
Preparation of Mn-modified Porous Carbon Microspheres
Y L Xu
1,2, *
, Z F Liu
1,2
, B Ren
1,2
, S S Wang
1,2
and L H Zhang
1,2
1
Institute of Energy Resources, Hebei Academy of Sciences, Shijiazhuang 050081,
China
2
Hebei Engineering Research Center for Water Saving in Industry, Shijiazhuang
050081, China
Corresponding author and e-mail address: Y L Xu, xudalong.cool@163.com.
Abstract. Mixed / composite oxides of transition metals with microsphere structures have
promising potentials for different applications but their preparation still remain a big
challenge. Herein, a facile hydrothermal method was developed to construct Mn-modified
porous carbon microspheres. The composition of the porous carbon microsphere structure can
be adjusted by controlling the composition of the precursors using glucose and MnO
2
as raw
materials. The precursors were heat-treated at 120°C, 150°C, and 180°C, respectively. The
samples were characterized by X-ray diffraction, scanning electron microscopy, transmission
electron microscopy and X-ray photoelectron spectroscopy (XPS). The optimum conditions
of the measurement is 180 °C for 24 h and the specific surface area of as – prepared porous
carbon microspheres is 93 m
2
/g. The improvement of mixed / composite oxides of transition
metals preparation with a morphology of carbon-coated spherical materials exhibit a great
potential application in new materials field.
1. Introduction
Mixed / composite oxides of transition metals with porous carbon microsphere structures are ideal
candidates to improve Lithium ion batteries (LIBs) performance [1-4]. The addition of carbon into
the composite could enhance the integrated conductivity notably, contributing to higher utilization of
active material and better rate capability [5-7]. Carbon materials have been widely used in electrical
double layer capacitor electrodes because of good electronic conductivity and chemical stability.
High-surface-area microporous carbons (e.g. activated carbons) exhibit high capability for charge
accumulation at the electrode–electrolyte interface, which contributes to large capacitance. While
mesoporous carbons are favorable for high transportation speed of electrolyte ions, and thus show
better charge–discharge rates, especially under high loading current density. Besides, porous carbon
microspheres with regular morphology and adjustable porosity and diameter can decrease the
resistance of ion diffusion, and the package porosity among carbon spheres benefits the generation of
ion buffer reservoirs and reduces ion diffusion distance. Thus, it is desirable to design and fabricate
micro and mesoporous carbon microspheres with regular geometry and well-developed pore structure
for tailoring high performance electrodes to be used in electrical double layer capacitors. Meanwhile,
carbon spheres are widely applied in catalyst supports, lubricants, additives for reinforced plastics
and rubbers, electrode materials in fuel cells, and supercapacitors, as well as anodes in Li-ion
batteries [8-10].
Xu, Y., Liu, Z., Ren, B., Wang, S. and Zhang, L.
Preparation of Mn-Modified Porous Carbon Microspheres.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 471-477
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
471
In this work, we report the simple preparation of Mn-modified Porous Carbon Microspheres via
one-pot hydrothermal method. The hydrothermal time and temperature have been investigated and
the morphology of microspheres has been studied with different instruments.
2. Experimental
All reagents were purchased from Aladdin. All reagents were analytical reagent (AR) grade and were
used as-received without further treatment. Mn-modified porous carbon microspheres were prepared
via one-pot hydrothermal method using glucose and MnO
2
as raw materials. Briefly, glucose and
MnO
2
were mixed in a distilled water, which was transferred to a Teflon-lined autoclave and heated
to a temperature of 120 °C, 120 °C and 180 °C, respectively, for 12 h, 18 h and 24 h, respectively.
After cool down, the resulting slurry was filtered and washed in ethanol and distilled water. The
powder obtained after filtering and water - ethanol wash was vacuum-dried and carbonized at 550 °C
for 6 h in nitrogen condition.
Surface area measurements were obtained from an ASAP 2420 surface area analyzer
(Micromeritics, USA) with the Brunauer–Emmett–Teller (BET) method. The samples were
evacuated at 90°C for 1 h and at 250°C for 6 h before they were measured in a nitrogen atmosphere.
