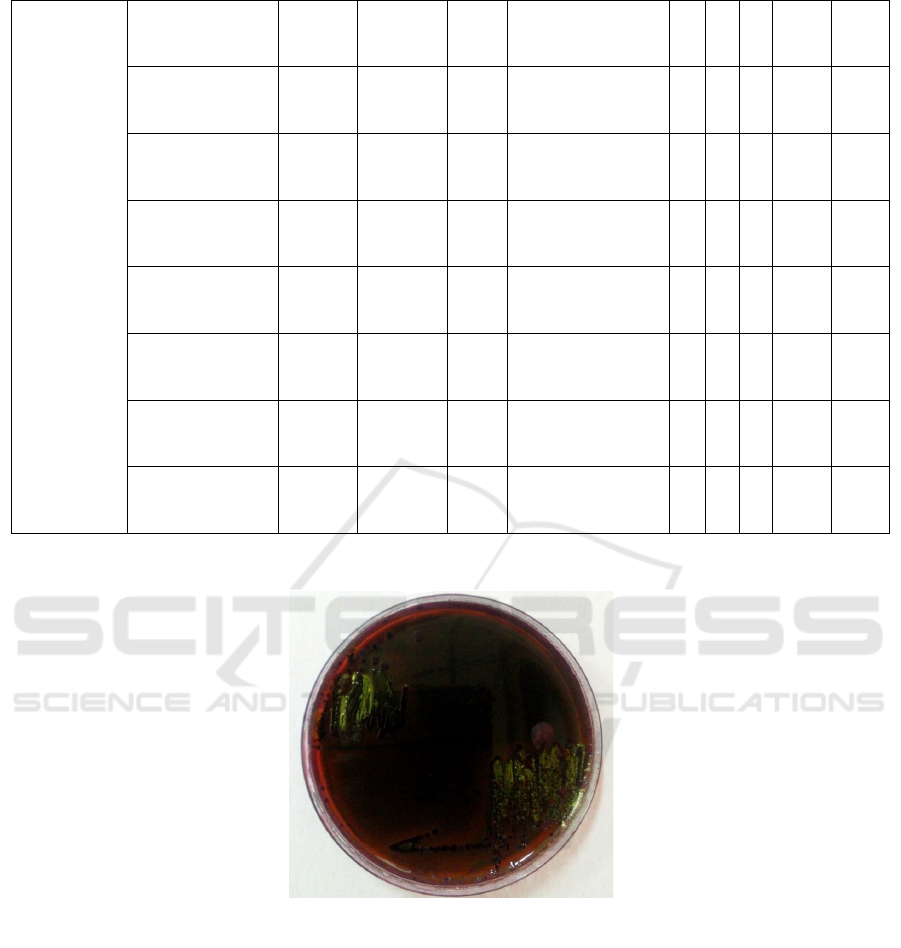
Colony of E. coli on EMBA medium showed
metallic green colour with uniform colony, with
circular colony shape, rough surface, low convex
elevation, and erose edges. EMBA medium is a
selective and differential medium for gram-negative
bacteria coliform containing eosin and methylene
blue which is an indicator of pH and positive gram
bacterial inhibitors. In addition, EMBA media can
distinguish coliform bacteria as normal flora and E.
coli in the presence of lactose and sucrose indicator.
All sugar tests resulted in yellow colour of the
medium due to the fermentation of sugar which
caused the pH of the media to turn into acid. In this
research, the result shows that no changing colour of
SCA medium indicated that E. coli bacteria did not
use citrate as carbon sources.
Figure 1 : Colony of E. coli on EMBA medium
Isolation and Detection of Escherichia Coli Trimethoprim Resistance Gene from Layer Chickens in East Java Province, Indonesia by
Polymerase Chain Reaction
297
In the SIM media, indol test aims to identify the
ability of bacteria to produce indole by using
tryptophanase enzyme (Leboffe, 2011). In this
research, observations on the SIM media found a
cloud clump around the streak area. This prompts
the movement of bacteria that grow around the
streak area. The movement of the bacteria due to
semisolid media (motility test) is designed by
reducing the concentration of agar to the media that
is about 0.4% on the medium which is only
sufficient to maintain its shape while allowing the
movement of bacteria (Leboffe, 2011).
Urease test is useful for identifying organisms
capable of hydrolyzing urea which can produce
ammonia and carbon dioxide, especially to know if
the microorganisms have urease enzyme or not.
Urease is a constitutive enzyme that hydrolyzes urea
into carbon dioxide and ammonia. In this research,
Urea Agar used was not pink, but a little orange in
colour and from the observational result the agar
media did not change in colour.
