
Synthesis and Electrochemical Performance of Keggin-type
Polyoxometalate Gels
Q Y Wu
*
, W S Dai, S B Cui and F W He
School of Biomedical and Chemical Engineering, Liaoning Institute of Science and
Technology, Benxi 117004, Liaoning, China
Corresponding author and e-mail: Q Y Wu, qywu@lnist.edu.cn
Abstract. Three Keggin-type vanadium-substituted polyoxo metalate gels, [PyPS]
6
PW
9
V
3
O
40
,
[PyPS]
4
PW
11
VO
40
and [PyPS]
4
PMo
11
VO
40
have been synthesized and characterizated. The
relationship between the component elements of the vanadium-substituted polyoxo metalate
gels and their electrochemical performance has been investigated. The results show that
[PyPS]
4
PMo
11
VO
40
has stronger oxidability than [PyPS]
6
PW
9
V
3
O
40
and [PyPS]
4
PW
11
VO
40
.
1. Introduction
Polyoxometalates (POMs), a class of nano-sized inorganic transition-metal oxide clusters with a
diverse range of fascinating properties, have attracted special interest in the fields of catalysis,
medicine, biology and materials science [1-6]. POMs can be modified by some other series of cations,
such as quaternary ammonium cations, to prepare many novel types of gel-type hybrid materials [7-
10]. Such POM-based gel-type materials can be easily shaped, and can maintain some significant
physical characteristics such as temperature-responsive behavior. Therefore, they have remarkable
potential applications, such as electrochemical supercapacitors and fuel cells [11, 12].
Herein, we report the synthesis and electrochemical performance of three Keggin-type vanadium-
substituted polyoxometalate gels, [PyPS]
6
PW
9
V
3
O
40
, [PyPS]
4
PW
11
VO
40
and [PyPS]
4
PMo
11
VO
40.
2. Experimental
2.1. Instruments and reagents
Infrared (IR) spectra were recorded on a NICOLET NEXUS 470 FT/IR spectrometer over the
wavenumber range 400–4000 cm
−1
using KBr pellet. X-ray powder diffraction analysis was obtained
on a BRUKER D8 ADVANCE X-ray diffractometer using a Cu tube operated at 50 kV and 200 mA
in the range of 2θ =4–40° at a scanning rate of 0.02° s
−1
. Inductively coupled plasma mass
spectrometry (ICP-MS) analysis was determined on a Shimadzu V-1012 ICP-MS spectrometer.
Electrochemical experiments were performed with a CHI660E Electrochemical Workstation in a
conventional three-electrode electrochemical cell using glass carbon (5 mm in diameter) as the
working electrode, platinum as the counter electrode, and a saturated calomel reference electrode in
organic media. The density of substrate was 0.25 mM and 0.2 M NaClO
4
was assigned as electrolyte.
All reagents were analysis grade and purchased from Aladdin, without further purification.
Wu, Q., Dai, W., Cui, S. and He, F.
Synthesis and Electrochemical Performance of Keggin-type Polyoxometalate Gels.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 625-629
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
625
2.2. Synthesis of polyoxometalate gels
1-(3-sulfonic group) propyl-pyridine (PyPS) was synthesized according to the literature [13].
H
6
PW
9
V
3
O
40
, H
4
PW
11
VO
40
and H
4
PMo
11
VO
40
were synthesized by modification of the method
according to the literatures [14-16]. The pre-synthesized PyPS and phosphorus-containing HPA,
H
6
PW
9
V
3
O
40
, H
4
PW
11
VO
40
and H
4
PMo
11
VO
40
were taken in 6:1, 4:1 and 4:1 mole ratio to give one
mole of [PyPS]
6
PW
9
V
3
O
40
, [PyPS]
4
PW
11
VO
40
and [PyPS]
4
PMo
11
VO
40
. PyPS was added to an
aqueous solution of HPA, and then the mixture was stirred for 10 h at room temperature. Water was
first evaporated in a 40°C water bath and then removed under vacuum to give highly viscous even
gel-state products. The obtained compounds are highly insoluble in tetrahydrofuran, acetone or ethyl
acetate, but soluble in water, N, N-dimethylformamide and dimethyl sulfoxide.