The as-prepared samples were characterized by X-ray diffraction (XRD, Ultima IV X-ray
diffractometer, Rigaku, Japan) with CuKa radiation (λ = 1.54 Å). The morphologies of the OM-CAs
were observed via scanning electron microscopy (SEM, TESCAN MAIA3, USA) and transmission
electron microscopy (TEM, JEM-2100F, JEOL, Japan). The surface compositions of samples were
obtained via X-ray photoelectron spectroscopy (XPS, PHI5600 Physical Electronics).
3. Results and discussion
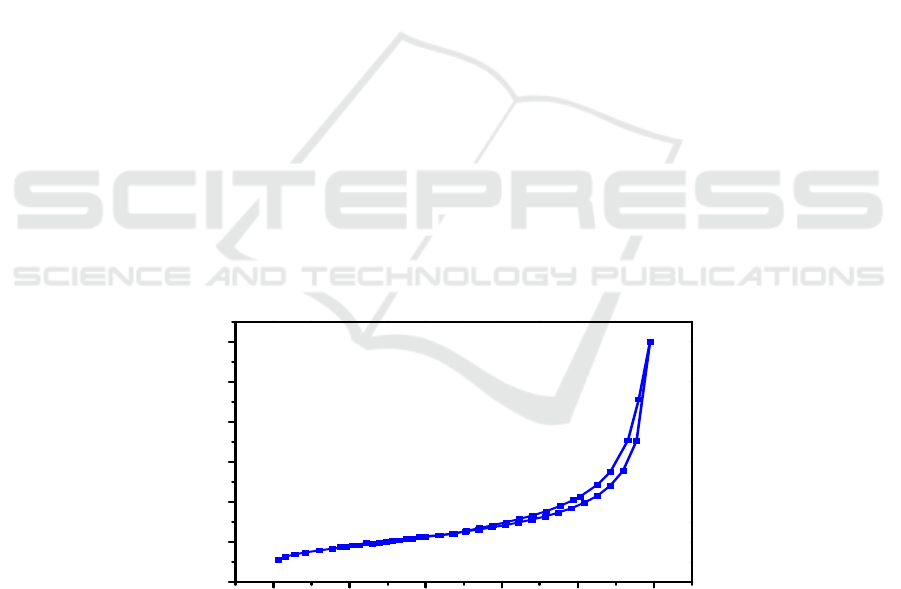
Nitrogen adsorption–desorption isotherms at 77 K for Mn-modified porous carbon microspheres is
shown in Figure 1. As seen in Figure 1, the isotherms exhibit a hysteresis loop shape in accordance
with type IV hysteresis according to the IUPAC classification scheme. As listed in Table 1, when the
hydrothermal time is up to 24 h, the sample exhibits a highest specific surface area, up to 93 m
2
/g.
The long hydrothermal time is beneficial for the formation of carbon microspheres.
