
Differences Expression of Caspase-3 In Hepar and Spleen of Rattus
norvegicus Infected with Methicillin Resistant Staphylococcus aureus
and Enterococcus faecalis
Sri Wahyuni
1
, Yoes Prijatna Dachlan.
1
and Agung Dwi Wahyu Widodo
2
1
Department of Immunology Postgraduate School, Universitas Airlangga, Surabaya, East Jawa, Indonesia
1
Department of Anatomical Pathology, Faculty of Medicine, Universitas Airlangga,Surabaya, East Jawa, Indonesia
2
Department of Clinical Microbiology, Faculty of Medicine, Universitas Airlangga, Surabaya, East Jawa, Indonesia
Keywords: Caspase-3, Methicillin Resistant Staphylococcus aureus (MRSA), Entrerococcus faecalis
Abstract: Bacteria are organisms that can cause infection. Methicillin Resistant Staphylococcus aureus (MRSA) and
Entrerococcus faecalis are gram-positive bacteria that can cause nosocomial infections. Virulence factors of
MRSA thus play a role in the infection process, such as are polysaccharide, surface proteins such as
adhesins, glycoprotein, hemagglutinin, and fibronectin, while enterococcus such as gelatinase, cytolysin,
enterococcal surface protein (Esp) and aggregation substance (AS) are the virulence factors of Enterococcus
that can cause infection. This research is True Experimental research with post-test only for the Control
Group Design. The MRSA and E. faecalis bacteria were injected intraperitoneally to R. norvegicus and
observed after 24 hours. Animals were placed into three groups: the control group, treatment with MRSA,
and Enterococcus faecalis. The hepar and spleen were isolated from the dead R. norvegicus and a
Immunohistochemistry (IHC) test was conducted to observe the expression of caspase-3 by light
microscope. The result showed that the caspase-3 expression increased in the infected group of MRSA and
Enterococcus faecalis compared with the control group. The expression of caspase-3 in the hepar was
higher than in the spleen. The hepar serves as the receiver of the portal and systemic circulation. The hepar
also plays an important role in the host defense that is exposed to and will catch pathogens, followed by
cleaning. The increased expression of caspase-3 suggests the cell death also increased.
1 INTRODUCTION
Infectious diseases still occupy the top causes of
morbidity and mortality in developing countries
(Triana, 2014). Bacteria is one of the organisms that
can cause infectious diseases (Kaufmann et al.,
2011). The spread of diseases is caused by various
intermediaries, including air, animals, objects,
humans themselves, and even unconsciously, the
hospital becomes a high-risk source of transmission
(Triana, 2014). These bacteria include MRSA
(Sandi, et al., 2015) and Enterococcus faecalis
(Chen and Zervos, 2009).
Increased incidence of the S. aureus infection,
especially MRSA with the phenomenon of antibiotic
resistance, is considered as one of the biggest
barriers to infection control. MRSA is a bacteria that
causes nosocomial infection (Sandi et al., 2015) and
Enterococcus faecalis (Tortora, 2016). Enterococcus
faecalis is a commonly found species of
Enterococcus (Chen and Zervos, 2009). MRSA and
Enterococcus faecalis are gram-positive bacteria that
can cause infections involving the death of cells
(Chen and Zervos, 2009; Sandi et al., 2015).
Apoptosis is the programming of cell death used
to prevent inflammation and limit cell damage
(Martinez, 2017). Increased toxins, produced by
bacteria, will increase apoptosis (Baudouin, 2008).
Apoptosis is also used for homeostasis and
protection against bacterial infections (Upton and
Chan, 2014).
The hepar is an organ that plays an important
role in the defense of the host against the invasion of
microorganisms (Talwani et al., 2013), while the
spleen is the main filter for pathogens and antigens
carried by blood. The spleen is an area of regulation
host immune response to initiate innate and adaptive
immune responses to pathogens and the
establishment of specific antigens in immune
Wahyuni, S., Dachlan, Y. and Wahyu Widodo, A.
Differences Expression of Caspase-3 In Hepar and Spleen of Rattus norvegicus Infected with Methicillin Resistant Staphylococcus aureus and Enterococcus faecalis.
DOI: 10.5220/0007540902530257
In Proceedings of the 2nd International Conference Postgraduate School (ICPS 2018), pages 253-257
ISBN: 978-989-758-348-3
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
253
responses that protect bacterial, viral, and blood-
borne fungal infections (Bronte and Pijtet, 2013).