Carbon, nitrogen, sulfur, phosphorus, tungsten, molybdenum and vanadium were analyzed by
elemental analysis. The results indicate that the actual measurement values are consistent with the
calculated values, which confirms the composition of three POM-Gels.
3. Results and discussion
3.1. IR spectra
Table1. The assignment of the vibration modes in the IR spectra of the gels.
Vibration modes
Wavenumber (cm
-1
)
[PyPS]
6
PW
9
V
3
O
40
[PyPS]
4
PW
11
VO
40
[PyPS]
4
PMo
11
VO
40
O-H stretching
3420
3413
3425
-CH
2
stretching
2953
2951
2935
H-O-H bending
1633
1637
1635
-CH
2
scissoring
1468
1488
1482
S=O bending
1171
1163
1148
P-O
a
stretching
1055
1053
1051
M-O
d
stretching
971
963
956
M-O
b
-M stretching
892
891
878
M-O
c
-M stretching
806
808
798
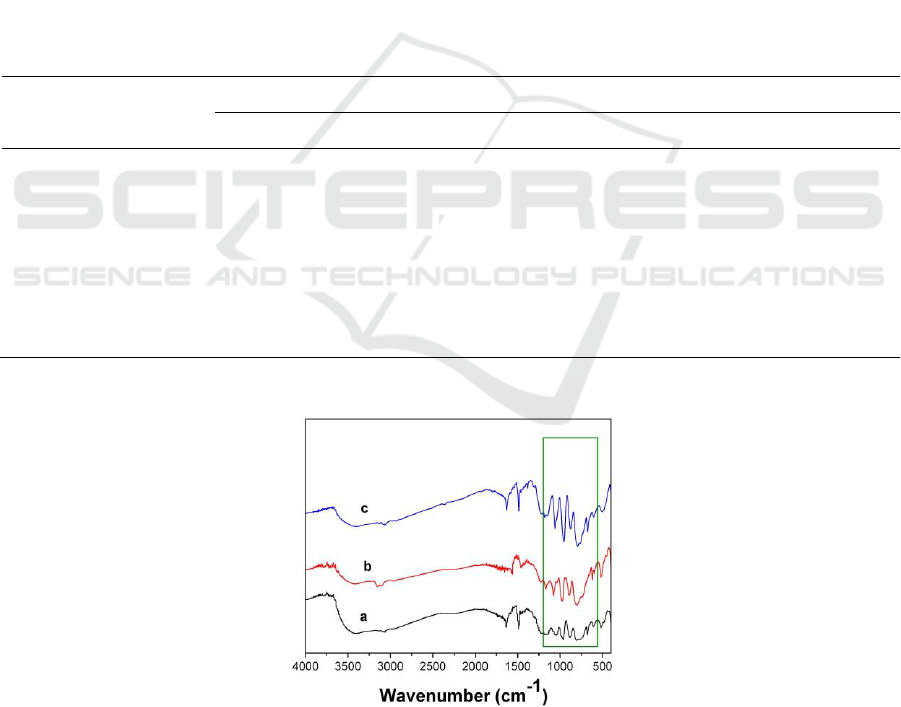
Figure 1.IR spectra of the products: (a) [PyPS]
6
PW
9
V
3
O
40
, (b) [PyPS]
4
PW
11
VO
40
, (c)
[PyPS]
4
PMo
11
VO
40.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
626
Compared with the pure acid, the characteristic bands of polyoxoanions have shifted (Figure 1). The
pure stretching vibration, M-O
d
vibrations, where the vibration frequency is influenced by the anion-
anion interactions, have decreased when sulf-group grafted ammoniums have been added with HPA
to make gel-type compounds. This is due to the weaker anion-anion electrostatic interaction as the
anion-anion distance increases. As M-O
b
-M and M-O
c
-M vibrations are not pure and cannot be free
from bending character, there is perhaps a competition of the opposite effects, which leads to an
increase in the vibratation comparing to the pure acid. The result reveals that this series of
compounds still maintain POM structures, which is consistent with those reported in the literature.
