
Synthesis of Phosphorus-based Polyoxometalates and Their
Corrosion Inhibition Performance
S B Cui, F W He, Q Y Wu
*
and W S Dai
School of Biomedical and Chemical Engineering, Liaoning Institute of Science and
Technology, Benxi 117004, Liaoning, China
Corresponding author and e-mail: Q Y Wu, qywu@lnist.edu.cn
Abstract. Polyo xo metalates corrosion inhib itors used in experiments were prepared based on
literatures, the synthesized products were characterized by infrared spectra and ultraviolet
spectra. The test results showed that the synthesized polyoxo metalates are of Keggin type.
The corrosion inhib ition perfo rmance was determined using A3 carbon steel specimen by
static weight-loss technique in this paper. The corrosion inhibition performance of Na
3
[PMo
12
O
40
], Na
3
[PW
12
O
40
], Na
4
[PW
11
VO
40
] and Na
5
[PMo
10
V
2
O
40
] was compared under
certain conditions of temperature, corrosion inhibitor concentrations and pH values, and the
effects of polyoxo metalate concentration and temperature on the corrosion inhibition
performance we re studied. The experimental results show that all of the syn thesized
polyoxo metalates have a good corrosion resistance, and that the corrosion inhibition
efficiency of Na
4
[PW
11
VO
40
] is the best of all, and it increase with the inhibitors
concentration and with the temperature.
1. Introduction
Since the 21st century, the development and application of corrosion inhibitor have been confronted
with new challenges because of the deepening of the thought of sustainable development and the
increasing awareness of environmental protection. The development of non-toxic harmless, green
inhibitors which do not damage the environment will become the focus of the research and
development of corrosion inhibitor [1-3]. Polyoxometalates are a type of metal oxide cluster, formed
through inorganic metal-oxygen cluster anions, with a variety of structures, compositions, and
functionalities [4]. The phosphorus based polyoxometalates is a kind of good corrosion and scale-
inhibiting water treatment agent [5].
The aim of the present work is to synthesize the four phosphorus based heteropoly compounds
and compare their corrosion inhibition properties in 3.5% sodium chloride solutions.
2. Experimental
2.1. Instruments and reagents
Na
2
WO
4
•2H
2
O, Na
2
MoO
4
•2H
2
O, Na
3
PO
4
•12H
2
O, Na
2
HPO
4
, NH
4
VO
3
•2H
2
O, H
2
SO
4
, HClO
4
, HCl,
NaOH, NaCl and diethyl ether were commercially bought, and the deionized water made in our lab.
All reagents except deionized water are of analytical grade.
450
Cui, S., He, F., Wu, Q. and Dai, W.
Synthesis of Phosphorus-Based Polyoxometalates and Their Corrosion Inhibition Performance.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 450-455
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

DF-101S type heat-collecting constant temperature heating magnetic stirrer, SC-DC-220AB type
electronic balance, PHS-3E type pH meter were commercially bought in China.
2.2. Synthesis and structural characterization of phosphorus-based polyoxometalates
2.2.1. Synthesis of phosphorus-based polyoxometalates. The four hybrid type of heteropoly acids of
H
3
[PMo
12
O
40
], H
3
[PW
12
O
40
], H
4
[PW
11
VO
40
] and H
4
[PMo
10
V
2
O
40
] were synthesized by literature
[6,7], respectively.
2.2.2. IR Spectra of Synthesized products. The IR spectra of several compounds were measured by a
WQF-510 FT-IR spectrophotometer as KBr disks by a 769YP-15A powder pressing machine at
ambient temperature.
2.2.3. UV spectra of Synthesized products. The ultraviolet spectra of several compounds were
detected by a TU-1901 type double beam ultraviolet visible spectrophotometer.
2.3. Corrosion inhibition performance of synthesized polyoxometalates
To test for corrosion inhibition of synthetic heteropoly acids, the methods described in literatures [8]
are used. A3 steel pieces (50mm×25mm×2 mm) were mechanically polished with 4/0 emergy papers.
The surface of the steel specimens was washed with hydrochloric acid, deionized water and
anhydrous ethanol and finally with anhydrous alcohol. Then the steel specimens were stored in a
dryer to dry.