Caspase is the leading cause of cell death by
dividing cell proteins to disassemble cells that will
die. In the arrangement, it is important that caspase
be maintained to avoid unexpected cell death
(Parrish et al., 2013). Caspase-3 is a member of the
caspase family that plays a role in the execution
phase in the apoptosis (Prakosa et al., 2013).
Caspase-3 is also the best effector caspase and an
apoptotic signal, if there is activation of caspase-3,
there is no cell rescue (Parrish et al., 2013).
2 MATERIALS AND METHOD
2.1 Animal model
The animal model used in the research was the
Rattus novergicus with the criteria of being male,
healthy, age three months, and a body weight of
150–200 grams.
2.2 Bacteria
The bacteria of MRSA and Entrerococcus faecalis
were obtained from the Installation of Clinical
Microbiology RSUD Dr. Soetomo Surabaya. The
concentration was 10
5
CFU bacteria with Phosphate-
buffer saline (PBS).
2.3 Sample
The animal models were placed in three groups,
each consisting of four rats. The groups were
infected with MRSA and Entrerococcus faecalis, as
a control were given PZ (1 ml in the peritoneum of
each rat; the concentration was 10
5
CFU bacteria
with PBS). Observation was performed for 24 hours
post infection. Rats were sacrificed for removal of
the hepars and spleens. Organs were fixed in
formalin buffer and preparation (formalin fixed and
paraffin embedded section were performed) and
Immunohistochemistry for Caspase-3 using a rabbit
and mouse antibody. The expression cells were
observed under a light microscope with an objective
100x in a five field of view. The analysis of data
using was carried out using GraPhad prism.
3 RESULTS
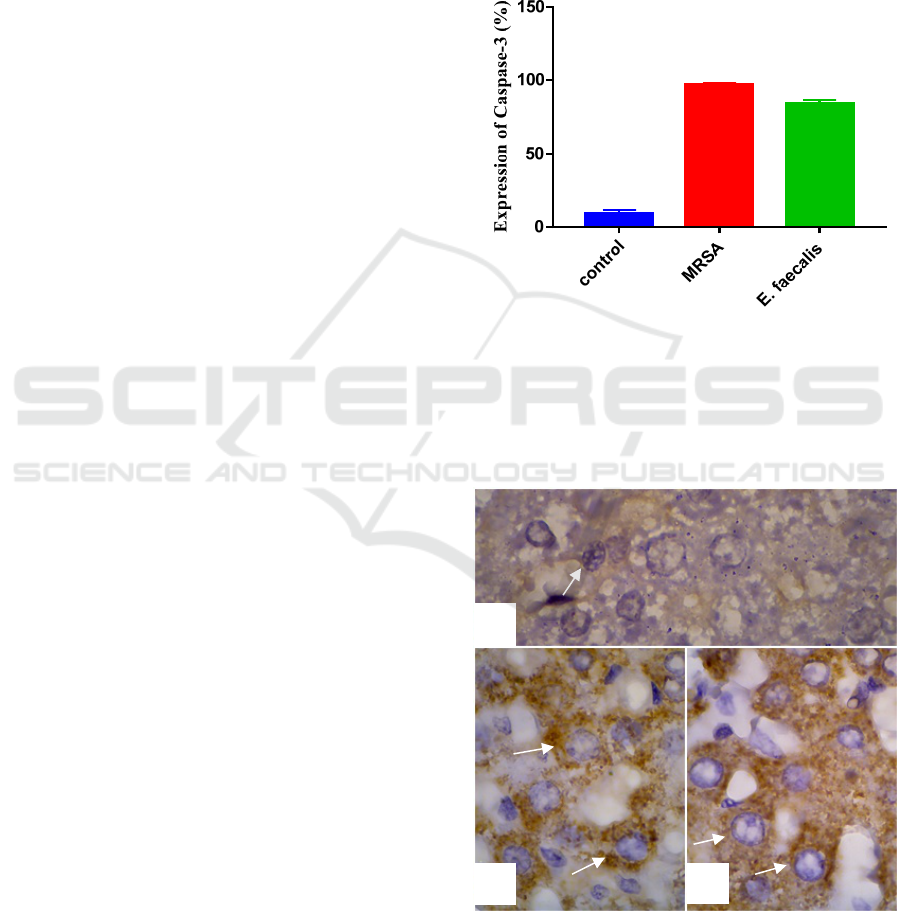
3.1 Expression of Caspace-3 in Hepar
Of the mean number of the cells expressed in the
caspase-3 in hepar, the group infected with MRSA
were higher than the group infected with
Entrerococcus faecalis (Figure 1).