0.0 0.2 0.4 0.6 0.8 1.0
0
20
40
60
80
100
120
Volume adsorbed(cm
3
g
-1
)
Relative pressure(P/P
0
)
Figure 1. Nitrogen adsorption–desorption isotherms of as – prepared sample.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
472
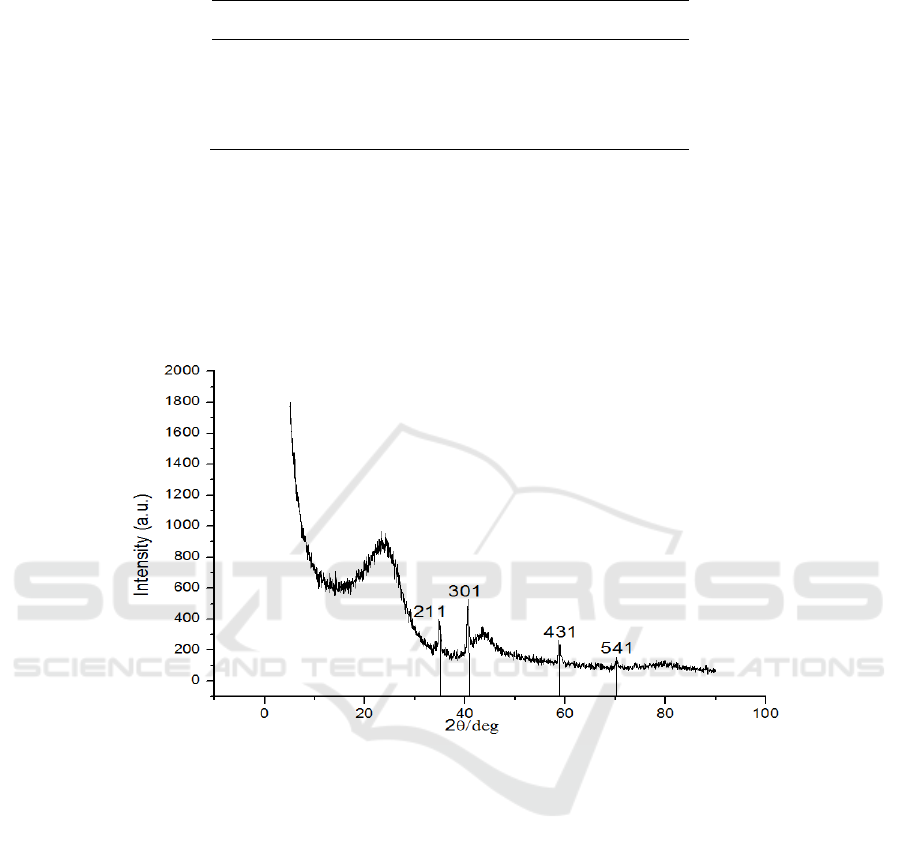
Table 1. The specific surface area (S
BET
) of all samples.
Entry
a
Time (h) S
BET
(m
2
/g)
1 12 45
2 16 56
3 20 72
4 24 93
a: the hydrothermal temperature is 180 °C
XRD pattern of sample prepared under 180 °C for 24 h is presented in Figure 2. As shown in
Figure 2, the curves clearly exhibits two diffraction peaks at a 2θ of 24° and 42°, which are attributed
to the planes (002) and (100), respectively. This result implies that the composite is composed of
graphite carbon and amorphous carbon. (211), (301), (431), and (541) are attributed to Mn
2
O
3
, which
implies the presence of Mn.
Figure 2. XRD patterns of as – prepared sample.
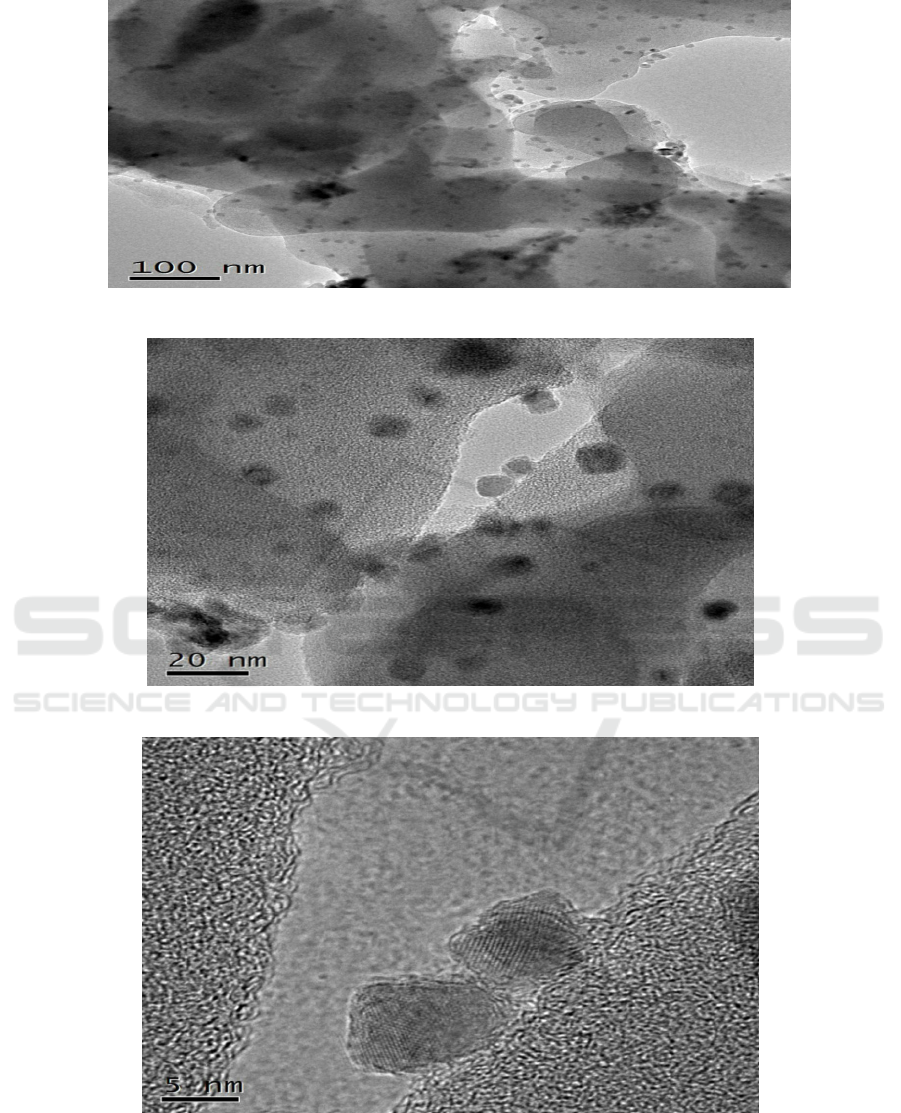
The SEM and TEM images of sample prepared under 180°C
for 24 h are shown in Figure 3. As
presented in Figure 3 (a), porous carbon microspheres can be clearly seen. Meanwhile, as seen in