In the high wavenumber region, each spectrum of the gels exhibits two other peaks at around
3420cm
-1
and 1630cm
-1
. These are assigned to the stretching vibration of O-H bonds and the bending
vibration of H-O-H bonds, respectively.
In addition, there are some other characteristic peaks of sulf-group grafted ammoniums such as
ν
S=O
,ν
C-H
of CH
2
. These sulf-group grafted ammoniums cations also maintain their structure, which
indicates the successful assembly and existence of the POM structure units and organic ammonium
cations in the compounds without depolymerization or degradation.
3.2. XRD
patterns
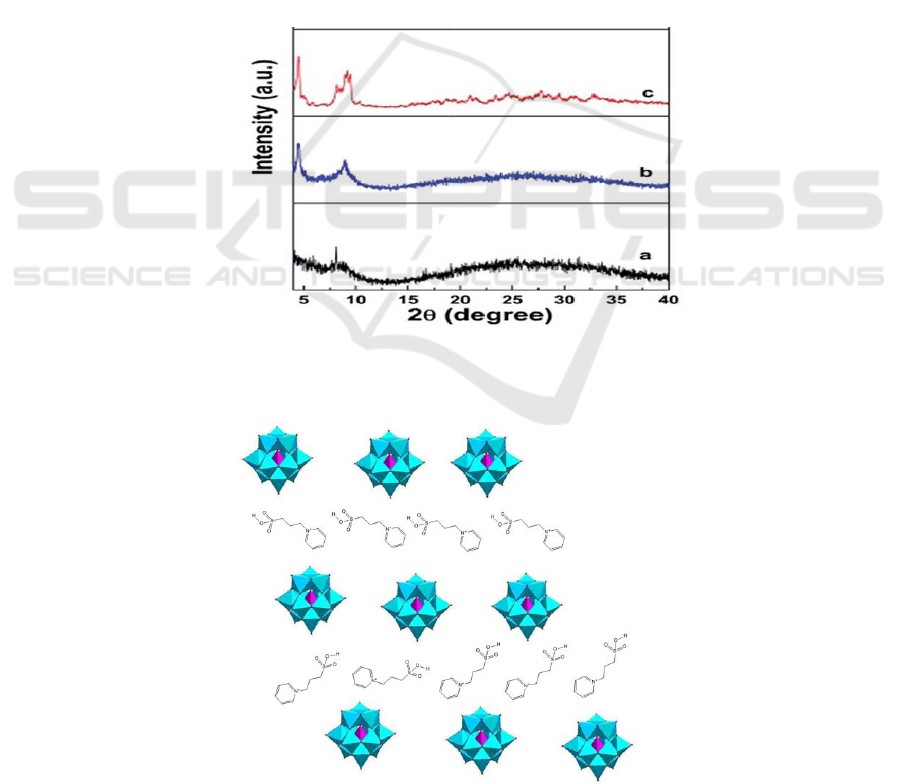
Figure 2.XRD patterns (a) [PyPS]
6
PW
9
V
3
O
40
, (b) [PyPS]
4
PW
11
VO
40
, (c) [PyPS]
4
PMo
11
VO
40.
Figure 3.Schematic illustration of organized structures of the POM-type layered materials.
Synthesis and Electrochemical Performance of Keggin-type Polyoxometalate Gels
627
The XRD patterns (Figure 2) of [PyPS]
6
PW
9
V
3
O
40
, [PyPS]
4
PW
11
VO
40
and [PyPS]
4
PMo
11
VO
40
are in
marked contrast to that of the pure heteropoly acids and consistent with the gel-state appearance.
According to the recent paper and considered intense peaks in the XRD patterns of these compounds
in small angles area, we can assume that an organized layer-type structure exists in this series of
compounds, as illustrated in Figure 3, and the height of each layer can be calculated by the intense
peaks in small angles area. Meanwhile, the strong diffraction peak at 7~10° can be considered as the
POM anion structure and a wide diffraction peak appears in the wide-angle region, indicating that
these compounds have a gel-type phase at room temperature, which is caused by weak connections of
the layers, rather than an identified shape in total like the pure acid.