Figure 1 : Bar-graph Expression of Caspase-3 in hepar.
The highest expression of caspase-3 that infected
was the MRSA group (96.75%), than the
Entrerococcus faecalis (84.25%), and finally the
control group (9.5%) (Figure 1).
Figure 2 : Expression of caspase-3 in hepar, (a) control
group of R. norvegicus, (b) Entrerococcus faecalis
group,
(c) MRSA group. Magnification x1000.
a
c
b
ICPS 2018 - 2nd International Conference Postgraduate School
254
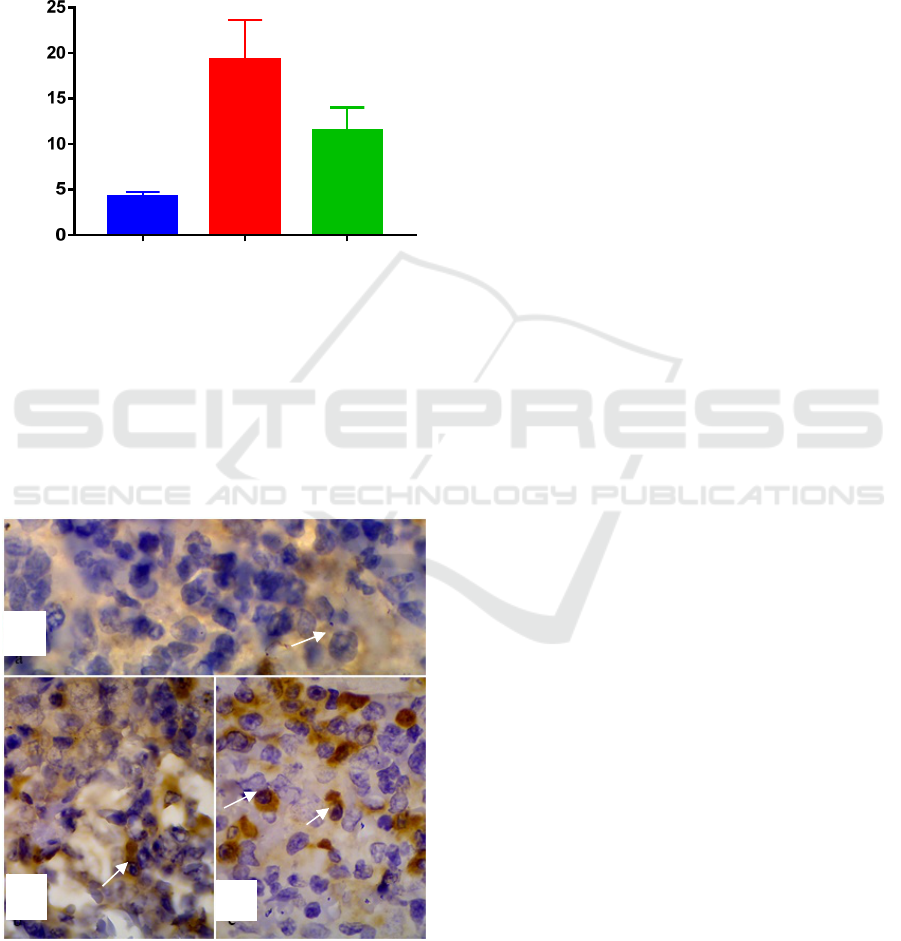
3.2 Expression of caspase-3 in the Spleen
The mean number of cells was expressed by
caspase-3 in the spleen; the group infected with
MRSA were higher than the group infected with
Entrerococcus faecalis (Figure 3).
c
o
ntrol
MRS
A
E. f
a
ecalis
Expression of Caspase-3 (%)
Figure 3 : Bar-graph Expression of Caspase-3 in the
Spleen.
The highest expression of caspase-3 was MRSA
(19.25%) then Enterococcus faecalis (11.5%), and
then the control group (4.25%) (Figure 4).
Figure 4 : Expression of caspase-3 in the spleen, (a)
control group of R. norvegicus, (b) Entrerococcus faecalis
group, (c) MRSA group. Magnification x1000.
4 DISCUSSION
Apoptosis plays a major role in removing infected
cells, mutating (or causing damage during
development), tissue homeostasis, and the effects of
aging. Apoptosis is associated with morphological
and biochemical changes, including the release of
cytochrome c, the activation of protease caspase,
chromatin condensation, and DNA fragmentation
(Jafari et al., 2015).