Figure 3 (b and c), metal oxides uniformly stick to the surface of carbon microspheres. As shown in
TEM images, manganese oxides are very noticeable. Metal lattice fringe can be clearly observed in
Figure 3 (f), which proved the metal oxides were Mn
2
O
3
.
The XPS spectrum of sample prepared under 180
°C for 24 h is shown in Figure 4. As seen in
Figure 4 (a), sample prepared under 180 °C for 24 h mainly contains C, O, and Mn elements. As
shown in Figure 4 (b), Csp
2
and Csp
3
peaks are located at 284.6 eV and 285.3 eV, respectively. The
285.9 eV (C-O bonds), 287.4 eV (C=O bonds) and 289.1 eV (O-C=O bonds) peaks are obviously
observed in the C 1s spectrum. Three peaks were clearly observed in the O 1s spectrum, and they are
assigned to the C=O bonds (531.0 eV), COOH bonds (532.0 eV) and C-O-C bonds (535.0 eV),
respectively. This result implies the presence of bulk oxygen-containing groups on the surface of the
as-prepared carbon aerogels. In the Mn 2p spectrum, the peaks at 641.8 eV and 653.4 eV were easily
distinguished.
Preparation of Mn-Modified Porous Carbon Microspheres
473

(a)
(b)
(c)
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
474
(d)
(e)
(f)
Figure 3. (a) SEM and (b) TEM images of as – prepared samples.