For testing the corrosion behaviour of steel, specimens were immersed in 200 mL water
containing 3.5% sodium chloride solutions, different heteropoly acids were added in the testing
solutions respectively. After the finishing of the tests which lasted 70 h, the specimens were taken out
of the testing solution and corrosion pr oducts were cleaned as following. a) The specimens were
washed with tap water and then the metallic surface rust was scratched with knife. b) The specimens
were washed with 10% hydrochloric acid and 0.5% hexamethylenamine. c) The specimens were
washed with tap water, then washed twice with deionized water, finally washed twice with anhydrous
alcohol.
The specimens were dried with a blower and weighed. The corrosion rate determined by the rate
of loss of the steel owing to corrosion products removal is calculated as the equation
v = ( m
1
- m
2
) / t·A.
Where v is the metallic corrosions rate (g·m
- 2
·h
- 1
), m
1
is the metallic gravity before experiment
(g), m
2
is the metallic gravity after experiment (g), t is the immersion time (h), A is surface area of
sample(m
2
).
The corrosion inhibition efficiency of the inhibitor E was calculated from the following equation
E = (v
1
- v
2
) / v
1
× 100%.
Where E corrosion inhibition efficiency, v
1
metallic corrosion rate without its inhibitor, v
2
metallic
corrosion rate with its inhibitor.
3. Results and discussion
3.1. IR Spectra of Synthesized products
The synthesized products are detected by a WQF-510 FT-IR spectrophotometer, and the results are
shown in Figure 1.
As shown in Figure 1, there are four characteristic absorption peaks of Keggin structure from 700
cm
-1
to 1100cm
-1
for the four kinds of synthesized heteropoly acids: the peak at from 1050cm
-1
to
(1)
(2)
Synthesis of Phosphorus-Based Polyoxometalates and Their Corrosion Inhibition Performance
451
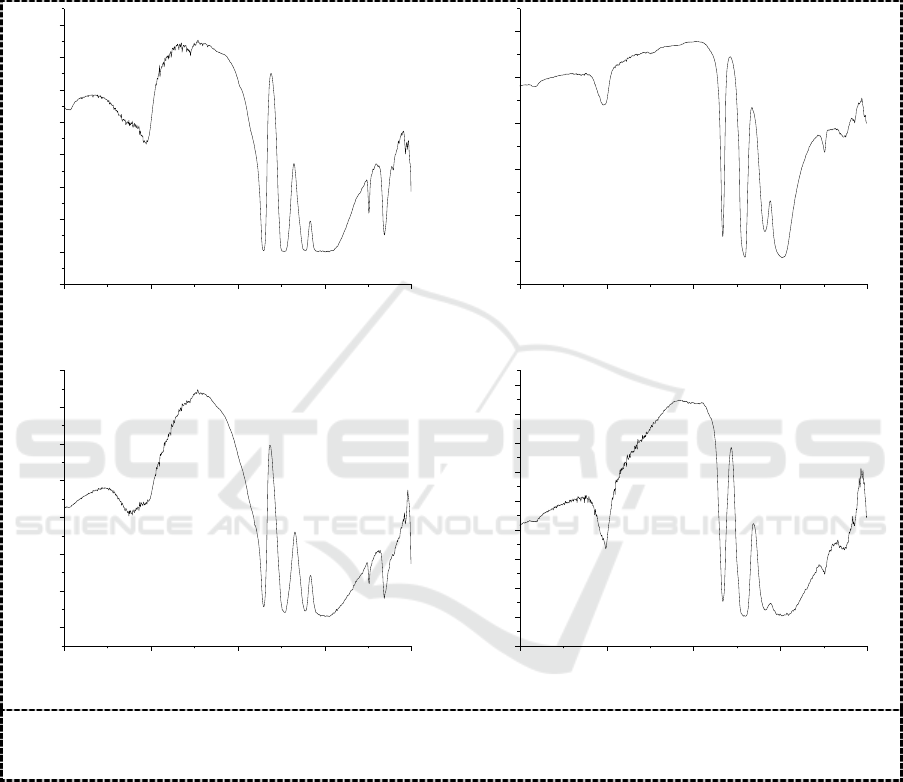
1100 cm
-1
is attributed to the asymmetric stretching vibration of P-O bond, the peak at from 900 cm
-1
to1000 cm
-1
is attributed to the asymmetric stretching vibration of M-O (M=Mo, W) bonds, and the
ones at from 850 cm
-1
to 900 cm
-1
and at from 750 cm
-1
to 800 cm
-1
, is to the ones of the M-O
b
-M
bridge bond and of the M-O
c
-M bridge bonds. In addition, the peak near to 1620 cm
-1
is attributed to
the bending vibration of H-O-H bonds in the water molecule, The IR spectra show that the prepared
compounds are of Keggin type [9].