The virulence of MRSA plays a role in an
infection, such as polysaccharides and surface
proteins (Gordon and Lowy, 2008). The surface
protein is responsible for colonization.
Polysaccharides and protein-A are inhibit
phagocytosis by polymorphonuclear leukocytes. The
enzyme catalase is also a factor that supports the
bacteria for survival inside phagocytic cells (Sandi et
al., 2015). Bacteria produces various virulence
factors that allows them to escape from the body’s
immunity, infecting and spreading to remote organs
(Ramachandran, 2014).
The Virulence of Enterococcus faecalis causes
infections such as gelatinase, enterococcal surface
protein (Esp), aggregation substance (AS) and
cytolysine (Uysal, 2013). Cytolysine can lyse
various target cells; it can lyse erythrocyte cells,
polymorponuclear neutrophils, and macrophages.
Cytolysine may also increase toxicity and
bacteriocin activity for colonization. Other factors,
such as gelatinase may help bacteria to avoid the
immune system (Tyne et al., 2013). Aggregation
substance promotes direct binding, independent
opsonin to polymorphonuklear leukococyte can
survive in different phagocytes. Enterococcal
surface proteins with multiple repetitive motives in
the encoding gene may be important in immune
avoidance. Products that cause direct tissue damage
are cytolysine and gelatinase (Waar, 2004).
Apoptosis can within or outside the host cell
because of stimulation of microbial infections,
oxidative stress, DNA damage or DNA errors or
when the cells have reached the final cycle. Started
enzymes form a cascade signal, send a danger signal
through the caspase initiator and continue to capase-
3 as the executor. The enzyme initiates cell
disassembly by degrading the DNA. The toxins of
bacteria cause inflammation and increased caspase-3
and apoptosis (Wall and Beth, 2014). The molecular
strategies by bacteria to interact with the host can be
unique to a particular pathogen (Wilson et al., 2002).
Hepar is the portal defense for bloodstream
infections. Hepar contains immune cells to bind and
clean pathogens (Talwani et al., 2013). The spleen is
a
b
c
Differences Expression of Caspase-3 In Hepar and Spleen of Rattus norvegicus Infected with Methicillin Resistant Staphylococcus aureus
and Enterococcus faecalis
255

the filter for pathogens and the antigen carried in
blood. Establishment of the immune system to
specific antigens from bacterial, viral, and fungal
infections. The spleen uses the regulation of immune
response (Bronte and Pijttet, 2013) and filters to
remove blood cells due to aging or pathological
changes (Pivkin et al., 2016).
MRSA and Enterococcus are bacteria that can
cause tissue damage especially for hepar. During
infection in the bloodstream, the majority of bacteria
are sequestered immediately by Kupffer cells in the
hepar and increased enzymes in the hepar
(Kolaczkowska et al., 2015). Abnormal enzyme
levels may indicate hepar damage (Giannini et al.,
2005). Response hepar damage to infection is occurs
increased caspase-3 and apoptosis (Kolaczkowska et
al., 2015).
5 CONCLUSIONS
In conclusion, there was an increase of expression
caspase-3 in the hepar and spleens of R. norvegicus
when the group infected with MRSA was higher
than that infected with Enterococcus faecalis.
Increased expression of caspase-3 suggests the cell
death also increased.
ETHICS APPROVAL
All documents for ethics approval as well as
proposal of the research have been reviewed by the
ethics committee of Universitas Airlangga Faculty
of Dental Medicine, as described on the ethical
approval No. 265/HRECC.FODM/X/2017.
REFERENCES
Baudouin, S.V. 2008. Sepsis. Springer: London.
Bronte, V., and Pijttet, M. 2013. The spleen in local and
systemic regulation of immunity. Immunity. November
14; 39(5): 806–818.
doi:10.1016/j.immuni.2013.10.010.
Chen, A.Y., and Zervos, M.J. 2009. Antimicrobial Drug
Resistance Volume 2 Clinical and Epidemiological
Aspects. Humana Press: New York.
Giannini, E.G., Testa, Roberto and Savarino, Vincenzo.
2005. Liver enzyme alteration: a guide for clinicians.
CMAJ. Feb 1; 172(3): 367–379.
doi: 10.1503/cmaj.1040752.
Gordon, R.J., and Lowy, F.D. 2008. Pathogenesis of
methicillin-resistant Staphylococcus aureus infection.
Clin. Infect. Dis., 46: 350-359. Doi: 10.1086/533591.