Preparation of Mn-Modified Porous Carbon Microspheres
475
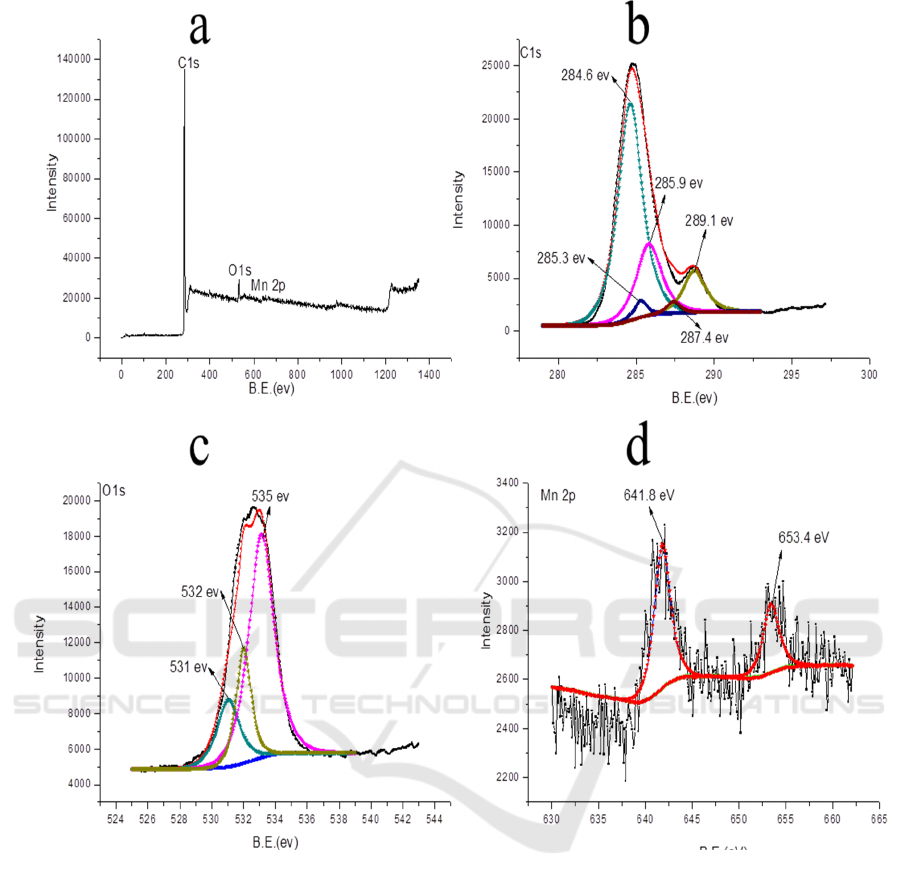
Figure 4. XPS spectra: (a) full-scan spectrum (b) C 1s, (c) O 1s, and (d) Mn 2p peak.
4. Conclusions
In summary, Mn-modified porous carbon microspheres have been synthesized by a facile method.
The porous carbon microspheres exhibited a high specific surface area, up to 93 m
2
/g. The optimal
preparation condition is 180°C
for 24 h. Our facile strategy has made it possible to optimize the
electrode conjuration of future energy storage devices and can also be extended to synthesize other
metal oxides carbon microspheres materials with excellent performance.
Acknowledgments
This work was financially supported by the Foundation of Key R&D Program of Hebei Province
(18393616D) and science and technology projects of Hebei Academy of Sciences (18707).
References
[1] Liang G, Qin X, Zou J, Luo L, Wang Y, Wu M, Zhu H, Chen G, Kang F and Li B 2018
carbon 127 424
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
476

[2] Jeong M, Du H, Islam M, Lee J, Sun Y and Jung H 2017 Nano Lett 17 5600
[3] Zhu G, Liu H, Zhuang J, Wang C, Wang Y and Xia Y 2011 Energy Environ. Sci, 4 4016
[4] Zhang W, Hu J, Guo Y, Zheng S, Liang-Shu Zhong, Song W and Wan L 2008 Adv. Mater 20
1160
[5] Yoshio M, Wang H, Fukuda K, Umeno T, Abe T and Ogumi Z 2004 J. Mater. Chem 14 1754
[6] Ye H, Xin S, Yin Y and Guo Y 2017 Adv. Energy Mater 7 L 1700530
[7] Jung H, Myung S, Yoon C, Son S, Oh K, Amine K, Scrosati B and Sun Y 2011 Energy
Environ. Sci. 4 1345
[8] Han W, Qin X, Wu J, Li Q, Liu M, Xia Y, Du H, Li B, and Kang F 2018 Nano Res. 11, 892
[9] Yan M, Zhang L, He R and Liu Z 2015 J. Porous Mater. 22 699
[10] Yang I, Kim S, Kwon S, Lee J, Kim M and Jung J 2016 Current Applied Physics 16 665
Preparation of Mn-Modified Porous Carbon Microspheres
477