2000 1600 1200 800 400
-20
0
20
40
60
80
100
120
140
T (%)T (%)
Wavenumber (cm
-1
)
A
2000 1600 1200 800 400
0
20
40
60
80
100
Wavenumber (cm
-1
)
T (%)
B
2000 1600 1200 800 400
10
20
30
40
50
60
70
80
Wavenumber (cm
-1
)
T (%)
C
2000 1600 1200 800 400
-5
0
5
10
15
20
25
30
35
40
Wavenumber (cm
-1
)
T (%)
D
Figure 1. IR spectra of H
3
[PW
12
O
40
] (A), H
5
[PMo
10
V
2
O
40
] (B), H
4
[PW
11
VO
40
] (C) and
H
5
[PMo
10
V
2
O
40
] (D).
3.2. UV spectra of Synthesized products
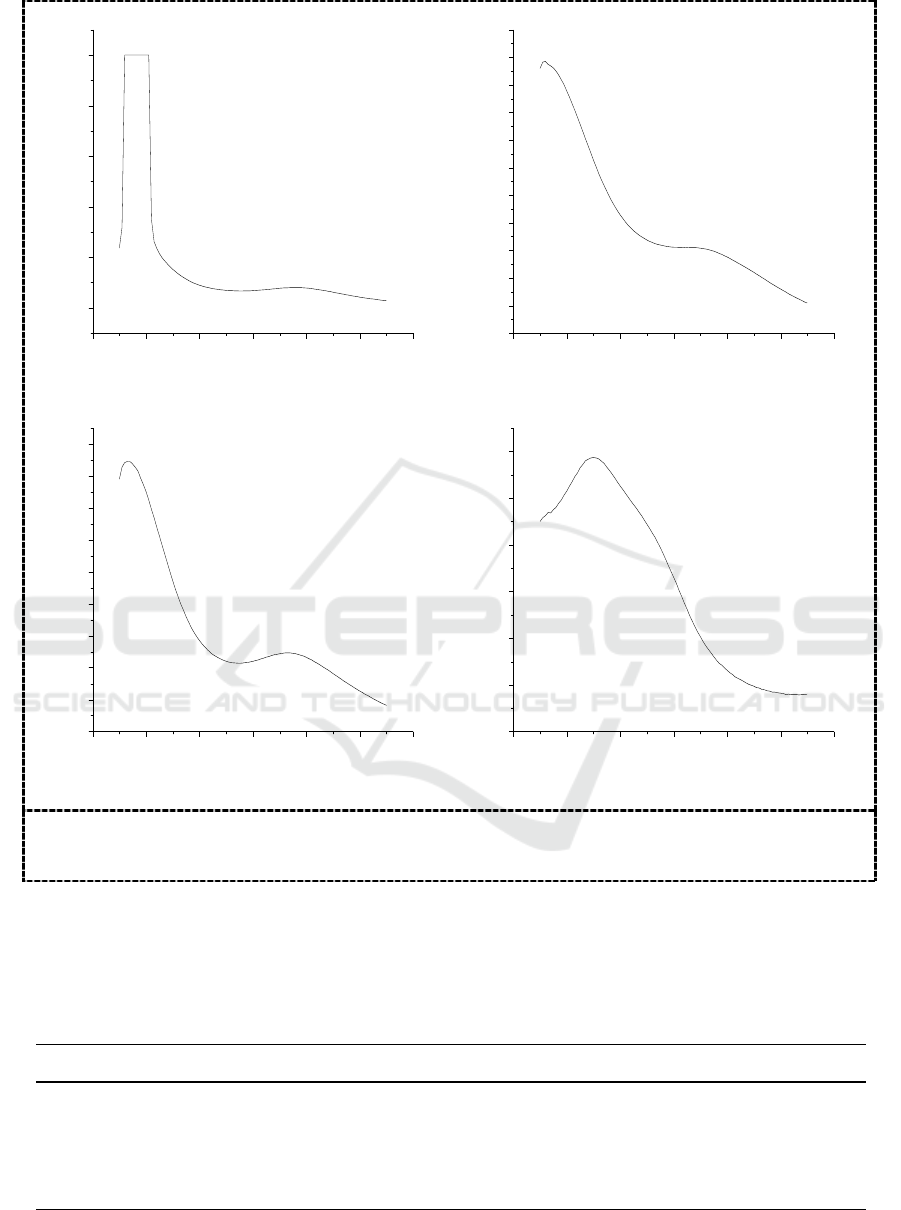
The synthesized products are detected by a double beam ultraviolet visible spectrophotometer, and
the results are shown in Figure 2.