Jafari, M., Shanaz, S., Seyed, H., Sadraie, Gholamreza, K.,
Majid, N. 2015. The Role of Apoptosis in the Cellular
Response of Liver and Spleen of BALB/c Mice in
Cutaneous Leishmaniasis. J Med Sci March; Vol 40
No 2.
Kaufmann, S.H., Rouse, B.T., Sacks, D.L. 2011. The
immune response to infection. ASM Press American
Society for Microbiology 1752 N Street, N.W.
Washington, DC 20036-2904.
Kolaczkowska, E., Craig, N., Jenne, B.G., Surewaard,
A.T., Woo,Y.L., Maria, J., Kerri, M., Ghislain, O., and
Paul, K. 2015. Molecular mechanisms of NET
formation and degradation revealed by intravital
imaging in the liver vasculature. Nature
Communications (6:6673) DOI:
10.1038/ncomms7673.
Martinez, Jennifer. 2017. Apoptotic and Non-apoptotic
Cell Death. Springer: Tokyo.
Parrish, A.B., Freel, C.D., Kornbluth, S. 2013. Cellular
Mechanisms Controlling Caspase Activation and
Function. Cold Spring Harb Perspect Biol; 5: a008672.
Pivkin, I.V., Zhangli, P., Geong, E.K., Pierre, A.B., Ming,
D., Subra, S. 2016. Consequences for Physiology and
diseases. PNAS. July vol. 113. N. 28.
Prakosa, T., Askandar, B., Fauziah, D. 2013. Ekspresi p53
Mutan dan caspase 3 sebagai Faktor Prediksi terhadap
Operabilitas Kanker Serviks IIB setelah Mendapat
Kemoterapi Neoajuvan. Indonesian Journal of Cancer
Vol. 7, (2).
Ramachandran, G. 2014. Gram-positive and gram-
negative bacterial toxins in sepsis A brief review. pp.
213–218.
Sandi, N.A., Tenri, A.W., Isabel, M., Siti, I.O., Basofi,
A.M., and Asmarani, K. 2015. Staphylococcus aureus
Vaccine Candidate from MRSA Isolates: The Prospect
of a Multivalent Vaccine. American Journal of
Infectious Diseases. Vol 11, issue 3. Pp: 54-62. Doi:
10.3844/ajidsp.2015.54.62.
Talwani, R.., Bruce L.G., and Charles H., 2013. Infectious
Diseases and the Liver. NIH Public Access. May 21
doi:10.1016/j.cld.2010.09.002. Infectious. 15(1), pp.
111–130.
Tortora, G.J., B.R., Funke, dan C.L., Case. 2016.
Microbiology. Twuelfth Edition. US: Pearson
Education, Inc.
Triana, D. 2014. Frekuensi β-Lactamase Hasil
Staphylococcus aureus Secara Iodometri Di
Laboratorium Mikrobiologi Fakultas Kedokteran
Universitas Andalas. Jurnal Gradien Vol. 10 (2) Juli :
992-995.
Tyne, D.V., Martin, M.J., and Gilmore, M.S. 2013.
Structure, Function, and Biology of the Enterococcus
Faecalis Cytolysin. Toxins. 5. 895-
911;doi:10.3390/toxins5050895.
Upton, J.W., and Chan, F.K. 2014. Staying Alive: Cell
Death in Anti-Viral Immunity. Mol Cell. April 24;
54(2): 273–280. doi:10.1016/j.molcel.2014.01.027.
ICPS 2018 - 2nd International Conference Postgraduate School
256

Uysal, H., Ciftci, G., Cifti, H. 2013. Determination of
antigenic glycoproteins of Enterococcus faecalis
strains distinguished with aggregation substance,
gelatinase and cytolysine. Revue Méd. Vét., 2013, 164,
7, 374-381.
Waar, Karola. 2004. Pathogenesis of nosocomial
infections with Enterococcus faecalis. Universsity of
Groningen.
Wall, D.M., and Beth, A.M. 2014. Bacterial Secreated
Effectors and Caspase-3 Interactions. 16(2). 1746-
1756. Doi: 10.1111/cmi.12368.
Wilson, J.M., Schurr, C., LeBlanc, Ramamurthy, R.,
Buchanan, K., and Nickerson, C., 2002. Mechanisms
of bacterial pathogenicity. PMC. Apr;78(918) pp.
216–225. Doi: 101136/pmj.78.918.216.
Differences Expression of Caspase-3 In Hepar and Spleen of Rattus norvegicus Infected with Methicillin Resistant Staphylococcus aureus
and Enterococcus faecalis
257