In this research, results from observations for the
TSIA test showed A/A with negative H
2
S and
presence of gas. The yellow colour of the entire
medium is due to the fermentation of carbohydrates
and will bring up the gas as a gap in the media or
will lift the agar from the bottom of the tube
(Leboffe, 2011).
Using the PCR method can give faster, cheaper
and more efficient results. If using another method
such as dilution with MIC (Minimum Inhibitory
Concentration) and MBC (Minimum Bacteriosid
concentration) that takes longer time to see the
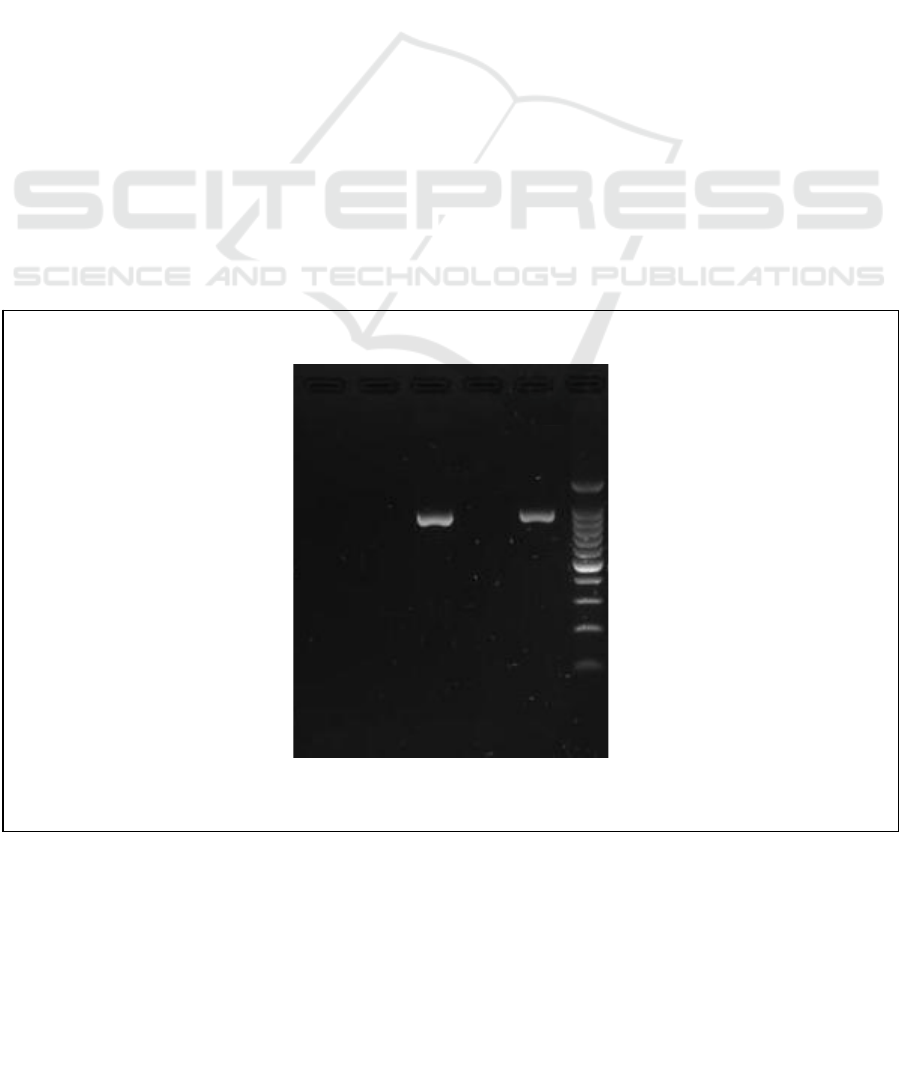
result. Based on the results of resistance test
antibiotic trimethoprim using PCR it can be seen
that the results of the Bojonegoro and Blitar districts
show positive antibiotic resistance trimethoprim.
This is indicated by the emergence of bands on
electrophoresis results at 861 bp. The presence of
bands that appear on the results indicates that there
are bacteria that have gene matches with the
existing primary pieces. This means that the bacteria
have adapted from the previous environment so that
it has a gene that can withstand the sensitivity of the
trimethoprim antibiotic that matches the existing
primer. With the emergence of this band, it can be
concluded that bacteria found in Bojonegoro and
Blitar districts have genetic compatibility with the
primary TEM gene, which indicates antibiotic
resistance to trimethoprim in Escherichia coli.