It is shown in Figure 2 that there are the two characteristic absorption peaks at about 200 nm and
at about 260 nm in the four kinds of heteropoly acids. The one at about 200 nm is generated by the
charge-transfer of Od→M, and the other at about 260 nm are generated by the charge-transfer of
Ob/Oc→M [10]. The ultraviolet spectra further confirmed that the four synthesized heteropoly acids
are of Keggin type.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
452
180 200 220 240 260 280 300
0
2
4
6
8
10
absorbsance
Wavelength (nm)
A
180 200 220 240 260 280 300
0.0
0.2
0.4
0.6
0.8
1.0
1.2
1.4
1.6
1.8
2.0
2.2
B
absorbsance
180 200 220 240 260 280 300
0.0
0.2
0.4
0.6
0.8
1.0
1.2
1.4
1.6
1.8
C
absorbsance
Wavelength (nm)
180 200 220 240 260 280 300
0.0
0.1
0.2
0.3
0.4
0.5
0.6
D
Wavelength (nm)
absorbsance
Wavelength (nm)
Figure 2. UV spectra of H
3
[PW
12
O
40
] (A), H
5
[PMo
10
V
2
O
40
] (B), H
4
[PW
11
VO
40
] (C) and
H
5
[PMo
10
V
2
O
40
] (D).
3.3. Analysis on corrosion inhibition performance of synthesized products
The test results for corrosion resistance of synthesized products are shown in Table 1, Table 2 and
Table 3.
Table 1. The corrosion rate and corrosion inhibition efficiency in different solutions
a
.
inhibitor
pH
value
Weight loss
(g)
corrosion rate
(g·m
- 2
·h
- 1
)
corrosion inhibition efficiency (%)
without inhibitor
8.13
0.148
0.7551
_
Na
3
[PMo
12
O
40
]
8.09
0.058
0.2951
60.92
Na
3
[PW
12
O
40
]
8.11
0.051
0.2602
65.54
Na
4
[PW
11
VO
40
]
8.12
0.040
0.2041
72.97
Na
5
[PMo
10
V
2
O
40
]
8.11
0.042
0.2143
71.62
a
Under the condition of the temperature of 20°C, the solution of 500 mg·l
-1
, and the immersing time of 70h.
Synthesis of Phosphorus-Based Polyoxometalates and Their Corrosion Inhibition Performance
453

Table 2. The corrosion rate and corrosion inhibition efficiency in different concentration of
solutions
a
.
inhibitor
Concentration
(mg·l
-1
)
Weight
loss (g)
corrosion rate (g·m
-
2
·h
- 1
)
corrosion inhibition
efficiency (%)
without inhibitor
0.148
0.7551
_
Na4[PW11VO40]
300
0.063
0.3214
57.44
Na4[PW11VO40]
800
0.025
0.1276
83.10
Na5[PMo10V2O40]
300
0.065
0.3316
56.09
Na5[PMo10V2O40]
800
0.026
0.1327
82.43
a
Under the condition of the temperature of 20°C , the pH of 8.0, and the immersing time of 70h.
Table 3. The corrosion rate and corrosion inhibition efficiency at 40°C
a
.
inhibitor
Concentration
(mg·l
-1
)
Weight loss
(g)
corrosion rate
(g·m
- 2
·h
- 1
)
corrosion inhibition
efficiency (%)
blank
800
0.187
0.9541
_
Na
3
[PMo
12
O
40
]
800
0.031
0.1582
83.42
Na
3
[PW
12
O
40
]
800
0.024
0.1224
87.17
Na
4
[PW
11
VO
40
]
800
0.023
0.1173
87.71
Na
5
[PMo
10
V
2
O
40
]
800
0.025
0.1276
86.63
a
Under the condition of the pH of 8.0, and the immersing time of 70h.