3.3. The cyclic voltammetry
The cyclic voltammetry studies of [PyPS]
4
PW
11
VO
40
, [PyPS]
6
PW
9
V
3
O
40
and PyPS]
4
PMo
11
VO
40
are
shown in Figure 4 and Table 2.
-0.3 0.0 0.3 0.6
-18
-12
-6
0
6
Ⅰ'
Ⅰ
Ⅰ'
Ⅰ
Ⅰ'
Ⅰ
I/
E/V vs Hg
2
Cl
2
/Hg
PyPS
4
PW
11
VO
40
PyPS
6
PW
9
V
3
O
40
PyPS
4
PMo
11
VO
40
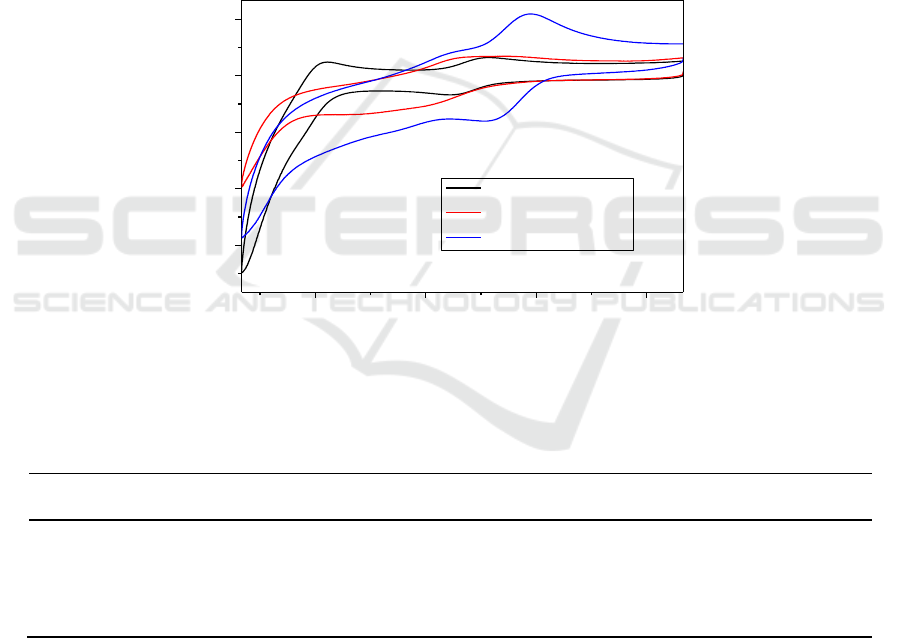
Figure 4. Cyclic voltammetry of [PyPS]
4
PW
11
VO
40
, [PyPS]
6
PW
9
V
3
O
40
and [PyPS]
4
PMo
11
VO
40
with
a scanning rates of 50mv·s
-1
in DMF.
Table 2. The half-wave potentials, E
1/2
, for the redox couples observed in Figure 4.
Compounds
E
1/2
(I/ I’) [mv]
[PyPS]
4
PW
11
VO
40
131
[PyPS]
6
PW
9
V
3
O
40
206
[PyPS]
4
PMo
11
VO
40
245
In [PyPS]
4
PW
11
VO
40
,
[PyPS]
6
PW
9
V
3
O
40
and [PyPS]
4
PMo
11
VO
40
, the reduction of the vanadium
is shown as follows: .