The resistance rate of trimethoprim among
Escherichia coli from layer chickens in East Java,
Indonesia was 6.66% based on the result of this
research. The resistance occurs because bacteria
produce enzyme decomposers antibiotics so that
antibiotics become inactive. These bacteria encode
genes that produce enzymes that break down
antibiotic molecules before they kill bacteria. An
example is the lactamase beta enzyme, this enzyme
will describe the beta structure of lactam in
antibiotics, so antibiotics become inactive again and
cannot kill bacteria. It reduces the accumulation of
C- E4 E5 E6 E7 M
Figure 3 : Trimethoprim Resistance by Polymerase Chain Reaction Result
Explanation:
Target band 861 bp, 2 positive
samples
M : Marker DNA 100bp
C- : Negative control
E4 : Sidoarjo
E5 : Bojonegoro
E6 : Jombang
E7 : Blitar
ICPS 2018 - 2nd International Conference Postgraduate School
298

intracellular antibiotics by decreasing permeability
and/or enhancing the active efflux of antibiotics.
The efflux mechanism occurs when a resistant gene
encodes a protein that actively pushes antibiotics out
of a bacterial cell, resulting in low levels of
antibiotics in the cell and inability to kill bacteria.
5 CONCLUSION
Based on biochemical examination, 50% of samples
from six districts in East Java, Indonesia were
Escherichia coli positive, and 6.66% were
trimethoprim resistant.
REFERENCES
Anyanwu, M. U, et. al. 2014. Case Report of
Misdiagnosis of Avian ColibacillosisIn Laying Birds.
Animal Research International (2014) 11(2): 1998-
2003.
Barnes HJ and Yoder HW. 1984. Disease of Poultry. 8
th
ed. Iowa State University Press. 692-708.
Bettelheim K. A and L. Beutin, 2003. Rapid
laboratory identification and characterization of
verocytotoxigenic (Shiga toxin producing)
Escherichia coli (VTEC/STEC). Journal of Applied
Microbiology. 95: 2: 205-217
Bettelheim KA, 1996. Enterohaemorrhagic Escherichia
coli: a new problem, an old group of organisms. Aust
Vet J 73:20-26
Bettelheim KA, Beutin L, 2003. Rapid Laboratory
Identification and Characterization of
Verocytotoxigenic (Shiga toxin producing )
Escherichia coli (VTEC/STEC). J App Microbiol
95:205-217
Beutin L, Montenegro MA, Ørskov I, Ørskov F, Prada J,
Zimmermann S, Stephan R, 1989. Close association
of verocytotoxin (Shiga-like toxin) production with
enterohemolysin production in strains of Escherichia
coli. J Clin Microbiol 27:2559-2564
Brooks GF, Butel JS, Morse SA. 2005. Medical
Microbiology. McGraw-Hills Companies Inc.
Dibner, J. J & J. D. Richards. 2005. Antibiotics Growth
Promoters in Agriculture: history and mode of action.
Poult. Sci. 84:634-643
Elfahmi. 2006. Phytochemical and biosynthetic studies of
Lignans, with a focus on Indonesian medicinal plants
(dissertation). University of Groningen. 2006.
Gordon, R.F.1997. Poultry Disease. Isted. Bailliere
Tindall and Cox. London.
Gross, T., J. Faull., S. Ketteridge., D. Springham. 1995.
Introductory Microbiology. Chapman and Hall. Great
Britain. 51.
Harborne, J. 1987. Metode Fitokimia, Edisi Kedua. ITB.
Bandung.
Hayashi T, Makino K, Ohnishi M, Kurokawa K, Ishii K,
Yokoyama K, Han CG, Ohtsubo E, Nakayama K,
Murata T, Tanaka M, Tobe T, Iida T, Takami H,
Honda T, Sasakawa C, Ogasawara N, Yasunaga T,
Kuhara S, Shiba T, Hattori M, Shinagawa H, 2001.
Complete genome sequence of enterohemorrhagic
Escherichia coli O157:H7 and genomic comparison
with a laboratory strain K-12. DNA Res. 8:11-22
Kardinan A. dan Kusuma FR. 2004. Meniran penambah
daya tahan tubuh alami. Jakarta: Agromedia Pustaka,
2004; 6-18.
Kester, Mark., K.D. Karpa, K.E. Vrana. 2012. Elsevier’s
Integrated Review: Pharmacology. Second edition.
Philadelphia. Book AID International. Sabre
Foundation.
Krisnaningsih, M.M.F., Widya A., M. H. Wibowo. 2005.