Table 1 shows the corrosion behaviour of steel without and with 500 mg·l
-1
heteropoly
compounds solutions containing 3.5% sodium chloride for 70 h at 20°C . It is observed that all of
heteropoly compounds have some inhibition, and that the corrosion inhibition efficiency of
heteropoly compounds containing vanadium are better than of the others, but the corrosion inhibition
efficiency of Na
4
[PW
11
VO
40
] is the highest of all, that is, 72.97%. On the one hand, the corrosion
resistance of multicomponent polyoxometalates is higher than that of bicomponent polyoxometalates.
On the other hand, the oxidation of tungsten (VI) is weaker than that of molybdenum (VI), so that the
tungstophosphoric polyoxometalates and the molybdophosphorates have different effects on metal
materials. The tungstophosphoric polyoxometalates is less corrosive to metallic materials and easy to
form a protective film on the surface of metal material to protect metallic materials from corrosion
[11]. However, molybdophosphoric polyoxometalates has strong oxidizing effect, it can form a
passivation film on the surface of metal material, and it will produce deeper corrosion to metal
material. So, the corrosion inhibition efficiency of Na
4
[PW
11
VO
40
] is higher than that of
H
5
[PMo
10
V
2
O
40
].
Table 2 summarizes the results of steel corrosion with the change of concentration of
Na
4
[PW
11
VO
40
] and Na
5
[PMo
10
V
2
O
40
] in 3.5% sodium chloride solutions for 70h at 40°C . It is
evident that the increase in concentration of Na
4
[PW
11
VO
40
] or Na
5
[PMo
10
V
2
O
40
] has considerably
reduced (up to about 10%) the corrosion rate. This is interpreted that the increase in concentration of
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
454

heteropoly compounds favor the formation of a protective film on the surface of metal material to
protect the surface of metallic materials from corrosion.
Table 3 lists the corrosion rate and the corrosion inhibition efficiency without and with 800 mg·l
-1
heteropoly compounds solutions containing 3.5% sodium chloride for 70h at 40°C . It is easily to
known that the corrosion inhibition properties of each of heteropoly compounds is almost equal when
the solution contains 800 mg·l
-1
. This can be explained that the rate of the formation of a protective
film on the surface of metal material from corrosion in all kinds of polyoxometalates solution is only
related to the concentration of various ions, but not to the oxidation properties of ions.
4. Conclusions
(1) The synthesized polyoxometalates of Na
3
[PMo
12
O
40
], Na
3
[PW
12
O
40
], Na
4
[PW
11
VO
40
] and
Na
5
[PMo
10
V
2
O
40
] have good corrosion inhibition. When the concentration is low, the corrosion
inhibition efficiency of Na
4
[PW
11
VO
40
] is the highest of all.
(2) The inhibition property of each of polyoxometalate increases with the increase of its
concentration, and the corrosion inhibition of various polyoxometalates was close to a certain
concentration.
Acknowledgements
This work was supported by the Liaoning Provincial Natural Science Foundation of China
(201602404), the Scientific Research Foundation of Liaoning Institute of Science and Technology
(RXYJ2015001) and the College students’ innovative entrepreneurial training project
(201711430000084).
References
[1] Herrmann S, Kostrzewa M, Wierschem A and Streb C 2014 Angew. Chem. Int. Ed. 53 13596
[2] Raja P B and Sethuraman M G 2008 Mater. Lett. 62 13
[3] Fu J, Chen S Y and Zhou Y L 2012 Chem. Engineer 11 45
[4] Wu X F, Wu W, Wu Q Y and Yan W F 2017 Langmuir 33 4242
[5] Zhang J S and Huang K Y 2000 Mater. Protection 33 11
[6] Wu Q Y 2010 Modern Inorganic Synthesis and Preparation Chemistry Beijing: Chemical
Industry Press p 60
[7] Tong X, Tian N Q, Zhu W M, Wu Q Y, Cao F H and Yan W F 2012 J. Alloys Compd. 544 37
[8] Ajmal M, Mideen A S and Quraishi M A 1994 Corrosion Sci. 36 79
[9] Rocchiccioli D C, Michel F, Raymonde F and Thouvenot R 1983 Inorg. Chem. 22 207
[10] Cai H X, Wu X F, Wu Q Y and Yan W F 2016 Dalton Trans. 45 14238
[11] Verma C, Ebenso E E and Quraishi M A 2017 J. Mol. Liq. 248 927
Synthesis of Phosphorus-Based Polyoxometalates and Their Corrosion Inhibition Performance
455