45
11 40 11 40
(V) (IV)PW V O e PW V O
Ⅰ /Ⅰ ’
69
9 3 40 9 3 40
(V) 3 (IV)PWV O e PWV O
Ⅰ /Ⅰ ’
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
628

45
11 40 11 40
(V) (IV)PMo V O e PMo V O
Ⅰ /Ⅰ ’
Table 2 reveals that [PyPS]
4
PMo
11
VO
40
has stronger oxidability than [PyPS]
6
PW
9
V
3
O
40
and
[PyPS]
4
PW
11
VO
40
,
while the tri-substituted compound, [PyPS]
6
PW
9
V
3
O
40
, has stronger oxidability
than the mono-substituted one, [PyPS]
4
PW
11
VO
40
. In other words, it can be concluded that when it
comes to the vanadium-substituted molybdophosphorates and tungstophosphorates, it is the POM
component elements that mainly determine the oxidability rather than the number of the substituted
atoms, as [PyPS]
4
PMo
11
VO
40
has stronger oxidability than [PyPS]
6
PW
9
V
3
O
40
and [PyPS]
4
PW
11
VO
40
,
while there are more vanadium atoms in [PyPS]
6
PW
9
V
3
O
40
.
4. Conclusions
In this paper, we have mainly reported the synthesis and electrochemical performance of a series of
POM-type gels, [PyPS]
6
PW
9
V
3
O
40
, [PyPS]
4
PW
11
VO
40
and [PyPS]
4
PMo
11
VO
40
. The relationship
between the component elements of the vanadium-substituted polyoxometalate gels and their
electrochemical performance has been investigated. The results show that [PyPS]
4
PMo
11
VO
40
has
stronger oxidability than [PyPS]
6
PW
9
V
3
O
40
and [PyPS]
4
PW
11
VO
40
. They can be promising materials
for supercapacitors.
Acknowledgements
This work was supported by the Liaoning Provincial Natural Science Foundation of China
(201602404) and the Scientific Research Foundation of Liaoning Institute of Science and
Technology (RXYJ2015001).
References
[1] Sadeghi O, Zakharov L N and Nyman M, 2015. Science 347 1359.
[2] Blasco-Ahicart M, Soriano-Lopez J, Carbo J J, Poblet J M and Galan-Mascaros J R, 2018. Nat.
Chem. 10 24.
[3] Chen X L, Zhou Y, Roy V A L and Han S T, 2018. Adv. Mater. 30 1703950.
[4] Wu Y, Shi R, Wu Y L, Holcroft J M, Liu Z, Frasconi M, Wasielewski M R, Li H and Stoddart
J F, 2015. J. Am. Chem. Soc. 137 4111.
[5] Khenkin A M, Somekh M, Carmieli R and Neumann R, 2018. Angew. Chem. Int. Ed. 57
5403.
[6] Shen F C, Wang Y R, Li S L, Liu J, Dong L Z, Wei T, Cui Y C, Wu X L, Xu Y and Lan Y Q
2018. J. Mater. Chem. A 6 1743.
[7] Wu X F, Tong X, Wu Q Y, Ding H and Yan W F , 2014. J Mater Chem A 2 5780.
[8] Li Y Y, Wu X F, Wu Q Y, Ding H and Yan W F, 2014. Ind. Eng. Chem. Res. 53 12920.
[9] Huang T P, Tian N Q, Wu Q Y and Yan W F, 2015. Soft Matter 11 4481.
[10] Wu X F, Cai H X, Wu Q Y and Yan W F, 2016. Dalton Trans. 45 11256.
[11] Wu X F, Wu W, Wu Q Y and Yan W F, 2017. Langmuir 33 4242.
[12] Xie Z R, Wu Q Y and Ai L M, 2018. Funct. Mater. Lett. 11 1850059.
[13] Leng Y, Wang J, Zhu D R, Ren X Q, Ge H Q and Shen L, 2009. Angew. Chem. Int. Ed. 48 168.
[14] Tong X, Tian N Q, Zhu W M, Wu Q Y, Cao F H and Yan W F, 2012. J. Alloys Compd. 544
37.
[15] Tong X, Tian N Q, Wu W, Zhu W M, Wu Q Y, Cao F H, Yan W F and Yaroslavtsev A B,
2013. J. Phys. Chem. C, 117, 3258.
[16] Tong X and Thangadurai V, 2015. J Phys Chem C 119 7621.
Synthesis and Electrochemical Performance of Keggin-type Polyoxometalate Gels
629