UjiSensitivitasIsolatEscherichia coli Patogen pada
Ayam Terhadap Beberapa Jenis Antibiotik. J. Sain
Vet. Vol. 1.
Leboffe, M. and Pierce, B. (2011) A Photographic Atlas
for the Microbiology Laboratory. 4th Edition, Morton,
Englewood.
Ma’at, S.1997. Phylanthus niruri L Sebagai
Immunostimulator Pada Mencit. Disertasi Program
Sarjana. Universitas Airlangga. Surabaya. Hal: 13-
174.
Madigan, M.T , Martinko, M.J , Parker. J. 2002. Brock
Biology of Microorganism. 10
th
Edition. Practice
Hall. USA. 375-378
Mathivanan R, Edwin SC, Amutha R and Viswanathan K.
2006. Panchagavya and Andrographis Panicuata as
Alternative to Antibiotic Growth Promoter on Broiler
Production and Carcass Characteristic. India.
Departement of Poultry Science, Veterinary College
and Research Institute. Namakkal-637001.
Matsuda, K., Atul A. C., John Hwa L. 2010. Avian
Colibacillosis caused by an intestinal pathogenic
Escherichia coli isolate from calf diarrhea. Research
in Veterinary Science 89: 150-152.
Matsuda, K., Atul A. C., John Hwa L. 2010. Avian
Colibacillosis caused by an intestinal pathogenic
Escherichia coli isolate from calf diarrhea. Research
in Veterinary Science 89: 150-152.
Nataro JP, Kaper JB, 1998. Diarrheagenic Escherichia
coli. Clin Microbiol Rev 11:142-201
Pelczar, J. Michael dan chan, E.C.S. 1988. Dasar-dasar
Mikrobiologi 2. Penerbit UI Press. Jakarta.
Robinson, T. 1995. Kandungan Organik Tumbuhan
Tinggi. Institut Teknologi Bandung, Bandung.
Rohimah. 1997. Identifikasi flavonoid yang Memiliki
Antifungal dari Damar (Hope mangarawan) dan
Shoren eptosula. [Tesis]. FMIPA-IPB. Bogor.
Sunarno. 2007. Efek Phyllanthus Niruri L Pada
Prosentase Neutrofil, Koloni Bakteri Limpa, dan
Histopatologi Hepar Balb/C yang Diinfeksi
Salmonella [Tesis]. Universitas Diponegoro
Semarang.
Tizard, IR. 1996. Veterinary Immunology an Introduction.
Fifth Edition, WB Sounders Company, a Division of
Harcourt Brace and Company. The Curtis Center
Isolation and Detection of Escherichia Coli Trimethoprim Resistance Gene from Layer Chickens in East Java Province, Indonesia by
Polymerase Chain Reaction
299

Independence Square West, Philadelphia,
Pennsylvania.
Tjandrawinata, R.R., S. Maat and D. Noviarny, 2005.
Effect of standardized Phyllanthus niruri extract on
changes in immunologic parameters: correlation
between preclinical and clinical studies. Medika
XXXI (6): 367-371.
Tjay, dan Raharja. 2002, Obat-Obat Penting: Khasiat
Penggunaan dan Efek-Efek Sampingnya, Edisi
Kelima, Cetakan Kedua, Penerbit PT. Alex Media
Komputindo, Jakarta.
Wahyuningsih. 2008. Pengaruh Pemberian Ekstrak Herba
Meniran (Phyllanthus Niruri L.) Terhadap Penurunan
Kadar Asam Urat Darah Tikus Putih Jantan
Hiperurisemia. Lampung.
Widayati, P. 2008. Efek Ekstrak Etanol Herba Meniran
(Phyllanthus niruri L.) Terhadap Penurunan Kadar
Asam Urat Mencit Putih Jantan Galur Balb-C
Hiperurisemia [Skripsi]. Universitas Muhammadiyah
Surakarta.
Wijayakusuma dan Hembing H.M. 2003. Ramuan
Tradisional Untuk Pengobatan Darah Tinggi. Penebar
Swadaya. Jakarta. 64-65.
Willey J.M, Sherwood L.M, Woolverten C.J. 2009.
Prescott’s Principle of Microbiology. McGraw-Hill
Higher Education. New York.
Zhu P, S. Li, C-M. Tang, D. Shelton. 2008. Sensitive and
Rapid Detection of Escherichia coli O157:H7 by
Coupling of Immunomagnetic Separation with
Fluorescence Immunoassay. American Society for
Microbiology
ICPS 2018 - 2nd International Conference Postgraduate School
300
